In this issue of Blood, Klein et al report the first genetically engineered mouse model of natural killer (NK)-cell leukemia, unlocking in vivo preclinical investigation of disease biology and therapeutics in NK-cell malignancies.1
NK-cell malignancies include NK-cell large granular lymphocytic leukemia (NK-LGLL), also known as chronic lymphoproliferative disorder of NK cells, as well as the more aggressive extranodal NK/T-cell lymphoma (ENKTL) and aggressive NK-cell leukemia (ANKL) associated with Epstein-Barr virus infection.
Preclinical models to study the disease biology and evaluate new treatments in the rare NK-cell malignancies have thus far been lacking, as genetically engineered mouse models of NK-cell leukemia have been unavailable. Earlier murine models have relied on transplantation of malignant cells, such as patient-derived xenografts of ENKTL.2 Although such models are useful for assessing therapeutic regimens, they offer limited insights into mechanisms of disease initiation and progression.
Klein and colleagues built their model on the most common N642H mutation occurring in the signal transducer and activator of transcription 5B (STAT5B). Mutations in STAT5B are found in 5% of patients with NK-LGLL, and the JAK-STAT pathway is genetically activated in a third of patients.3,4 Given the relative simplicity of genetics in NK-LGLL, a murine model harboring a single oncogenic alteration can potentially accurately represent human disease.
The more aggressive NK-cell neoplasms, ENKTL and aggressive ANKL, also carry alterations in the JAK-STAT pathway, including STAT5B.5,6 However, these cancers are positive for Epstein-Barr virus and typically harbor several driver alterations, suggesting more complex disease biology involving the interplay of viral and host genetic mechanisms.
In addition to NK cells, STAT5B mutations occur in several T-cell malignancies, including T-cell LGLL, T-cell acute lymphoblastic leukemia, T-cell prolymphocytic leukemia, enteropathy-associated T-cell lymphoma, γδ T-cell lymphoma, and hepatosplenic T-cell lymphoma. In LGLL, the STAT5BN642H mutation has been linked to a more aggressive clinical course.3
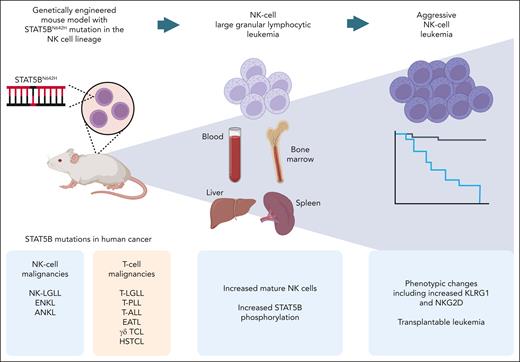
The mice with STAT5BN642H placed under the control of the Ncr1 (NKp46) promoter, expressed specifically in NK cells, displayed increased NK cell counts and STAT5B phosphorylation, recapitulating features of human disease (see figure). The resulting chronic NK-cell lymphoproliferation showed a mature phenotype and high expression of cytolytic molecules, consistent with an NK-LGLL phenotype.
Summary of the mouse model developed by Klein et al. The STAT5BN642H mutation, found in the indicated human cancers, is expressed in the NK-cell lineage, leading to a chronic NK-cell lymphoproliferation and progressing over time to ANKL in a subset of the mice. EATL, enteropathy-associated T-cell lymphoma; HSTCL, hepatosplenic T-cell lymphoma; T-ALL, T-cell acute lymphoblastic leukemia; T-LGLL, T-cell large granular lymphocytic leukemia; T-PLL, T-cell prolymphocytic leukemia; γδ TCL, γδ T-cell lymphoma. Figure created with BioRender.com.
Summary of the mouse model developed by Klein et al. The STAT5BN642H mutation, found in the indicated human cancers, is expressed in the NK-cell lineage, leading to a chronic NK-cell lymphoproliferation and progressing over time to ANKL in a subset of the mice. EATL, enteropathy-associated T-cell lymphoma; HSTCL, hepatosplenic T-cell lymphoma; T-ALL, T-cell acute lymphoblastic leukemia; T-LGLL, T-cell large granular lymphocytic leukemia; T-PLL, T-cell prolymphocytic leukemia; γδ TCL, γδ T-cell lymphoma. Figure created with BioRender.com.
Upon aging, a third of the mice developed an aggressive leukemic variant of the disease resembling ANKL. The development of an aggressive disease accompanied by a switch in cellular phenotype over time in a subset of the animals is a particularly interesting feature of the model. The phenotype of the aggressively proliferating NK cells included downregulation of CD11b and upregulation of CD27, potentially implying skewing toward the murine equivalent of the more immature CD56bright NK-cell subset in humans. This observation is consistent with the high CD56 expression reported in STAT5B-mutated NK-LGLL4 and ANKL.7
The progression to aggressive disease of the model provides several interesting directions for further inquiry that may shed light on human disease. Do spontaneous genetic or epigenetic alterations drive the progression? How does the molecular state of the malignant cells change, and which regulatory networks are responsible for the switch to aggressive growth? Are there immunological alterations in the nonmalignant immune compartment associated with disease progression?
Although transformation of human NK-LGLL to aggressive leukemia is rare with only individual cases reported,8 T-cell and NK-LGLL harboring STAT5BN642H have been reported to display a rapidly progressing chemorefractory phenotype possibly reflected by the murine model.3 The model may also give insight into the molecular mechanisms driving aggressive growth in ANKL and ENKTL, although these usually do not arise from an NK-LGLL background.
STAT5B mutations are found in various T- and NK-cell neoplasms, raising questions about the cell of origin: does the mutation drive differentiation of the transformed cells toward a certain lineage, or is the phenotype dictated by the cell in which the mutation originally occurred?
The authors’ findings provide evidence that a gain-of-function mutation in STAT5B occurring at the stage of differentiated NK cells is sufficient to drive leukemogenesis. Extrapolated to humans, the findings would indicate that STAT5B mutations found in human NK-cell malignancies would have occurred at a stage committed to the NK lineage. In contrast, overexpression of STAT5BN642H at the hematopoietic stem cell level leads to predominantly T-cell proliferations in mice.9 Collectively, the findings support a model in which a gain-of-function mutation in STAT5B occurring at an earlier hematopoietic progenitor stage drives T-cell leukemogenesis, whereas the same mutation occurring in differentiated NK cells induces chronic NK-LGLL, with potential for later aggressive transformation.
The new mouse model opens up several opportunities to explore the disease pathobiology of NK-cell malignancies in future studies. Given the strong association of LGLL with autoimmune phenomena, the model can help shed light on whether genetic alterations in the JAK-STAT pathway exacerbate immune-mediated pathologies. Beyond NK-cell malignancies, the model could enable investigation of the potential of genetic alterations such as STAT5BN642H to enhance antitumor function of NK cells, in light of recent work on exploiting naturally occurring mutations in cell therapies.10
Overall, the model developed by Klein and colleagues unlocks NK-cell malignancies to in vivo investigation of therapeutic regimens and prevention of transformation to an aggressive phenotype, as well as in-depth phenotypic and lineage-tracing studies of disease progression. The work points the way toward more complex models involving cooperating genetic alterations, enabling interrogation of therapeutic regimens for improved treatment of NK-cell malignancies.
Conflict-of-interest disclosure: O.D. reports research funding from Gilead Sciences and Incyte and personal fees from Sanofi (all outside the submitted work).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal