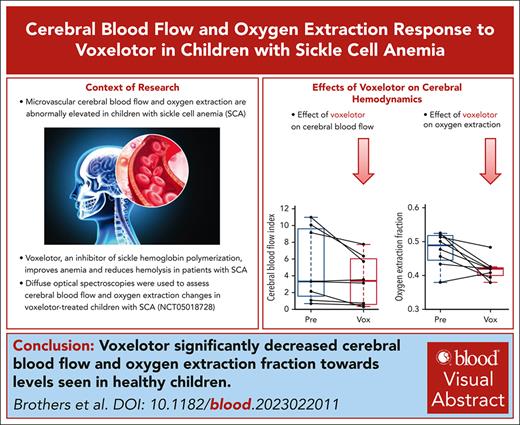
Optical measurements of cerebral blood flow are validated against perfusion MRI in pediatric SCA.
Voxelotor significantly decreases OEF and cerebral blood flow toward levels seen in healthy children.
Visual Abstract
Voxelotor is an inhibitor of sickle hemoglobin polymerization that is used to treat sickle cell disease. Although voxelotor has been shown to improve anemia, the clinical benefit on the brain remains to be determined. This study quantified the cerebral hemodynamic effects of voxelotor in children with sickle cell anemia (SCA) using noninvasive diffuse optical spectroscopies. Specifically, frequency-domain near-infrared spectroscopy combined with diffuse correlation spectroscopy were used to noninvasively assess regional oxygen extraction fraction (OEF), cerebral blood volume, and an index of cerebral blood flow (CBFi). Estimates of CBFi were first validated against arterial spin–labeled magnetic resonance imaging (ASL-MRI) in 8 children with SCA aged 8 to 18 years. CBFi was significantly positively correlated with ASL-MRI–measured blood flow (R2 = 0.651; P = .015). Next, a single-center, open-label pilot study was completed in 8 children with SCA aged 4 to 17 years on voxelotor, monitored before treatment initiation and at 4, 8, and 12 weeks (NCT05018728). By 4 weeks, both OEF and CBFi significantly decreased, and these decreases persisted to 12 weeks (both P < .05). Decreases in CBFi were significantly correlated with increases in blood hemoglobin (Hb) concentration (P = .025), whereas the correlation between decreases in OEF and increases in Hb trended toward significance (P = .12). Given that previous work has shown that oxygen extraction and blood flow are elevated in pediatric SCA compared with controls, these results suggest that voxelotor may reduce cerebral hemodynamic impairments. This trial was registered at www.ClinicalTrials.gov as #NCT05018728.
Introduction
In sickle cell anemia (SCA), deoxygenated sickle hemoglobin (HbS) polymerization causes red blood cell (RBC) sickling, hemolysis, and altered rheology. These hematologic complications result in chronic severe anemia, increased inflammation, and endothelial dysfunction that has numerous detrimental effects on multiple organ systems. The most severe of these consequences is the high risk of infarction, both silent and overt, that significantly affects neurologic morbidity and quality of life.1,2
Several studies have shown that cerebral blood flow (CBF) is chronically elevated in SCA to compensate for decreases in oxygen delivery caused by severe anemia.3-5 Further compensatory elevations in cerebral oxygen extraction fraction (OEF), that is, the fraction of oxygen extracted by the tissue as blood travels from arteries to veins, have also been observed.5-7 It is hypothesized these chronic elevations in both CBF and OEF leave patients ill-equipped to respond to acute increases in oxygen metabolic demand, for example, due to infection, fever, or injury.3 Indeed, elevated OEF has been associated with increased risk of infarction.5-7
Voxelotor is an inhibitor of sickle Hb polymerization that is approved by the US Food and Drug Administration for treatment of sickle cell disease in patients aged ≥4 years. Clinically, voxelotor has been shown to significantly improve anemia by reducing hemolysis and increasing blood Hb levels.8-10 However, the clinical benefit of voxelotor on end-organ protection has been a topic of debate. On long-term follow-up, voxelotor was reported as well tolerated.11 Treatment-emergent adverse events were mostly mild and self-limiting. However, there is concern that voxelotor–bound HbS is unable to offload its oxygen to the tissue, offsetting any improvements caused by reduction in sickling or increases in Hb.12,13 This concern is particularly relevant in the cerebrovasculature, given the state of chronic cerebral hemometabolic stress that these patients experience.
In this study, we aimed to determine the effects of voxelotor treatment in children with SCA on a battery of cerebral hemodynamic parameters, including OEF, CBF, and cerebral blood volume (CBV). We focused our investigation on children due to the high risk of infarction.5,14,15 To assess these effects, we used 2 noninvasive diffuse optical techniques: (1) frequency-domain near-infrared spectroscopy (FDNIRS) and (2) diffuse correlation spectroscopy (DCS). FDNIRS is a well-established technique to assess regional blood volume and oxygen saturation,16-20 whereas DCS is an emerging technique used to quantify an index of regional microvascular CBF (CBFi). DCS measures of CBFi have been validated in children without SCA16,21-23 but not in children with SCA. Because RBCs are the primary contrast agent in DCS and SCA alters RBC concentration, morphology, and rheology, DCS also needs to be validated in SCA patients. Thus, we first compare DCS–measured CBFi with perfusion magnetic resonance imaging (MRI)–assessed CBF. Next, we completed a single-center, open-label pilot study in children with SCA on voxelotor for 12 weeks. Given that voxelotor improves anemia, we hypothesized that voxelotor would decrease OEF and CBFi from pretreatment levels. Furthermore, we hypothesized that reductions in OEF and CBFi would be inversely associated with increases in Hb.
Methods
Study design
This work consisted of 2 separate study protocols. The first protocol was used to validate DCS measures of CBFi against MRI measures of CBF, and the second was used to assess the cerebral hemodynamic effects of voxelotor. Patients were recruited separately for the 2 protocols; there was no overlap in participants between the protocols. Both studies were approved by the Emory University Institutional Review Board, and all participants and/or their guardians provided informed consent.
DCS validation against MRI
In DCS, a near-infrared light source is placed centimeters away from a light detector on the tissue surface. Simple analytical models are used to relate fast intensity fluctuations of the detected light to an index of CBF, that is, CBFi (in units of cm2/s) that reflects regional microvascular blood flow in the tissue under the sensor. DCS–measured CBFi has been extensively validated in vivo in numerous patient cohorts against other perfusion modalities, including arterial spin–labeled MRI (ASL–MRI)21,23,24 and xenon-enhanced computed tomography.25 Moreover, DCS has been used successfully in children with SCA to detect expected elevations in CBFi compared with controls, inverse associations between CBFi and Hb concentration, and decreases in CBFi with transfusion.26,27 However, the primary contrast agent in DCS is the RBC, and SCA alters RBC morphology, rheology, and concentration. Thus, to provide further confidence in this optical estimation of CBF in SCA, we first compare DCS–measured CBFi with CBF measured by ASL–MRI in a cohort of children with SCA.
Children enrolled in this study were aged from 8 to 18 years (inclusive), with SCA (genotype HbSS), and undergoing a clinically indicated head MRI at Children’s Healthcare of Atlanta. Exclusion criteria were known significant vasculopathy, defined as >50% stenosis of the circle of Willis vessels on a prior magnetic resonance angiography, overt stroke, prior transient ischemic attack, major head injury requiring a visit to the emergency department, moyamoya, or a previous revascularization procedure. Further exclusion criteria included illness within the past month, neurologic disorder not related to SCA, claustrophobia, metal orthodontia, or any other contraindication for MRI. FDNIRS/DCS measurements of regional CBFi were obtained within 1 hour of the MRI.
Cerebral hemodynamic effects of voxelotor
Children aged 4 to 17 years with SCA (genotypes HbSS or HbSβ0-thalassemia) were enrolled at Children’s Healthcare of Atlanta (ClinicalTrials.gov identifier: NCT05018728). Eligible participants had a Hb level ≤10.5 g/dL and transcranial doppler ultrasound (TCD) flow velocities of ≤200 cm/s during screening. Primary exclusion criteria were changes in hydroxyurea dose within the last 3 months, the occurrence of a vaso-occlusive event during screening, or RBC transfusion within 60 days of signing informed consent (full exclusion criteria in supplemental Material, available on the Blood website). Participants who met study criteria started open-label voxelotor at 1500 mg or weight equivalent dose daily, taken either as dispersible tablets or dissolvable powder. Participants were monitored before treatment initiation, as well as at 4, 8, and 12 weeks.
Laboratory markers associated with anemia (complete blood count and reticulocyte count), vital signs, and peripheral oxygen saturation were obtained at each visit (screening, Pre/0, 4, 8, and 12 weeks). Laboratory markers associated with hemolysis (indirect bilirubin level and lactate dehydrogenase level [LDH]) were performed at weeks 0 and 12. FDNIRS/DCS measurements of regional CBFi, OEF, and CBV were made at each visit.
FDNIRS/DCS experimental protocol and analysis
Details of the FDNIRS/DCS hardware, theory, and analytical methods used to measure regional OEF, CBFi, and CBV are as previously described.27 Data were acquired with a customized FDNIRS system (Imagent, ISS Inc) and an in-house built DCS system operating at 852 nm. For each measurement, an optical sensor with FDNIRS source-detector spacings of 2.0, 2.5, 3.0, and 3.5 cm and DCS spacing of 2.5 cm was manually held over the right and left forehead for 3- to 5-second intervals. The sensor was slightly repositioned 3 times per hemisphere to account for local inhomogeneities under the tissue surface, and all repetitions were averaged to yield a global mean of each measured parameter (OEF, CBFi, and CBV).
ASL–MRI experimental protocol and analysis
All patients were scanned on a 3T Magnetom Vida (Siemens Healthineers, Erlangen, Germany) MRI system. To measure CBF, a 2-dimensional pseudocontinuous arterial spin labeling scan was added to the clinical scan (repetition time [TR], 4800 ms; echo time [TE], 13 ms; flip angle, 90 degrees; labeling duration, 1800 ms; postlabeling delay, 1500 ms; voxel size, 3.4 × 3.4 × 5.0 mm3; 18 slices). For comparison with DCS, mean frontal gray matter CBF was estimated using the MR ASL Perfusion Analysis application v1.2.0 (Siemens Healthineers) with automated segmentation, labeling efficiency (α) = 0.6528 and blood T1 = 1818 ms.29
Statistics
Summary statistics are reported as median (interquartile range [IQR] first quartile and third quartile) for continuous measurements and count (percentage) for categorical measurements. For DCS validation against MRI, a linear regression model was used to assess association between ASL–MRI and DCS measures of CBF. For the voxelotor cohort, changes in laboratory assessments of Hb, percent reticulocytes, white blood cell count, LDH, and total bilirubin were reported as the difference between posttreatment (4, 8, and 12 weeks) and pretreatment levels (eg, dHb4w = Hb4w – Hbpre). Changes in FDNIRS/DCS cerebral hemodynamic outcomes measures (OEF, CBFi, and CBV) were defined as the relative percent change from pretreatment levels (eg, rOEF8w = [OEF8w − OEFpre]/OEFpre × 100%). Because of the small sample size, Wilcoxon signed-rank tests, a nonparametric counterpart of the paired t test, were used to detect whether laboratory or cerebral hemodynamic outcome measures were significantly different from pretreatment levels at each posttreatment time point separately. Linear mixed-effects regression models were used to examine the association of each repeated-measure hemodynamic outcome and Hb with random-intercept at the patient level. These models were 2-level and accounted for repeated measures nested within each patient. All statistical analyses were performed with CRAN R (version 3.4.1; R Foundation). Significance was assessed at P value of .05.
Results
Association of MRI and DCS measures of CBF
Eleven children with SCA were consented for the validation of DCS against MRI. Of these, 3 were excluded because of either improper placement of the ASL labeling plane (n = 1), low DCS signal-to-noise ratio (n = 1), or corruption of DCS data (n = 1). Thus, a total of 8 children were included for analysis. Patients were primarily female (6/8 [75%]) with a median age of 14.1 years (IQR, 11.0-17.2). The majority (n = 7) were receiving hydroxyurea therapy. All patients had HbSS; 1 patient received prior curative therapy (bone marrow transplant).
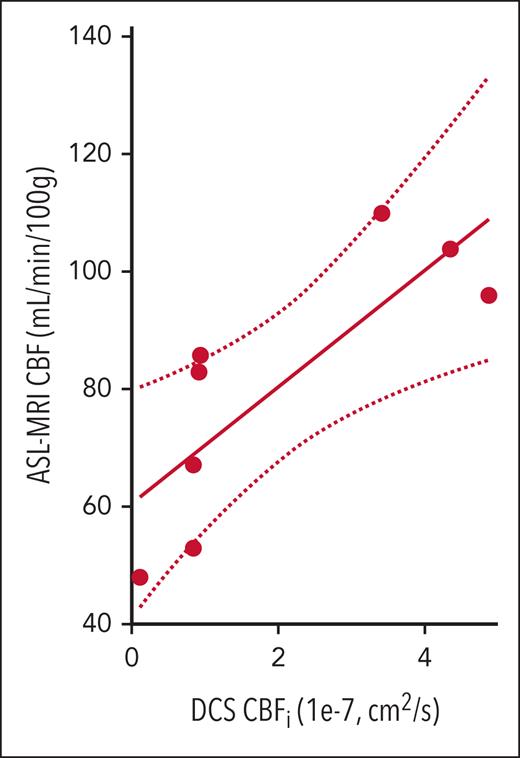
Average frontal gray matter CBF in this cohort ranged from 45 to 110 mL per minute per 100 g, with a median of 84 mL per minute per 100 g (IQR, 64-98), as estimated by ASL–MRI. ASL measures of CBF were significantly positively correlated with DCS measures of CBFi (R2 = 0.651; P = .015, Figure 1).
Relationship between DCS and ASL–MRI measures of blood flow. CBF (mL/min per 100 g) measured with ASL-MRI vs CBFi (1 × 10−7 cm2/s) measured with DCS (n = 8 patients). Strong correlation between the 2 measures was observed (R2 = 0.651; P = .015). The solid line denotes the best linear fit to the data, and the dotted lines indicate the 95% CI to this fit. The slope of the line of best fit was 1.00 (95% CI, 0.97-1.03), and the intercept was 60.6 (95% CI, 55.7-65.4). CI, confidence interval.
Relationship between DCS and ASL–MRI measures of blood flow. CBF (mL/min per 100 g) measured with ASL-MRI vs CBFi (1 × 10−7 cm2/s) measured with DCS (n = 8 patients). Strong correlation between the 2 measures was observed (R2 = 0.651; P = .015). The solid line denotes the best linear fit to the data, and the dotted lines indicate the 95% CI to this fit. The slope of the line of best fit was 1.00 (95% CI, 0.97-1.03), and the intercept was 60.6 (95% CI, 55.7-65.4). CI, confidence interval.
Effects of voxelotor on cerebral hemodynamics
To determine the effects of voxelotor on cerebral hemodynamics, 11 patients were consented and screened for study eligibility. One patient subsequently failed screening because of an acute complication (osteomyelitis of femur) before drug initiation. Eight of the remaining 10 patients completed planned treatment and assessments; 2 patients were withdrawn during the study based on persistent patient-reported failure to comply with daily study medication. Baseline characteristics of the 8 patients included in the analysis are summarized in Table 1. All patients were HbSS on stable dose of hydroxyurea. The cohort was majority male (n = 5 [63%]), with a median age of 9 years (IQR, 7-12).
Summary of participant characteristics
| . | DCS vs MRI . | Voxelotor . |
|---|---|---|
| n | 8 | 8 |
| Age, y | 14 (11-17) | 9 (7-12) |
| Male, n (%) | 2 (25) | 5 (63) |
| Weight, kg | 60 (45-71) | 33 (23-43) |
| Hb, g/dL | 9.9 (8.3-11.1) | 9.1 (8.2-9.9) |
| % Reticulocyte count | — | 8.1 (5.2-11.0) |
| % White blood cell count | — | 7.5 (5.0-10.0) |
| LDH, U/L | — | 455 (268-571) |
| Bilirubin, μmol/L | — | 2.3 (1.4-3.2) |
| HbF, % | — | 22.4 (10.7-34.1) |
| HbSS genotype, n (%) | 8 (100) | 8 (100) |
| Current hydroxyurea use, n (%) | 7 (88) | 8 (100) |
| . | DCS vs MRI . | Voxelotor . |
|---|---|---|
| n | 8 | 8 |
| Age, y | 14 (11-17) | 9 (7-12) |
| Male, n (%) | 2 (25) | 5 (63) |
| Weight, kg | 60 (45-71) | 33 (23-43) |
| Hb, g/dL | 9.9 (8.3-11.1) | 9.1 (8.2-9.9) |
| % Reticulocyte count | — | 8.1 (5.2-11.0) |
| % White blood cell count | — | 7.5 (5.0-10.0) |
| LDH, U/L | — | 455 (268-571) |
| Bilirubin, μmol/L | — | 2.3 (1.4-3.2) |
| HbF, % | — | 22.4 (10.7-34.1) |
| HbSS genotype, n (%) | 8 (100) | 8 (100) |
| Current hydroxyurea use, n (%) | 7 (88) | 8 (100) |
Data are reported as median (IQR) or count (percentage) for the DCS validation against MRI cohort (n = 8, first column) and voxelotor cohort at the time of screening (n = 8, second column).
HbF, fetal hemoglobin.
Before treatment, patients had a median blood Hb level of 9.1 g/dL (IQR, 8.2-9.9). At 4 weeks, Hb levels increased significantly by 1.2 g/dL (IQR, 0.4-1.5; P = .023; Table 2). These increases persisted at weeks 8 and 12 (both P < .05 compared with pretreatment; Table 2). At 12 weeks, total bilirubin changed by −0.9 μmol/L (IQR, −1.3 to −0.5; P = .008), whereas no significant changes in LDH were observed (P = .24).
Changes in laboratory measures on voxelotor
| . | Wk 4 . | Wk 8 . | Wk 12 . |
|---|---|---|---|
| dHb, g/dL | 1.2 (0.4-1.5)∗ | 0.9 (0.4-1.2)∗ | 0.8 (0.2-1.4)∗ |
| dRetic, % | −2.8 (−3.3 to −0.7)† | −1.2 (−4.2 to 0.1)† | −1.4 (−2.5 to −0.4)† |
| dWBC, 1000/μL | −1.0 (−1.2 to −0.2)† | 0.4 (−2.0 to 1.4) | −0.8 (−1.6 to −0.1)∗ |
| dLDH, U/L | — | — | 27 (−10 to 64) |
| dBilirubin, μmol/L | — | — | −0.9 (−1.3 to −0.5)‡ |
| . | Wk 4 . | Wk 8 . | Wk 12 . |
|---|---|---|---|
| dHb, g/dL | 1.2 (0.4-1.5)∗ | 0.9 (0.4-1.2)∗ | 0.8 (0.2-1.4)∗ |
| dRetic, % | −2.8 (−3.3 to −0.7)† | −1.2 (−4.2 to 0.1)† | −1.4 (−2.5 to −0.4)† |
| dWBC, 1000/μL | −1.0 (−1.2 to −0.2)† | 0.4 (−2.0 to 1.4) | −0.8 (−1.6 to −0.1)∗ |
| dLDH, U/L | — | — | 27 (−10 to 64) |
| dBilirubin, μmol/L | — | — | −0.9 (−1.3 to −0.5)‡ |
Data are reported as median (IQR). P values were obtained from a 2-sided paired Wilcoxon signed-rank test.
dBilirubin, changes from pretreatment levels in bilirubin at 4, 8, and 12 wk of treatment; dHb, changes from pretreatment levels in Hb at 4, 8, and 12 wk of treatment; dLDH, changes from pretreatment levels in LDH at 4, 8, and 12 wk of treatment; dRetic, changes from pretreatment levels in reticulocytes at 4, 8, and 12 wk of treatment; dWBC, changes from pretreatment levels in white blood cell count at 4, 8, and 12 wk of treatment.
P < .05.
P < .01.
P < .005.
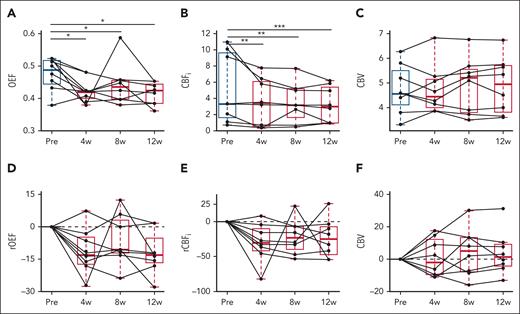
Compared with pretreatment levels, OEF and CBFi were significantly decreased at week 4, and these decreases persisted at weeks 8 and 12 (all P < .05; Figure 2A-B; Table 2). Specifically, median OEF decreased by 12% at week 4 (P = .007), 14% at week 8%, and 13% at week 12, compared with pretreatment levels (Figure 2A; Table 3). Median CBFi decreased by 30% at week 4 (P < .001), 33% at week 8%, and 24% at week 12, compared with pretreatment levels (Figure 2B; Table 3). No significant changes in CBV were observed at any time point (Figure 2C; Table 3).
Effects of voxelotor on cerebral hemodynamic markers. (A-C) Box plots of OEF, CBFi (1 × 10−7 cm2/s), and CBV (mL per 100 g) from pretreatment (black) to 4, 8, and 12 weeks after treatment (red). ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001 compared with pretreatment levels using 2-sided paired Wilcoxon signed-rank test. (D-F) Box plots of relative percent changes from pretreatment levels of oxygen extraction fraction (rOEF), cerebral blood flow (rCBFi), and cerebral blood volume (rCBV) from pretreatment. The horizontal gray dotted line at 0 denotes no change from pretreatment levels. For each box plot, the central line denotes the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points not considered outliers. Individual connected gray dots represent each pretreatment and posttreatment matched pair for the 8 patients.
Effects of voxelotor on cerebral hemodynamic markers. (A-C) Box plots of OEF, CBFi (1 × 10−7 cm2/s), and CBV (mL per 100 g) from pretreatment (black) to 4, 8, and 12 weeks after treatment (red). ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001 compared with pretreatment levels using 2-sided paired Wilcoxon signed-rank test. (D-F) Box plots of relative percent changes from pretreatment levels of oxygen extraction fraction (rOEF), cerebral blood flow (rCBFi), and cerebral blood volume (rCBV) from pretreatment. The horizontal gray dotted line at 0 denotes no change from pretreatment levels. For each box plot, the central line denotes the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points not considered outliers. Individual connected gray dots represent each pretreatment and posttreatment matched pair for the 8 patients.
Relative changes in cerebral hemodynamic markers on voxelotor
| . | Wk 4 . | Wk 8 . | Wk 12 . |
|---|---|---|---|
| rOEF, % | −11.5 (−17.9 to −5.1)∗ | −13.6 (−21.4 to −5.7)∗ | −12.9 (−18.1 to −7.8)∗ |
| rCBFi, % | −29.9 (−52.7 to −7.1)† | −32.8 (−40.8 to −24.8)‡ | −24.1 (−40.0 to −8.2)† |
| rCBV, % | 0.9 (−10.1 to 11.0) | 4.4 (−6.2 to 15.0) | 3.8 (−2.8 to 10.4) |
| . | Wk 4 . | Wk 8 . | Wk 12 . |
|---|---|---|---|
| rOEF, % | −11.5 (−17.9 to −5.1)∗ | −13.6 (−21.4 to −5.7)∗ | −12.9 (−18.1 to −7.8)∗ |
| rCBFi, % | −29.9 (−52.7 to −7.1)† | −32.8 (−40.8 to −24.8)‡ | −24.1 (−40.0 to −8.2)† |
| rCBV, % | 0.9 (−10.1 to 11.0) | 4.4 (−6.2 to 15.0) | 3.8 (−2.8 to 10.4) |
Data are reported as median (IQR). P values were obtained from a 2-sided paired Wilcoxon signed-rank test.
rCBFi, relative changes in CBFi from pretreatment levels at wk 4, 8, and 12; rCBV, relative changes in CBV from pretreatment levels at wk 4, 8, and 12; rOEF, relative changes in OEF levels from pretreatment levels at wk 4, 8, and 12.
P < .05.
P < .01.
P < .005.
Finally, we quantified the relationship between OEF, CBFi, and CBV and Hb levels. After accounting for repeated measures within subjects, bivariable analysis revealed a significant inverse relationship between Hb and CBFi (P = .024; Table 4). Similar negative trends with Hb were seen with OEF, although the results did not reach statistical significance (P = .129). CBV was not correlated with Hb (P = .79).
Bivariable analysis of the association between Hb and hemodynamic markers
| . | Estimate (95% CI) . | P value . |
|---|---|---|
| OEF | −0.013 (−0.035 to 0.006) | .129 |
| CBFi | −0.76 (−1.37 to −0.10) | .024 |
| CBV | 0.04 (−0.35 to 0.33) | .792 |
| . | Estimate (95% CI) . | P value . |
|---|---|---|
| OEF | −0.013 (−0.035 to 0.006) | .129 |
| CBFi | −0.76 (−1.37 to −0.10) | .024 |
| CBV | 0.04 (−0.35 to 0.33) | .792 |
Slope estimate and associated 95% CI of the linear mixed-effects models are reported.
CI, confidence interval.
Bold P value indicates statistical significance.
Discussion
Both CBF and OEF are elevated in children with SCA compared with healthy controls3,5,6,26 (supplemental Figure 1). In this study, we used noninvasive FDNIRS/DCS to demonstrate that voxelotor decreases regional CBFi and OEF in children with SCA, suggesting the drug improves these parameters toward the level of healthy controls. These changes were observable by 4 weeks and persisted to study end (12 weeks; Figure 2; Table 3). Of note, all participants were on stable hydroxyurea therapy before and during voxelotor treatment with baseline median percentage fetal Hb of 22.4 (IQR, 10.7-34.1; Table 1). Changes in CBFi were significantly associated with changes in Hb (P = .024; Table 4). Changes in OEF trended with changes in Hb (P = .129; Table 4).
The decreases in OEF and CBFi on voxelotor were expected because of the documented improvements in Hb and reductions in HbS polymers on voxelotor9,30 that likely act to increase oxygen delivery, along with literature reporting similar decreases in OEF and CBF with other treatments known to improve anemia, for example, chronic transfusion3,4,27 and hydroxyurea.5,31-33 Although decreases in either CBFi or OEF in isolation could be indicative of impaired oxygen delivery at the tissue level, the combined decreases in both OEF and CBFi, coupled with the absence of clinical symptoms of central nervous system hypoxia in our cohort and in published literature,9,10,34 suggest that voxelotor is inducing beneficial effects on cerebral hemodynamics. Although many studies, including ours (see supplemental Figure 1), show increases in OEF in SCA compared with healthy controls,1,3,5,7,31,35-38 some studies have reported decreased OEF in SCA.39-41 If OEF were decreased in patients with sickle cell, the further decreases in OEF that we observe on voxelotor could be an adverse effect of the medication instead of a benefit. However, publications that report reductions in OEF in SCA compared with healthy controls used different imaging modalities with different vascular sensitivities.42 Additionally, the cohorts differ in age and genotype, making it difficult to draw meaningful conclusions by comparing our study with these previous works.
When normalized to the increase in Hb, the relative percent decreases in OEF and CBFi appear to be more pronounced with voxelotor than other anemia treatments. For example, previous work with FDNIRS/DCS reported median (IQR) decreases in OEF and CBFi after transfusion of 1.2% per g per dL (IQR, 0.8-2.1) and 4.3% per g per dL (IQR, 2.6-5.9), respectively,27 whereas voxelotor induced changes of 15% per g per dL (IQR, 11-21) and 37% per g per dL (IQR, 33-42) at 4 weeks. There are numerous possible reasons for this robust change. The timing of the measurements was different, that is, the effects of transfusion27 were assessed immediately after blood was given, whereas the effects of voxelotor were assessed at steady state. Additionally, other factors besides improvements in Hb concentration could be contributing to OEF and CBF reductions on voxelotor, including decreases in systemic inflammation caused by decreases in circulating free heme and reticulocytosis,8,9,43 increases in Hb oxygen affinity,8,9,11 or prolonged RBC half-life.9,11,44
This study demonstrates the use of FDNIRS/DCS as a routine screening tool of cerebral hemodynamic markers in children with SCA. Although FDNIRS/DCS is limited in spatial resolution and regional sensitivity, these noninvasive optical tools present numerous advantages over other neuroimaging modalities, including lower cost, bedside portability, rapid data acquisition, and microvascular sensitivity. A well-known limitation of FDNIRS/DCS is the influence of extracerebral layers (skull, scalp, and cerebrospinal fluid),45 whereas the strong correlation we observed between DCS and MRI suggests that this influence is not appreciable in this patient cohort (Figure 1), although certainly the 2 modalities should not be viewed as interchangeable. Given the low-cost, bedside nature of the technology, FDNIRS/DCS has the potential to shed light onto the complex interplay between anemia, hemolysis, inflammation, and oxygen delivery in the brain in SCA.
This study has several limitations. It was a pilot, open-label study with a small sample size and no control group. Additionally, it relied on patient-reported compliance with the medication. Intermittent compliance was an issue that likely increased the heterogeneity of the data set. Ongoing work will expand the sample size to determine factors that contribute to the hemodynamic alterations induced by voxelotor (eg, age and RBC health). Due to the short duration of treatment, it remains to be seen whether these cerebral hemodynamic effects persist beyond the time window of this study. However, the hemoglobin oxygen affinity modulation to inhibit HbS polymerization (HOPE) and HOPE Kids 1 study found that hematologic benefits persisted for the duration of the study period, which was >1 year of treatment.10,11 Technical limitations include the limited regional sensitivity of the optical measurements. More work is needed to confirm that the trends we observed persist in regions of the brain most susceptible to hypoxic injury.6 Moreover, FDNIRS estimation of OEF relies on the assumption that γ, the fraction of the signal that arises from the venous compartment, remains constant across the treatment duration. Errors in this assumption due to redistribution of blood within the vasculature could lead to inaccuracies in our estimation of OEF that are not accounted for here. Furthermore, we did not quantify the influence of voxelotor on oxygen metabolism or oxygen delivery. In the interest of being conservative, we chose not to report these parameters because their estimation relies on several assumptions that could be altered with voxelotor treatment. Future work should directly quantify the effects of voxelotor on metabolism and delivery using positron emission tomography.
In summary, voxelotor decreases CBF and OEF. Given that previous work has shown that these parameters are elevated in children with SCA compared with healthy controls, these results suggest that voxelotor may reduce cerebral hemodynamic impairments. Further investigations are warranted to validate these findings using alternative modalities and to determine whether there is an association between these hemodynamic alterations and functional outcomes in patients receiving voxelotor therapy.
Acknowledgments
The authors thank the Aflac clinical research office, especially Katyria Thornton and Nwanna Ifendu, for their valuable support.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant R01HL152322 (E.M.B.), Global Blood Therapeutics, Pfizer, and the National Science Foundation Graduate Research Fellowship Program under grant 1937971 (R.O.B.).
Authorship
Contribution: E.M.B. and R.C.B. conceived and designed the study; R.O.B., H.Z., R.A.J., J.K.-S., S.S.M., K.B.T., M.A., S.T., T.M.U., and A.T. collected data; all authors had access to data; R.O.B., H.Z., R.A.J., A.G.-Y., S.B., and E.M.B. analyzed the data; S.B. helped with statistical analysis; R.O.B., R.C.B., and E.M.B. drafted the manuscript; R.O.B., R.C.B., A.T., R.A.J., A.G.-Y., S.B., and E.M.B. edited the manuscript; and all authors contributed to the study and approved the submitted version of manuscript.
Conflict-of-interest disclosure: During the conduct of this study, R.C.B. was employed at Emory University. R.C.B. is currently employed by and has equity ownership with Pfizer; is a former employee of Global Blood Therapeutics; has received consultant fees from Global Blood Therapeutics, Imara, and Novartis; and has received research funding from Forma Therapeutics, Global Blood Therapeutics, Imara, Novartis, and Pfizer. E.M.B. has received research funding from Pfizer, Global Blood Therapeutics, and Novartis. A.G.-Y. has received research funding from Pfizer, Global Blood Therapeutics, and Novartis. The remaining authors declare no competing financial interests.
The current affiliation of R.C.B. is Pfizer, Atlanta, GA.
Correspondence: Erin M. Buckley, Department of Biomedical Engineering, Emory University and Georgia Institute of Technology, 1760 Haygood Dr NE, Suite E100, RM E106, Atlanta, GA 30322; email: erin.buckley@emory.edu.
References
Author notes
Deidentified individual participant data are available upon request from author Rowan O. Brothers (rowan.oakley.brothers@emory.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal