In this issue of Blood, Locke et al1 analyze outcomes of patients in the ZUMA-7 trial, but now stratified by metabolic tumor volume (MTV). The authors show that tumor burden, as measured by MTV, is prognostic of responses to second-line therapies for large B-cell lymphoma (LBCL).
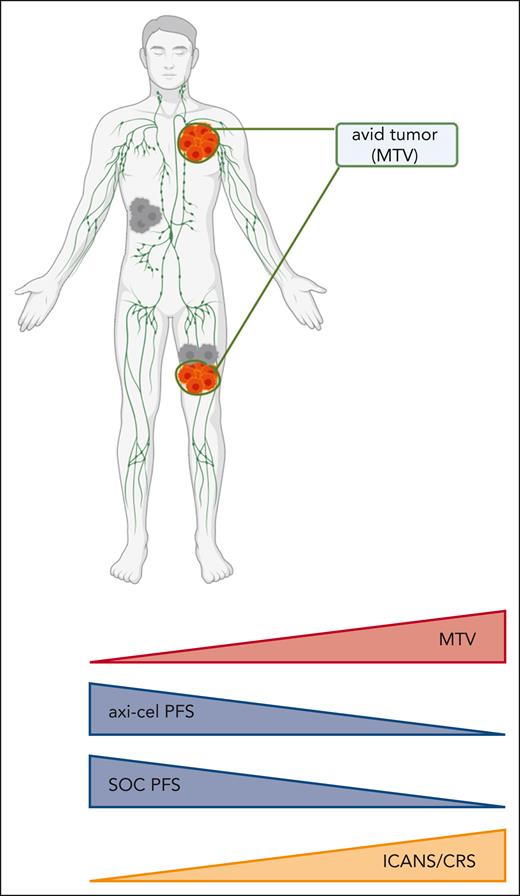
The pivotal ZUMA-7 trial demonstrated superiority of the CD19-directed chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (axi-cel) over previous standard-of-care (SOC) chemotherapy with autologous stem cell transplant for second-line treatment of LBCL.2 Despite remarkable responses, over half of the patients treated with axi-cel have experienced refractory or relapsed disease,3 prompting investigation into prognostic factors to risk-stratify patients and improve delivery of therapy. High tumor burden (as measured by sum of the product diameters) has been described as a negative prognostic factor for response to axi-cel in refractory LBCL.4 In their analysis of patients in the ZUMA-7 trial, Locke et al report that high tumor burden, as measured by MTV (see figure)---summative volume quantification of positron emission tomography–avid lesions---was associated with inferior duration of response in both the axi-cel and SOC chemotherapy cohorts of ZUMA-7. Importantly, axi-cel continued to demonstrate superior efficacy to SOC in both MTV-high and MTV-low patient cohorts. This finding supports the continued use of CAR T therapies in second-line management of LBCL for patients with high or low tumor burden.
Correlation of metabolic tumor burden with clinical outcomes in the ZUMA-7 trial. ICANS/CRS, incidence of grade 3+ immune effector cell–associated neurotoxicity syndrome/cytokine-release syndrome; MTV, metabolic tumor volume; PFS, progression-free survival; SOC, standard-of-care high-dose chemotherapy with autologous stem cell transplant. Figure created with BioRender.com.
Correlation of metabolic tumor burden with clinical outcomes in the ZUMA-7 trial. ICANS/CRS, incidence of grade 3+ immune effector cell–associated neurotoxicity syndrome/cytokine-release syndrome; MTV, metabolic tumor volume; PFS, progression-free survival; SOC, standard-of-care high-dose chemotherapy with autologous stem cell transplant. Figure created with BioRender.com.
Locke and colleagues report median tumor burden was increased in axi-cel–treated patients who experienced severe toxicity (grade 3+ immune effector cell–associated neurotoxicity or cytokine-release syndrome) compared with those with mild or no toxicity. This finding has significant implications for ongoing efforts5 to move cellular therapies to the outpatient setting in low-risk patients. Current CAR T therapies are typically administered on an inpatient basis, but the ability to deliver these therapies in the outpatient setting is appealing for the potential to increase access and decrease burden on both patients and hospital systems. Despite significant progress in management of adverse events from cellular therapies, the risk of high-grade toxicities in the days after CAR T infusion remains the most significant barrier to outpatient protocols.6 Assessment of metabolic tumor burden as a risk factor for CAR T-related toxicities represents a promising addition to the prognostic variables used to predict risk of adverse events. Effective risk stratification of patients and efforts to prophylactically treat high-risk patients7 to prevent toxicity are crucial to the success of transitioning cellular therapies to the outpatient setting.
Using continuous-variable analysis of MTV, Locke et al show that increasing tumor burden had a similar degree of negative impact on the efficacy of patients treated with axi-cel and SOC, implying that the decrement to efficacy described in patients with high tumor burden is not specific to a mechanism of axi-cel or chemotherapy. In contrast, a recent analysis using gene-expression signatures from pretreatment tumor biopsies of patients in ZUMA-7 elucidated degree of CD19 expression and certain tumor microenvironment contextures as prognostic variables for efficacy of axi-cel, but not SOC.8 Further work is needed to delineate the individual contribution of these variables to the efficacy of cellular therapies, including understanding whether these factors are dependent on treatment sequencing and/or the cellular therapy product used. Understanding the interaction of tumor burden, microenvironment, target antigen expression, and other relevant clinical factors will aid in the delivery of optimized CAR T-cell products.
Locke and colleagues’ findings, as well as data from other studies highlighting increased tumor burden as a negative prognostic factor in LBCL, raise the question of whether reduction of tumor burden with bridging therapy before CAR T infusion would improve efficacy. Some retrospective analyses have corroborated this finding, showing that bridging therapy improves responses in LBCL.9 However, there is a need for more prospective data, particularly in patients with high tumor burden. Understanding whether bridging regimens targeted at tumor burden reduction before CAR T treatment can abrogate the negative effects of high-burden disease, and, if particular regimens are more effective in doing so, would inform clinical practice.
Although Locke et al clearly demonstrate how metabolic volume of tumor is an important prognostic variable in LBCL, translation of these findings in the ZUMA-7 cohort to real-world patient populations is limited by a need for standardization of measurement of MTV. Positron emission tomography–avidity quantification can vary between machines/institutions,10 and MTV is not routinely calculated in clinical practice. Furthermore, recent findings from ZUMA-7 show that tumor burden measured by sum-of-product diameters does not correlate with axi-cel outcomes,8 indicating that incorporation of a metabolic measurement is necessary to capture the biologically relevant tumor burden. Future work to standardize metabolic tumor burden assessments and define broadly applicable cut-offs for high/low-burden disease will facilitate incorporation of this important factor into clinical prognostication for cellular therapies.
In conclusion, Locke et al provide valuable insight into the effect of pretreatment tumor burden on responses to modern therapies for relapsed/refractory LBCL. Their data add to a growing body of evidence that tumor burden can be used to risk-stratify patients receiving cell therapies, aiding efforts to triage patients to outpatient CAR T therapy and highlighting the need for prospective trials targeting tumor reduction before CAR T therapy in high-burden patients. Standardization of metabolic tumor burden assessments will aid in the translation of these important findings to the clinic.
Conflict-of-interest disclosure: M.V.M. is an inventor on patents related to adoptive cell therapies, held by Massachusetts General Hospital (some licensed to Promab) and the University of Pennsylvania (some licensed to Novartis); has received grant/research support from Kite Pharma and Moderna; has served as a consultant for multiple companies involved in cell therapies; holds equity in 2SeventyBio, A2Bio, Cargo, Century Therapeutics, Neximmune, Oncternal, and TCR2; serves on the board of directors for 2Seventy Bio; has been or is a consultant for A2Bio (scientific advisory board), Adaptimmune, Agenus/Mink Therapeutics, Allogene, Arcellx, Astellas, AstraZeneca, Atara, Bristol Myers Squibb, Cabaletta Bio (scientific advisory board), Cargo (scientific advisory board), CRISPR Therapeutics, In8bio (scientific advisory board), Intellia, GlaxoSmithKline, Kite Pharma, Maxyte (scientific advisory board), Neximmune, Novartis, Oncternal, Sanofi, Sobi, Synthekine, TCR2 (scientific advisory board), and Tmunity. E.P.D. declares no conflict of interest.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal