At clinically-relevant doses, selective JAK1 and JAK2 inhibitors are well-tolerated and suppress IFN-γ–mediated STAT1 phosphorylation.
Selective JAK1 vs JAK2 inhibition differentially affects the clinical and laboratory manifestations of disease in mouse models of HLH.
Visual Abstract
Hemophagocytic lymphohistiocytosis (HLH) comprises a severe hyperinflammatory phenotype driven by the overproduction of cytokines, many of which signal via the JAK/STAT pathway. Indeed, the JAK1/2 inhibitor ruxolitinib has demonstrated efficacy in preclinical studies and early-phase clinical trials in HLH. Nevertheless, concerns remain for ruxolitinib-induced cytopenias, which are postulated to result from the blockade of JAK2-dependent hematopoietic growth factors. To explore the therapeutic effects of selective JAK inhibition in mouse models of HLH, we carried out studies incorporating the JAK1 inhibitor itacitinib, JAK2 inhibitor fedratinib, and JAK1/2 inhibitor ruxolitinib. All 3 drugs were well-tolerated and at the doses tested, they suppressed interferon-gamma (IFN-γ)–induced STAT1 phosphorylation in vitro and in vivo. Itacitinib, but not fedratinib, significantly improved survival and clinical scores in CpG–induced secondary HLH. Conversely, in primary HLH, in which perforin-deficient (Prf1−/−) mice are infected with lymphocytic choriomeningitis virus (LCMV), itacitinib, and fedratinib performed suboptimally. Ruxolitinib demonstrated excellent clinical efficacy in both HLH models. RNA-sequencing of splenocytes from LCMV-infected Prf1−/− mice revealed that itacitinib targeted inflammatory and metabolic pathway genes in CD8 T cells, whereas fedratinib targeted genes regulating cell proliferation and metabolism. In monocytes, neither drug conferred major transcriptional impacts. Consistent with its superior clinical effects, ruxolitinib exerted the greatest transcriptional changes in CD8 T cells and monocytes, targeting more genes across several biologic pathways, most notably JAK-dependent proinflammatory signaling. We conclude that JAK1 inhibition is sufficient to curtail CpG-induced disease, but combined inhibition of JAK1 and JAK2 is needed to best control LCMV-induced immunopathology.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a severe inflammatory phenotype characterized by the excessive activation of CD8 T cells and macrophages that overproduce cytokines and mediate significant tissue damage.1 Primary (familial) HLH is caused by germ line mutations in genes required for lymphocyte cytotoxic function.1 In contrast, secondary (nonfamilial) HLH occurs in individuals without identifiable mutations, but after certain inciting triggers such as infections, malignancies, or autoimmune disorders.1 Despite current treatments, up to 40% of patients with HLH die due to disease or the complications of its treatment.1 Accordingly, there is a pressing need to develop better and safer therapies. Regardless of its etiology, a key factor driving the pathogenesis of HLH is the excessive secretion of proinflammatory cytokines, including interferon-gamma (IFN-γ), interleukins (IL)-2, IL-6, and granulocyte macrophage colony stimulating factor (GM-CSF), which signal via the Janus kinases (JAKs).2,3 Based on this property, JAK inhibition has been trialed as a novel therapeutic intervention in HLH, with studies in mice and humans demonstrating that the JAK1/2 inhibitor ruxolitinib significantly improves disease manifestations.4-8
Despite these promising results, hematologic toxicities occur after administration of ruxolitinib, and they pose a potential challenge for patients with HLH who often present with cytopenias at diagnosis. Anemia and/or thrombocytopenia have been reported after ruxolitinib treatment in patients with myelofibrosis9,10 and in those with graft-versus-host disease.11 These cytopenias are hypothesized to result from blockade of hematopoietic growth factors that signal via JAK2, such as erythropoietin, thrombopoietin, granulocyte colony stimulating factor (G-CSF), and GM-CSF.12 Alternatively, they might result from incomplete blockade of IFN-γ or other marrow suppressive cytokines such as tumor necrosis factor (TNF).
Concerns for toxicity were also raised in a previous study, in which ruxolitinib but not AZD4205 (a selective JAK1 inhibitor) decreased the survival of lymphocytic choriomeningitis virus (LCMV)–infected Prf1−/− mice (a model of primary HLH). The poorer outcome of ruxolitinib-treated mice was proposed to result from blockade of JAK2 signaling.13 To our knowledge, the hematopoietic effects of in vivo JAK2 inhibition have not been well established in naïve mice. However, in mouse models of JAK2–driven myeloproliferative disorders, JAK2 inhibition using fedratinib resulted in significant and dose-dependent leukopenia and anemia.14 Given these concerns, our aim was to assess whether blocking JAK1 signaling, while sparing JAK2 signaling, might lessen inflammation and minimize hematologic toxicities using mouse models of HLH.
Methods
Mice
Perforin-deficient (C57BL/6J Prf1tm1Sdz/J) and wildtype (WT; C57BL/6J) mice were from The Jackson Laboratory. Sex- and age-matched animals of 8 to 12 weeks were used for these studies. Mice were housed in specific pathogen-free facilities and experiments conducted with approval of the institutional animal care and use committee at the St Jude Children’s Research Hospital.
JAK inhibitors
Itacitinib adipate (Incyte Corporation) was prepared in 0.5% weight/volume (w/v) methylcellulose (FujiFilm) using salt factor of 1.26 and overnight agitation at 4°C. Fedratinib hydrochloride hydrate (MedChem Express) was prepared in 0.5% w/v methylcellulose using salt factor of 1.173. Ruxolitinib (provided by Ross Levine, Memorial Sloan Kettering Cancer Center, New York, NY) was prepared fresh daily in citrate buffer (0.1 M, pH 3.5) with captisol (20% w/v), as previously described.4
Plasma PK
Plasma pharmacokinetics (PK) studies were conducted after single free base equivalent doses of 120 mg/kg fedratinib or itacitinib, and 90 mg/kg ruxolitinib. For each drug, a staggered survival sampling design was used to collect blood samples at 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, and 24 hours. JAK inhibitor plasma levels were quantified using qualified liquid chromatography–tandem mass spectrometry and analyzed by the St. Jude Preclinical Pharmacokinetics Shared Resource (see also supplemental Methods, available on the Blood website).
Detection of STAT1 phosphorylation in macrophages
Samples of bone-marrow–derived and thioglycolate–elicited peritoneal macrophages (see supplemental Methods) were fixed with warmed BD Pharmingen Phosphoflow Fix Buffer I, followed by staining with anti-CD11b (M1/70, Tonbo) and anti-F4/80 (BM8.1, Tonbo) for 10 minutes at room temperature (bone marrow–derived macrophages) or anti-F4/80 antibody on ice for 30 minutes (peritoneal macrophages), then permeabilized with BD Pharmingen Phosphoflow Fix Buffer III. Cells were washed and stained with antiphosphorylated-STAT1 antibody (pSTAT1, pY701, BD Biosciences) for 1 hour at room temperature. Anti-mouse IgG2a antibody was used as a negative control. Samples were collected using a LSR II flow cytometer (BD Biosciences) and data analyzed using FlowJo (v10.8.1).
Tolerability of JAK inhibitors in vivo
WT mice were treated with 0.5% methylcellulose vehicle, 120 mg/kg itacitinib, 60 mg/kg fedratinib, or 90 mg/kg ruxolitinib administered in 200 μL volume by oral gavage twice daily for 30 days. On day 30, mice were humanely euthanized for analysis.
Modeling HLH in mice
For the secondary (nonhereditary) model, WT mice were injected intraperitoneally with CpG 1826 (50 μg per mouse; Integrated DNA Technologies) and 0.2 mg of an IL-10 receptor-blocking antibody (αIL-10R; clone 1B1.3A; Bio X Cell) on days 0, 2, 4, and 7, as previously reported.15,16 Mice were treated with 0.5% methylcellulose vehicle, itacitinib (120 mg/kg), fedratinib (60 mg/kg), or ruxolitinib (90 mg/kg) in 200 μL volume twice daily via oral gavage on days 4 through 8 (or 9 for survival study/clinical scores) after the first CpG/αIL-10R injection. Mice were then humanely euthanized for tissue collection and analysis on day 9 (or 10). Clinical scores were calculated in a blinded fashion as previously described.17
For the primary (hereditary) model,Prf1−/− mice were infected intraperitoneally with 2 × 105 plaque forming units LCMV. Mice were treated with 0.5% methylcellulose vehicle, itacitinib (120 mg/kg), fedratinib (60 mg/kg), or ruxolitinib (90 mg/kg) in 200 μL volume via oral gavage twice daily on days 4 through 8 (short term experiment) or days 4 through 29 (survival study) after LCMV infection.4,5,13 Mice were humanely euthanized on day 9 or day 30 post-infection (PI) for analysis of laboratory parameters or survival/clinical scores, respectively. For both models, disease was assessed as described6,18 (supplemental Methods).
Cell sorting and RNA sequencing
CD8 T cells and monocytes were sort-purified from the spleens of Prf1−/− mice collected on day 9 PI, 1 hour after receiving the 11th dose of JAK inhibitor or vehicle. For RNA-sequencing analysis, we used R package voom-limma19 for count normalization and differential gene expression analysis. A false discovery rate of <0.05 and fold change >2 were used to determine significance. Overrepresentation analysis of genes within each cluster of heat maps was performed using hallmark gene sets. Separately, ranked gene lists were used to run gene set enrichment analysis20 (see also supplemental Methods).
Statistical analyses
Outliers were removed with Grubb’s test using GraphPad Prism outlier calculator. Mean outcome measurements were compared across the groups by 1-way analysis of variance (ANOVA) and post-ANOVA comparisons were performed using Tukey’s multiple comparisons test. For survival studies, the log-rank test was performed to determine statistical significance between treated groups and vehicle control. For clinical score studies, statistical significance was determined using 2-way ANOVA followed by Tukey’s multiple comparisons test.
Experiments involving mice were conducted under the approval of the institutional animal care and use committee and institutional biosafety committee at the St. Jude Children’s Research Hospital.
Results
Plasma PK of JAK inhibitors in C57BL/6 mice
Because the prior preclinical studies of JAK inhibition for HLH have not included PK analyses, it has been challenging to interpret whether poor clinical responses or toxicities were due to inadequate or supratherapeutic drug levels, respectively. Further, without PK information it is difficult to know whether the plasma drug levels achieved in mice correlate with those seen in humans. To address these gaps, we performed PK analyses after administration of a single dose of itacitinib, fedratinib, or ruxolitinib to WT C57BL/6 mice. We observed rapid absorption, high apparent plasma clearance in excess of the hepatic blood flow, and high apparent volumes of distribution (Figure 1A; supplemental Table 1). Itacitinib and fedratinib reached Cmax at 0.25 hour and ruxolitinib at 0.125-hour post-dose. Plasma concentrations of itacitinib and fedratinib diminished in a bi-exponential manner, whereas ruxolitinib showed tri-exponential decay. The apparent terminal half-lives were 14.2 hours for itacitinib, 7.8 hours for fedratinib, and 1.51 hours for ruxolitinib.
Pharmacokinetic and pharmacodynamic studies of JAK1, JAK2, and JAK1/2 inhibition. (A) Plasma drug levels (μg/L) in naïve C57BL/6J mice after single oral dose of each inhibitor (120 mg/kg itacitinib, 120 mg/kg fedratinib, 90 mg/kg ruxolitinib). Mean values ± standard deviation (SD) are shown. (B) Intracellular STAT1 phosphorylation measured in bone marrow–derived macrophages (BMDM) cultured with vehicle (methyl cellulose) or itacitinib (2500, 500, or 50 nM), (C) vehicle (methyl cellulose) or fedratinib (1000, 300, or 30 nM), or (D) vehicle (captisol in citrate buffer) or ruxolitinib (1000, 50, or 1 nM) for 1 hour and then stimulated with IFN-γ (30 ng/mL) for 20 minutes. Representative overlayed histogram plots are shown below each graph. Experiments were performed using duplicates. Data are representative of 2 independent experiments. (E) Intracellular STAT1 phosphorylation measured in thioglycolate–elicited peritoneal macrophages after a single oral dose of JAK inhibitor and intraperitoneal (500 ng) injection of IFN-γ, shown as percentage of pSTAT1+ F4/80+ macrophages. F4/80lo macrophages were excluded from the gating. (F) Representative histograms (left) and fold-change mean fluorescence intensity (MFI) (right) compared to isotype control. Each data point in panels E-F represents 1 mouse. Data are representative of 2 independent experiments. ∗P < .05, ∗∗∗P < .001, or ∗∗∗∗P < .0001 by pairwise comparison.
Pharmacokinetic and pharmacodynamic studies of JAK1, JAK2, and JAK1/2 inhibition. (A) Plasma drug levels (μg/L) in naïve C57BL/6J mice after single oral dose of each inhibitor (120 mg/kg itacitinib, 120 mg/kg fedratinib, 90 mg/kg ruxolitinib). Mean values ± standard deviation (SD) are shown. (B) Intracellular STAT1 phosphorylation measured in bone marrow–derived macrophages (BMDM) cultured with vehicle (methyl cellulose) or itacitinib (2500, 500, or 50 nM), (C) vehicle (methyl cellulose) or fedratinib (1000, 300, or 30 nM), or (D) vehicle (captisol in citrate buffer) or ruxolitinib (1000, 50, or 1 nM) for 1 hour and then stimulated with IFN-γ (30 ng/mL) for 20 minutes. Representative overlayed histogram plots are shown below each graph. Experiments were performed using duplicates. Data are representative of 2 independent experiments. (E) Intracellular STAT1 phosphorylation measured in thioglycolate–elicited peritoneal macrophages after a single oral dose of JAK inhibitor and intraperitoneal (500 ng) injection of IFN-γ, shown as percentage of pSTAT1+ F4/80+ macrophages. F4/80lo macrophages were excluded from the gating. (F) Representative histograms (left) and fold-change mean fluorescence intensity (MFI) (right) compared to isotype control. Each data point in panels E-F represents 1 mouse. Data are representative of 2 independent experiments. ∗P < .05, ∗∗∗P < .001, or ∗∗∗∗P < .0001 by pairwise comparison.
We compared our in vivo PK results to available human data at approved or recommended phase 2 doses for each JAK inhibitor, accounting for differences in mouse vs human plasma exposure and interspecies plasma protein binding, to estimate the clinically-relevant doses for mice. Itacitinib PK compared well with previous findings in mice, with Cmax and area under the curve (AUC) within 25% of those expected from 80 mg/kg dosing.21 Although the itacitinib fraction unbound in plasma for mice has not been reported, it is assumed to be similar to that of humans at ∼0.3.22,23 Therefore, a clinically-relevant itacitinib dose for mice was calculated as 17.5 mg/kg PO twice daily. Nevertheless, prior studies have shown that higher doses up to 120 mg/kg are well-tolerated and effective in controlling inflammation in models of colitis, graft-versus-host disease, and cytokine release syndrome.21,24 Based on these reports, we chose a dose of 120 mg/kg twice daily for this study.
Fedratinib Cmax and AUC were ∼1.6-fold higher than previous findings in C57BL/6J mice dosed with 100 and 200 mg/kg.14 These higher exposures may have resulted from hemolysis in plasma samples or our use of an optimal salt and suspension formulation. In clinical studies, the total plasma AUC of fedratinib at steady state was 30 800 h∗μg/L with the FDA-approved dose of 400 mg PO once daily.25 Accounting for the estimated fraction of unbound fedratinib in humans and mice26 and the short half-life of fedratinib in mice compared with humans (7.8 vs 114 hours), 60 mg/kg PO twice daily was determined to be a clinically-relevant dose. Based on these PK studies, the fedratinib dose chosen for our studies was 60 mg/kg.
Ruxolitinib PK also compared well with previous findings in mice, although our AUC trended higher by ∼35%.27,28 In a phase 1 clinical trial of ruxolitinib for children with cancer, the mean Cmax and AUC values were 1170 μg/L and 5760 h∗μg/L, respectively, after a single oral dose of 50 mg/m2 ruxolitinib.29 Accounting for differences in unbound drug fraction in humans and mice, 66 mg/kg of ruxolitinib phosphate was determined to be a clinically-relevant dose. In prior studies completed in our laboratory, we observe comparable efficacy of ruxolitinib when dosed at 60 mg/kg using a chow formulation or 90 mg/kg twice daily given by oral gavage (not shown). Because all our prior experiments incorporating oral gavage used 90 mg/kg twice daily and ruxolitinib is being included here as a historical comparator, we chose 90 mg/kg twice daily herein.
Administration of selective JAK inhibitors blocks IFN-γ-induced STAT1 phosphorylation and is well-tolerated in vivo
To verify that the JAK inhibitors could dampen downstream signaling, STAT1 phosphorylation (pSTAT1) was assessed using bone marrow–derived macrophages pretreated in vitro with increasing concentrations of JAK inhibitors before stimulation with IFN-γ, which uses JAK1 and JAK2 for its signaling.3 Itacitinib, fedratinib, or ruxolitinib each inhibited STAT1 phosphorylation in a dose-dependent manner (Figure 1B-D). STAT1 phosphorylation of peritoneal macrophages was also assessed in vivo after intraperitoneal administration of a single dose of IFN-γ. Vehicle-treated mice had significantly higher percentages of pSTAT1-positive F480+ peritoneal macrophages compared with control mice that had not received IFN-γ (Figure 1E). Further, the macrophages from vehicle-treated mice exhibited a significantly higher mean fluorescence intensity of pSTAT1 compared to controls (Figure 1F). At the time point chosen, itacitinib (120 mg/kg), fedratinib (60 mg/kg), and ruxolitinib (90 mg/kg) each significantly decreased STAT1 phosphorylation when compared to vehicle (Figure 1E-F), confirming that in vivo blockade can effectively be achieved.
To test the tolerability of the JAK inhibitors in vivo, we treated WT naïve mice with itacitinib, fedratinib, or ruxolitinib at the chosen doses for 30 days. Treatment did not lead to any significant untoward effects on clinical score, body weight, bone marrow cellularity, or peripheral blood cell counts compared to vehicle-treated controls (supplemental Figure 1A-E and data not shown). Collectively, these studies reveal that at the doses chosen for this study, each JAK inhibitor leads to plasma levels that correlate with those achievable in humans and effectively inhibits JAK1/2 signaling with no overt baseline toxicity.
Selective JAK1 inhibition lessens disease manifestations in a model of secondary HLH
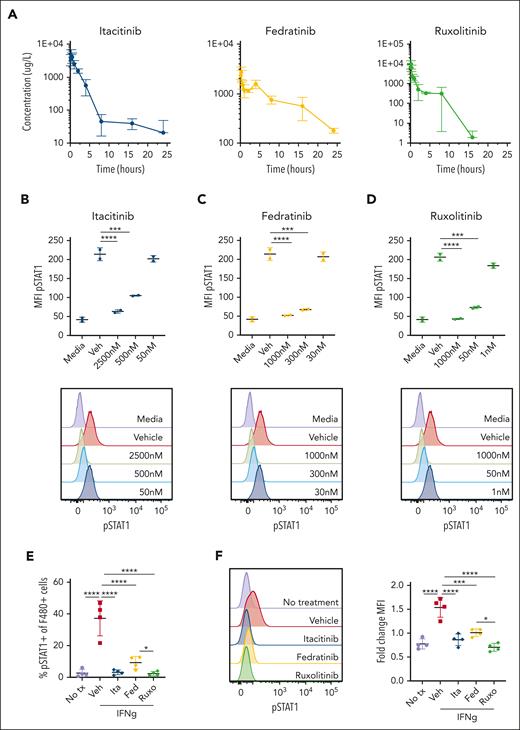
With clinically-relevant doses, functional in vivo JAK inhibition, and drug tolerability established, we next sought to determine the efficacy of these agents in a model of secondary HLH in which C57BL/6J mice are serially administered CpG DNA and anti-IL-10 receptor (αIL-10R) antibody.16 Mice receiving CpG/αIL-10R were treated with vehicle, itacitinib, fedratinib, or ruxolitinib and analyses were performed on day 9 or 10 after the first CpG/αIL-10R injection (Figure 2A). Treatment with itacitinib significantly prolonged survival and lessened clinical scores compared to treatment with vehicle, and it did so in a manner comparable to ruxolitinib (Figure 2B-C). In contrast, fedratinib failed to improve either of these clinical outcomes. Despite its beneficial clinical effects, splenomegaly and thrombocytopenia did not improve after itacitinib treatment and anemia was more severe than that observed in vehicle-treated animals (Figure 2D-G).
JAK1 inhibition improves survival and clinical scores in CpG/αIL-10R-induced HLH. (A) Experimental schema for modeling secondary HLH, wherein WT C57BL/6J mice receive injections of CpG and αIL-10R antibody on days 0, 2, 4, and 7, and are then analyzed on day 9 or 10 after the first injection. (B) Probability of survival (B) and clinical scores (C) from naïve, or CpG/αIL-10R-treated mice who received vehicle, itacitinib (120 mg/kg twice daily [bid]), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) from days 4 to 9 after first CpG/αIL-10R injection. Analysis was performed on day 10. Data are combined from 2 independent experiments (9-10 mice per group). (D) Spleen weights shown as percentage of final body weight in mice treated with vehicle, itacitinib (120 mg/kg bid), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) on days 4 to 8 after the first CpG/αIL-10R injection. Analysis was performed on day 9. Peripheral blood hemoglobin (E), hematocrit (F), and platelet counts (G). Data points in panels D-G represent single mice from 3 pooled experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, or ∗∗∗∗P < .0001 by pairwise comparison. For survival, log-rank test was performed to determine significance between treated groups and vehicle control. For clinical score, statistical significance was determined using two-way ANOVA.
JAK1 inhibition improves survival and clinical scores in CpG/αIL-10R-induced HLH. (A) Experimental schema for modeling secondary HLH, wherein WT C57BL/6J mice receive injections of CpG and αIL-10R antibody on days 0, 2, 4, and 7, and are then analyzed on day 9 or 10 after the first injection. (B) Probability of survival (B) and clinical scores (C) from naïve, or CpG/αIL-10R-treated mice who received vehicle, itacitinib (120 mg/kg twice daily [bid]), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) from days 4 to 9 after first CpG/αIL-10R injection. Analysis was performed on day 10. Data are combined from 2 independent experiments (9-10 mice per group). (D) Spleen weights shown as percentage of final body weight in mice treated with vehicle, itacitinib (120 mg/kg bid), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) on days 4 to 8 after the first CpG/αIL-10R injection. Analysis was performed on day 9. Peripheral blood hemoglobin (E), hematocrit (F), and platelet counts (G). Data points in panels D-G represent single mice from 3 pooled experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, or ∗∗∗∗P < .0001 by pairwise comparison. For survival, log-rank test was performed to determine significance between treated groups and vehicle control. For clinical score, statistical significance was determined using two-way ANOVA.
Innate immune activation is central to disease pathogenesis in the CpG/αIL-10R model.16,30 Therefore, we quantified the proportions, numbers, and activation status of splenic monocytes and neutrophils in CpG/αIL-10R-exposed mice (gating strategy as in supplemental Figure 2). Compared with naïve mice, mice exposed to CpG/αIL-10R and treated with vehicle exhibited elevated numbers of CD11b+Ly6C+Ly6G− monocytes (Figure 3A-B) and CD11b+Ly6G+ neutrophils (Figure 3C-D), many of which expressed the activation marker CD80. Itacitinib treatment did not decrease the frequencies or numbers of monocytes, but it significantly reduced the frequencies and numbers of neutrophils, including those expressing CD80 (Figure 3C-D). Paradoxically, despite its lack of clinical benefit, fedratinib significantly decreased the numbers of total and CD80+ monocytes as well as the numbers of total and CD80+ neutrophils (Figure 3A-D).
JAK1 and JAK2 inhibition differentially ameliorate CpG/αIL-10R-induced activation and accumulation of splenic monocytes and neutrophils, as well as serum cytokine levels. (A) Proportions and numbers of total Ly6C+CD11b+ splenic monocytes and (B) CD80+ splenic monocytes in naïve mice or CpG+aIL10R treated mice treated with vehicle, itacitinib (120 mg/kg bid), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) from days 4 to 8 after the first CpG+aIL10R injection. Analysis was performed on day 9. Proportions and absolute numbers of total Ly6G+CD11b+ splenic neutrophils (C) and CD80+ splenic neutrophils (D). (E-L) Serum cytokine levels. Data points represent single mice from 3 pooled experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, or ∗∗∗∗P < .0001 by pairwise comparison.
JAK1 and JAK2 inhibition differentially ameliorate CpG/αIL-10R-induced activation and accumulation of splenic monocytes and neutrophils, as well as serum cytokine levels. (A) Proportions and numbers of total Ly6C+CD11b+ splenic monocytes and (B) CD80+ splenic monocytes in naïve mice or CpG+aIL10R treated mice treated with vehicle, itacitinib (120 mg/kg bid), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) from days 4 to 8 after the first CpG+aIL10R injection. Analysis was performed on day 9. Proportions and absolute numbers of total Ly6G+CD11b+ splenic neutrophils (C) and CD80+ splenic neutrophils (D). (E-L) Serum cytokine levels. Data points represent single mice from 3 pooled experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, or ∗∗∗∗P < .0001 by pairwise comparison.
Finally, we examined whether itacitinib or fedratinib treatment might decrease CpG/αIL-10R-induced hypercytokinemia. Multiple proinflammatory cytokines increase in response to CpG/αIL-10R injections6 including IFN-γ, the IFN-γ-induced chemokine C-X-C motif chemokine ligand 10 (CXCL10), TNF, IL-12(p70), IL-6, GM-CSF, monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein (MIP)-1a (Figure 3E-L). Mice treated with itacitinib had significantly decreased levels of several of these cytokines, including CXCL10, IL-12(p70), IL-6, GM-CSF, MCP-1, and MIP1a (Figure 3F,H,J,L). In contrast, mice treated with fedratinib only showed reduction in the levels of IL-12(p70) (Figure 3H) and GM-CSF (Figure 3J). Together, these studies demonstrate that itacitinib, but not fedratinib, improves survival and decreases clinical scores in this model of secondary HLH, a finding associated with decreased tissue neutrophilia and reduced levels of proinflammatory cytokines and chemokines, many of which are produced by or involved in the recruitment and activation of myeloid cells – the cells central to this model.
Combined JAK1 and JAK2 inhibition is required for optimal amelioration of disease in primary HLH
We next turned our attention to a model of primary HLH in which perforin-deficient mice (Prf1−/−) are infected with LCMV. LCMV-induced immunopathology relies upon CD8 T cell production of IFN-γ.31,32 Nevertheless, levels of G-CSF (a JAK2-dependent cytokine) are elevated in the serum, and tissues demonstrate an influx of neutrophils.6 Therefore, we sought to determine whether selective JAK inhibition might affect these and other features of disease. Toward this end, we treated LCMV-infected Prf1−/− mice with itacitinib, fedratinib, ruxolitinib, or vehicle, and analyzed survival, clinical scores, and laboratory parameters (Figure 4A).
JAK1 inhibition partially improves survival and disease features in LCMV-induced HLH. (A) Experimental schema for modeling primary HLH, wherein Prf1−/− mice are infected with LCMV and analyzed day 9 or 30 PI. Probability of survival (B) and clinical scores (C) of naïve, or LCMV- infected Prf1−/− mice treated with vehicle, itacitinib (120 mg/kg bid), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) from days 4 to 29 PI (5 mice per group). Analysis was performed on day 30 PI. Peripheral blood hemoglobin (D), platelet counts (E), spleen weights (F) shown as percentage of final body weight, and (G-L) serum cytokine levels of mice analyzed on day 9 PI. Data points represent single mice from 5 pooled experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, or ∗∗∗∗P < .0001 by pairwise comparison. For survival, the log-rank test was performed to determine statistical significance between treated groups and vehicle control. For clinical score, statistical significance was determined using two-way ANOVA.
JAK1 inhibition partially improves survival and disease features in LCMV-induced HLH. (A) Experimental schema for modeling primary HLH, wherein Prf1−/− mice are infected with LCMV and analyzed day 9 or 30 PI. Probability of survival (B) and clinical scores (C) of naïve, or LCMV- infected Prf1−/− mice treated with vehicle, itacitinib (120 mg/kg bid), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) from days 4 to 29 PI (5 mice per group). Analysis was performed on day 30 PI. Peripheral blood hemoglobin (D), platelet counts (E), spleen weights (F) shown as percentage of final body weight, and (G-L) serum cytokine levels of mice analyzed on day 9 PI. Data points represent single mice from 5 pooled experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, or ∗∗∗∗P < .0001 by pairwise comparison. For survival, the log-rank test was performed to determine statistical significance between treated groups and vehicle control. For clinical score, statistical significance was determined using two-way ANOVA.
In these studies, itacitinib partially enhanced survival and lessened clinical scores, whereas fedratinib conferred no clinical benefit (Figure 4B-C). As we have previously observed,4,6,7,18 ruxolitinib was the most effective agent in promoting survival. Ruxolitinib also significantly improved clinical scores. Analyses on day 9 PI revealed that itacitinib improved splenomegaly (Figure 4F), but it had no effect on anemia or thrombocytopenia (Figure 4D-E), and it did not decrease the levels of proinflammatory cytokines, including IFN-γ, TNF, and sCD25 (Figure 4G-J). As with the secondary model, fedratinib appeared to improve multiple disease parameters despite showing minimal to no improvement in survival or clinical scores.
LCMV-infected, vehicle-treated mice exhibited significantly increased percentages and numbers of splenic total, CD44+ effector, and gp33-specific CD8 T cells (Figure 5A-C). Further, these CD8 T cells exhibited increased capacity to produce IFN-γ when stimulated ex vivo with gp33 peptide (Figure 5D-G). Although itacitinib ameliorated clinical readouts, we observed no reductions in CD8 T cells or IFN-γ production in itacitinib-treated mice (Figure 5A-G). Again, fedratinib, which performed poorly from a clinical perspective, significantly reduced the numbers of CD8 T cells (Figure 5B-C) and their ability to produce IFN-γ (Figure 5D-G), doing so in a manner comparable to ruxolitinib. Collectively, these findings reveal that combined inhibition of JAK1 and JAK2 with ruxolitinib is required to optimally promote survival, lessen clinical scores, and decrease the accumulation and activation of disease-promoting CD8 T cells in this model.
JAK1 inhibition is not sufficient to reduce CD8 T-cell number or capacity to produce IFN-γ. Proportions and numbers of total splenic CD8 T cells (A), CD44+ effector CD8 T cells (B), and gp33-specific CD8 T cells (C) in naïve mice or LCMV-infected Prf1−/− mice treated with vehicle, itacitinib (120 mg/kg), fedratinib (60 mg/kg), or ruxolitinib (90 mg/kg) from days 4 to 8 PI. Analysis was performed on day 9 PI. (D-E) Representative contour plots and summary data of cytokine producing CD44+ CD8 T cells (D-E) and gp33+ CD8 T cells (F-G) after in vitro restimulation with gp33 peptide. Data points represent single mice from 5 pooled experiments. ∗P < .05, ∗∗P < .01, or ∗∗∗∗P < .0001 by pairwise comparison.
JAK1 inhibition is not sufficient to reduce CD8 T-cell number or capacity to produce IFN-γ. Proportions and numbers of total splenic CD8 T cells (A), CD44+ effector CD8 T cells (B), and gp33-specific CD8 T cells (C) in naïve mice or LCMV-infected Prf1−/− mice treated with vehicle, itacitinib (120 mg/kg), fedratinib (60 mg/kg), or ruxolitinib (90 mg/kg) from days 4 to 8 PI. Analysis was performed on day 9 PI. (D-E) Representative contour plots and summary data of cytokine producing CD44+ CD8 T cells (D-E) and gp33+ CD8 T cells (F-G) after in vitro restimulation with gp33 peptide. Data points represent single mice from 5 pooled experiments. ∗P < .05, ∗∗P < .01, or ∗∗∗∗P < .0001 by pairwise comparison.
JAK1 vs JAK2 inhibitors uniquely influence immune cell transcriptional profiles
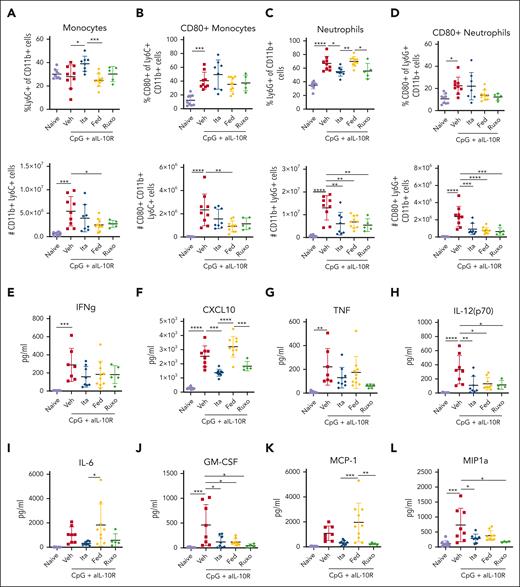
In the primary model, itacitinib modestly prolonged survival and improved clinical scores yet failed to ameliorate many immunologic parameters of HLH. Conversely, fedratinib had the opposite effects. To elucidate the mechanisms underlying these contradictory findings and place them in context with the superior results conferred by ruxolitinib, we performed RNA sequencing of splenic CD8 T cells and monocytes collected on day 9 PI. Unsupervised clustering showed modest separation of CD8 T cell and monocyte transcriptional profiles by treatment group (Figures 6A and 7A). Examination of significant differentially expressed genes (DEGs) in CD8 T cells revealed only 3 genes uniquely affected by itacitinib but 337 by fedratinib and 342 by ruxolitinib, as compared with vehicle (Figure 6B). Analysis of monocyte data revealed 3 genes uniquely affected by itacitinib, 2 by fedratinib, and 250 by ruxolitinib (Figure 7B). Overall, ruxolitinib led to the greatest number of significant DEGs in both cell types (supplemental Tables 2-5).
Changes in transcriptional profiles of splenic CD8 T cells from Prf1−/− mice infected with LCMV and treated with a JAK1, JAK2, or combined JAK1/2 inhibitor. (A) Unsupervised clustering using multidimensional scaling of transcriptional profiles of splenic CD8 T cells from mice infected with LCMV and treated with vehicle, itacitinib (120 mg/kg bid), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) with 3 mice per group. (B) Venn diagram depicting the numbers of DEGs in itacitinib, fedratinib, or ruxolitinib vs vehicle-treated groups (adjusted P value <.05, |Log2FC| >1). (C) Heat map across the significant DEGs in vehicle, itacitinib, fedratinib, or ruxolitinib treatment groups with numbered clusters labeled on the y-axis. To the right of each cluster the enriched hallmark pathways are shown. (D) Dot plot of significantly up- (red) and downregulated (blue) hallmark gene sets (MSigDB v7.5.1) in LCMV-infected mice treated with itacitinib, fedratinib, or ruxolitinib compared with vehicle control (adjusted P value <.05 and normalized enriched score [NES] >1). Gene sets in proliferation (purple), developmental (cyan), metabolic (pink), housekeeping (black), and immune (brown) pathways are colored respectively. (E-G) Gene set enrichment analysis (GSEA) enrichment plots of the top 2 pathways in LCMV-infected mice for itacitinib (E), fedratinib (F), or ruxolitinib (G) vs vehicle-treated controls. FC, fold change.
Changes in transcriptional profiles of splenic CD8 T cells from Prf1−/− mice infected with LCMV and treated with a JAK1, JAK2, or combined JAK1/2 inhibitor. (A) Unsupervised clustering using multidimensional scaling of transcriptional profiles of splenic CD8 T cells from mice infected with LCMV and treated with vehicle, itacitinib (120 mg/kg bid), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) with 3 mice per group. (B) Venn diagram depicting the numbers of DEGs in itacitinib, fedratinib, or ruxolitinib vs vehicle-treated groups (adjusted P value <.05, |Log2FC| >1). (C) Heat map across the significant DEGs in vehicle, itacitinib, fedratinib, or ruxolitinib treatment groups with numbered clusters labeled on the y-axis. To the right of each cluster the enriched hallmark pathways are shown. (D) Dot plot of significantly up- (red) and downregulated (blue) hallmark gene sets (MSigDB v7.5.1) in LCMV-infected mice treated with itacitinib, fedratinib, or ruxolitinib compared with vehicle control (adjusted P value <.05 and normalized enriched score [NES] >1). Gene sets in proliferation (purple), developmental (cyan), metabolic (pink), housekeeping (black), and immune (brown) pathways are colored respectively. (E-G) Gene set enrichment analysis (GSEA) enrichment plots of the top 2 pathways in LCMV-infected mice for itacitinib (E), fedratinib (F), or ruxolitinib (G) vs vehicle-treated controls. FC, fold change.
Changes in transcriptional profiles of splenic monocytes from Prf1−/− mice infected with LCMV and treated with JAK1, JAK2, or combined JAK1/2 inhibitor. (A) Unsupervised clustering using multidimensional scaling of transcriptional profiles of splenic CD11b+Ly6C+ monocytes from mice infected with LCMV and treated with vehicle, itacitinib (120 mg/kg bid), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) with 3 mice per group. (B) Venn diagram depicting the numbers of DEGs in itacitinib, fedratinib, or ruxolitinib vs vehicle-treated groups (adjusted P value <.05, |Log2FC| >1). (C) Heat map of the significant DEGs in vehicle, itacitinib, fedratinib, or ruxolitinib treatment groups with clusters labeled on the y-axis. To the right of each cluster the enriched hallmark pathways are shown. (D) Dot plot of significantly up- (red) and downregulated (blue) hallmark gene sets (MSigDB v7.5.1) in LCMV-infected mice treated with itacitinib, fedratinib, or ruxolitinib compared with vehicle control (adjusted P value <.05 and NES >1). Gene sets in proliferation (purple), metabolic (pink), housekeeping (black), DNA damage repair (cyan), and immune (brown) pathways are colored respectively. (E-G) GSEA enrichment plots of the top 2 pathways in CD11b+Ly6C+ monocytes for itacitinib (E), fedratinib (F), or ruxolitinib (G) vs vehicle-treated controls. FC, fold change.
Changes in transcriptional profiles of splenic monocytes from Prf1−/− mice infected with LCMV and treated with JAK1, JAK2, or combined JAK1/2 inhibitor. (A) Unsupervised clustering using multidimensional scaling of transcriptional profiles of splenic CD11b+Ly6C+ monocytes from mice infected with LCMV and treated with vehicle, itacitinib (120 mg/kg bid), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) with 3 mice per group. (B) Venn diagram depicting the numbers of DEGs in itacitinib, fedratinib, or ruxolitinib vs vehicle-treated groups (adjusted P value <.05, |Log2FC| >1). (C) Heat map of the significant DEGs in vehicle, itacitinib, fedratinib, or ruxolitinib treatment groups with clusters labeled on the y-axis. To the right of each cluster the enriched hallmark pathways are shown. (D) Dot plot of significantly up- (red) and downregulated (blue) hallmark gene sets (MSigDB v7.5.1) in LCMV-infected mice treated with itacitinib, fedratinib, or ruxolitinib compared with vehicle control (adjusted P value <.05 and NES >1). Gene sets in proliferation (purple), metabolic (pink), housekeeping (black), DNA damage repair (cyan), and immune (brown) pathways are colored respectively. (E-G) GSEA enrichment plots of the top 2 pathways in CD11b+Ly6C+ monocytes for itacitinib (E), fedratinib (F), or ruxolitinib (G) vs vehicle-treated controls. FC, fold change.
Through unsupervised clustering of the significant DEGs in the sorted cell populations, we defined 8 distinct gene clusters for CD8 T cells and 3 such clusters for monocytes. We then performed overrepresentation analysis of the genes within these clusters (Figures 6C and 7C). As expected, CD8 T cells from vehicle-treated mice exhibited upregulation of proinflammatory pathways involved in the IFN-γ response, IFN-α response, IL-2 STAT5 signaling, and IL-6 JAK/STAT3 signaling (Figure 6C, clusters 3, 4, and 5). Itacitinib treatment did not appear to consistently downregulate gene expression pathways within these clusters. However, fedratinib treatment resulted in partial downregulation of IFN-γ response genes (Figure 6C, cluster 5) although to a lesser degree than that observed after ruxolitinib treatment that showed the most consistent downregulation of proinflammatory gene pathways (Figure 6C, clusters 3, 4, and 5).
Monocytes from vehicle-treated mice exhibited upregulation of pathways associated with IL-2 STAT5 signaling, TNFα signaling via NF-κB, IL-6 JAK/STAT3 signaling, the IFN-α response, and the IFN-γ response (Figure 7C, cluster 3). Contrary to its effects on CD8 T cells, itacitinib appeared to have a greater impact downregulating proinflammatory gene expression pathways in monocytes, namely those in cluster 3 (Figure 7C), whereas fedratinib exerted minimal effects (Figure 7C). In alignment with CD8 T cell results, combined JAK1/2 inhibition with ruxolitinib showed the most consistent suppression of pathways in monocytes compared with those upregulated in vehicle-treated controls (Figure 7C, cluster 3).
To identify global changes in gene expression across multiple groups, we performed gene set enrichment analysis.20 Comparison of gene expression in vehicle-treated vs naïve CD8 T cells revealed a significant increase in G2M checkpoint, E2F target genes, IFN-γ response, and IL-2 STAT5 signaling, and a decrease in anti-inflammatory genes of the TGF-β pathway (supplemental Figure 3A-B), consistent with the phenotype of T cell proliferation in this model and prior sequencing results from our group.18 Comparison of gene expression in vehicle-treated vs naïve monocytes revealed similar results with a significant increase in E2F targets, IFN-α response, G2M checkpoint, IFN-γ response, IL-6 JAK/STAT3 signaling, and proinflammatory genes involved in allograft rejection pathways (supplemental Figure 3C-D).
Finally, we compared gene expression pathways in cells from each LCMV-infected, JAK inhibitor–treated cohort vs vehicle-treated controls. In CD8 T cells, itacitinib treatment led to downregulation of genes involved in proinflammatory pathways (eg, IL-2 STAT5 signaling, IFN-α response, and IFN-γ response) and pathways involved in heme metabolism (Figure 6D-E; supplemental Figure 4). Fedratinib treatment downregulated cell cycling and proliferation genes (eg, Myc targets, G2M checkpoint, and E2F targets), as well as genes involved in metabolic pathways (eg, oxidative phosphorylation, MTORC1 signaling, heme metabolism, and glycolysis), but it increased expression of genes involved in the IFN-α response (Figure 6D,F; supplemental Figure 5). Interestingly, ruxolitinib led to downregulation of genes across multiple pathways, including proinflammatory signaling (eg, TNFα signaling, IFN-γ response, IFN-α response, IL-6 JAK STAT3 signaling, IL-2 signaling, and complement), cell cycling (eg, Kirsten rat sarcoma virus [KRAS] signaling), and metabolism (eg, oxidative phosphorylation and MTORC1 signaling) (Figure 6D,G; supplemental Figure 6).
In monocytes, itacitinib downregulated proinflammatory pathways (eg, IL-6 JAK STAT3 signaling, IFN-α and IFN-γ response) but upregulated proliferation (eg, Myc targets), and metabolic (eg, oxidative phosphorylation) pathways (Figure 7D-E; supplemental Figure 7). Fedratinib conferred a lesser reduction in gene expression and targeted pathways involved in cell proliferation (eg, KRAS signaling and E2F targets) and heme metabolism (Figure 7D,F; supplemental Figure 8). Finally, ruxolitinib targeted pathways similar to itacitinib but the numbers of genes significantly affected were ∼10-fold higher (Figure 7B,D,G; supplemental Figure 9). From these studies, we conclude that combined inhibition of JAK1 and JAK2 exerts the broadest impact on transcriptional profiles in CD8 T cells and monocytes, a property that may explain the superior clinical performance of ruxolitinib in this model of LCMV-induced HLH.
Discussion
Recent studies demonstrate that the JAK1/2 inhibitor ruxolitinib lessens inflammation in mouse models and humans with HLH. Nevertheless, concerns remain for ruxolitinib-induced cytopenias due to the inhibition of JAK2, which functions downstream of hematopoietic growth factors. This conundrum underscores the need to better understand the tolerability and therapeutic effects of selective JAK1 vs JAK2 inhibition for the treatment of HLH. Herein, we used complementary mouse HLH models to elucidate the PK, pharmacodynamics, and therapeutic impacts of JAK1 inhibition with itacitinib vs JAK2 inhibition with fedratinib, while comparing outcomes to those obtained using the JAK1/2 inhibitor ruxolitinib. Through this investigation, we observe that each of the JAK inhibitors was well-tolerated and effectively suppressed IFN-γ-mediated STAT1 phosphorylation. Further, selective vs combined JAK inhibition differentially affected the manifestations of disease in the secondary and primary models. Altogether, these findings demonstrate that underlying disease pathophysiology dictates the clinical response to JAK inhibition. Accordingly, careful consideration must be made when choosing a JAK inhibitor for the treatment of HLH.
Despite the central role of CD8 T cell production of IFN-γ in primary HLH and the importance of JAK1 in perpetuating IFN-γ–induced signaling, we saw only modest clinical improvement in LCMV–infected Prf1−/− mice treated with itacitinib. In line with these results, RNA sequencing of immune cells isolated from LCMV-infected animals revealed only a minimal number of significant DEGs after itacitinib treatment. Further, although itacitinib reduced expression of genes involved in proinflammatory signaling, such as those induced by IFN-α and IFN-γ, these changes were less robust than those observed after treatment with ruxolitinib. Itacitinib treatment also led to upregulation of Myc targets in monocytes. Thus, itacitinib may also promote myeloid cell proliferation, a property potentially contributing to its poorer performance in this model.
Notably, WT uninfected mice treated with fedratinib for 30 days did not demonstrate any hematologic toxicity. Further, in both HLH models, fedratinib-treated mice did not fare worse than animals treated with vehicle. Thus, JAK2 inhibition does not appear to be toxic in our hands. Indeed, contrary to our expectations, fedratinib-treated mice had significantly improved anemia and thrombocytopenia in the primary HLH model. Neutralization of IFN-γ lessens anemia in LCMV-infected Prf1−/− mice6,31,32 and in our hands, fedratinib significantly decreased IFN-γ-induced STAT1 phosphorylation. Therefore, the beneficial effects of fedratinib on anemia may be due to its inhibitory effects on IFN-γ signaling in hematopoietic progenitors. Another possibility involves the role of G-CSF in skewing hematopoiesis. Toward this end, G-CSF inhibits differentiation of myeloid and megakaryocytic erythroid progenitors33 and its disinhibition after the blockade of JAK2 signaling may have allowed increased erythroid and megakaryocyte output.
Altogether, our data are consistent with observations made using an in vivo model of a JAK2-driven myelofibrosis in which fedratinib treatment improved thrombocytopenia.34 Patients with JAK2-positive myeloproliferative neoplasms, who are commonly treated with fedratinib, may develop hypercytokinemia, often to levels mirroring those seen in HLH.35,36 Our findings suggest that switching from a selective JAK2 to a combined JAK1/2 inhibitor might be most effective in dampening inflammation in these patients. Toward this end, several prior studies have shown that ruxolitinib effectively decreases inflammatory manifestations in patients with myeloproliferative neoplasms and in mouse models of the disease.10,37,38 In further support of the beneficial effects of ruxolitinib, it is important to note it did not worsen the anemia or thrombocytopenia, respectively, in either the primary (P = .4647 and P = .6106) or secondary (P = .9968 and P = .6498) HLH model. Further, there are multiple reports of human patients whose cytopenias improved significantly after ruxolitinib administration.39-41
Although IL-10 is typically classified as an anti-inflammatory cytokine, its role in states of hyperinflammation is less straightforward. Elevated IL-10 has been identified as a poor prognostic factor in patients with HLH or those with severe viral infection. Additionally, mouse models overexpressing IL-10 and IL-18 resulted in severe hyperinflammation, whereas overexpression of either cytokine alone did not lead to an inflammatory phenotype. Interestingly, only IL-10 blockade, not IL-18 blockade, improved survival in this model of hyperinflammation.42 The predominant role of IL-10 may depend upon its timing, levels, and the constellation of cytokines present during an inflammatory response. Thus, in our studies, the beneficial effects of ruxolitinib and itacitinib in the secondary HLH model may depend in part on their inhibition of IL-10-induced JAK1 signaling.
Altogether, our findings reveal that the efficacy of itacitinib, fedratinib, and ruxolitinib varies depending on the experimental model used. Nevertheless, in both models examined, JAK inhibitor–induced reductions in cytokine levels and/or proinflammatory signaling pathways were a common and central feature associated with improvement in the signs of disease, immunologic biomarkers, and overall survival. Further studies are warranted to determine whether similar effects are observed in humans with primary or secondary HLH.
Acknowledgments
The authors thank members of the St. Jude Children’s Research Hospital Preclinical Pharmacokinetics Shared Resource, Comparative Pathology Core, Flow Cytometry and Cell Sorting Core Facility, Animal Resource Center, Hartwell Center for Biotechnology, the Biostatistics Department, and Center for Applied Bioinformatics for their assistance with numerous technical aspects of this project. The authors also thank Incyte for providing itacitinib for experimental use.
This work was supported in part by funding from American Lebanese Syrian Associated Charities.
Authorship
Contribution: C.K. designed and performed experiments, interpreted data, and wrote the manuscript; S.A. designed and performed experiments, assisted with data interpretation and provided experimental oversight, and wrote and edited sections of the manuscript; N.O. performed RNA sequencing analysis; A.S. provided technical assistance; H.S.T. performed histologic evaluation and analysis of mouse tissues; Y.W. performed plasma processing and analysis for PK experiments; B.B.F. assisted with PK experimental design and data interpretation and helped write the manuscript; S.A.A. provided technical assistance; L.K.M. assisted with data interpretation and helped edit the manuscript; R.W. provided technical assistance; K.C.V. helped design experiments and assisted with data interpretation; Y.Z. performed statistical analysis; C.C. provided oversight for statistical analysis; K.E.N. oversaw the project, interpreted data, and edited the manuscript; and all authors contributed to reviewing and editing the manuscript.
Conflict-of-interest disclosure: K.E.N. receives research funding from Incyte Corporation. The remaining authors declare no competing financial interests.
Correspondence: Kim E. Nichols, Department of Oncology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS 1170, Memphis, TN 38105; email: kim.nichols@stjude.org; and Sabrin Albeituni, Department of Oncology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS 343, Memphis, TN 38105; email: sabrin.albeituni@stjude.org.
References
Author notes
All raw and processed RNA sequencing data associated with this manuscript have been deposited to Gene Expression Omnibus, with the accession number GSE230771.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![JAK1 inhibition improves survival and clinical scores in CpG/αIL-10R-induced HLH. (A) Experimental schema for modeling secondary HLH, wherein WT C57BL/6J mice receive injections of CpG and αIL-10R antibody on days 0, 2, 4, and 7, and are then analyzed on day 9 or 10 after the first injection. (B) Probability of survival (B) and clinical scores (C) from naïve, or CpG/αIL-10R-treated mice who received vehicle, itacitinib (120 mg/kg twice daily [bid]), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) from days 4 to 9 after first CpG/αIL-10R injection. Analysis was performed on day 10. Data are combined from 2 independent experiments (9-10 mice per group). (D) Spleen weights shown as percentage of final body weight in mice treated with vehicle, itacitinib (120 mg/kg bid), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) on days 4 to 8 after the first CpG/αIL-10R injection. Analysis was performed on day 9. Peripheral blood hemoglobin (E), hematocrit (F), and platelet counts (G). Data points in panels D-G represent single mice from 3 pooled experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, or ∗∗∗∗P < .0001 by pairwise comparison. For survival, log-rank test was performed to determine significance between treated groups and vehicle control. For clinical score, statistical significance was determined using two-way ANOVA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/23/10.1182_blood.2023021046/2/m_blood_bld-2023-021046-gr2.jpeg?Expires=1767716295&Signature=vuCcnyB98B5LTR7Tmf7PGRI-ASkgp8PAJP9eBWAjphq-HsvG-fivFr4Y-O-0BEvcA4EBGRr5qn5byc38WGU21RXjHOyr1xNps-6W5vvedQ8jT8~ILLvaSru84j-pbkeWPwnL1xaFOMyjIGlHsYRWLLMiKnIZLibqZ1EdrDjKu6hOLpeGGGTSpO1O38WSt-Cp6mT6enwPgxwnWwyBosABvgfppGRg9mh0bJcAnudtzS7EnfO5w4qW36970NF26aLu-gvF-1onBFfSYqgmisICqVOwu68ML2fFCWhNAE-ZVFl7T7cxIZ33c~OInbZhfYggV7ch8GeQhMc~gAB00haNkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Changes in transcriptional profiles of splenic CD8 T cells from Prf1−/− mice infected with LCMV and treated with a JAK1, JAK2, or combined JAK1/2 inhibitor. (A) Unsupervised clustering using multidimensional scaling of transcriptional profiles of splenic CD8 T cells from mice infected with LCMV and treated with vehicle, itacitinib (120 mg/kg bid), fedratinib (60 mg/kg bid), or ruxolitinib (90 mg/kg bid) with 3 mice per group. (B) Venn diagram depicting the numbers of DEGs in itacitinib, fedratinib, or ruxolitinib vs vehicle-treated groups (adjusted P value <.05, |Log2FC| >1). (C) Heat map across the significant DEGs in vehicle, itacitinib, fedratinib, or ruxolitinib treatment groups with numbered clusters labeled on the y-axis. To the right of each cluster the enriched hallmark pathways are shown. (D) Dot plot of significantly up- (red) and downregulated (blue) hallmark gene sets (MSigDB v7.5.1) in LCMV-infected mice treated with itacitinib, fedratinib, or ruxolitinib compared with vehicle control (adjusted P value <.05 and normalized enriched score [NES] >1). Gene sets in proliferation (purple), developmental (cyan), metabolic (pink), housekeeping (black), and immune (brown) pathways are colored respectively. (E-G) Gene set enrichment analysis (GSEA) enrichment plots of the top 2 pathways in LCMV-infected mice for itacitinib (E), fedratinib (F), or ruxolitinib (G) vs vehicle-treated controls. FC, fold change.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/23/10.1182_blood.2023021046/2/m_blood_bld-2023-021046-gr6.jpeg?Expires=1767716295&Signature=3reenxVKOcMuU4-xQB4hHDooMDJfsVI68vUMg4AkIoq-Tjra83WMRfIHMNw96QsG1o9AXCVqMkjqHrnlrVR~LVcLOq8USoWLuGhNlshJVnoFkdW5w~zmQXCYNLsBv8twYQFson8RpO~9XDS1xbRn-opvgyT5pvit~K3C9~i3~-oAHNErRbzptqVwvkX6w88ziVElXQ27bpewXzFOVgrmV640~9rzyg~Uwml~HRx~YfLKj~~aQloMZxMMdfY4Dg~s7F33OgSFk6R4ueLxq6Eb0pxk-txlYMFVx-JaWfQLrFZRQvHMTW9ywp0nPfWgNal5aPSCpVPr7lKZBlw1642E2A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Comments
Heterogeneous roles of non-selective JAK inhibitors, ruxolitinib and momelotinib, in immune bone marrow failure
Conflict-of-interest disclosure: H.W. declares no competing financial interests.
REFERENCES
1. Keenan C, Albeituni S, Oak N, et al. Differential effects of itacitinib, fedratinib, and ruxolitinib in mouse models of hemophagocytic lymphohistiocytosis. Blood. 2024;143(23):2386-2400.
2. Singer JW, Al-Fayoumi S, Taylor J, Velichko S, O'Mahony A. Comparative phenotypic profiling of the JAK2 inhibitors ruxolitinib, fedratinib, momelotinib, and pacritinib reveals distinct mechanistic signatures. PLoS One. 2019;14(9):e0222944.
3. Kong T, Yu L, Laranjeira ABA, et al. Comprehensive profiling of clinical JAK inhibitors in myeloproliferative neoplasms. Am J Hematol. 2023;98(7):1029-1042.
4. Groarke EM, Feng X, Aggarwal N, et al. Efficacy of JAK1/2 inhibition in murine immune bone marrow failure. Blood. 2023;141(1):72-89.
5. Asshoff M, Petzer V, Warr MR, et al. Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood. 2017;129(13):1823-1830.