In this issue of Blood, Brothers et al demonstrate that voxelotor lowers brain blood flow and oxygen extraction fraction (OEF) in children with sickle cell disease.1 Sickle cell disease causes both large and small blood vessels in the brain to remodel, leading to both overt and silent stroke. The baseline brain hemodynamic state in chronic anemias can be characterized by increased blood flow, decreased vascular flow reserve,2 normal to decreased whole brain OEF,3 and severely hypoxic deep white matter structures.3,4 Silent strokes and volume loss accumulate in the deep white matter, with concordant neurocognitive consequences.
The present study exploring the changes in cerebral physiology observed in 8 children before and following voxelotor treatment is novel for 2 reasons. To my knowledge, it is the first published cerebrovascular data in humans treated with voxelotor. It also exploits purely optical methods of assessing cerebral blood flow (CBF) and oxygen saturation. Diffusion correlation spectroscopy measures an index of CBF by detecting rapid light fluctuations produced by the flowing red blood cells, whereas frequency domain near-infrared spectrometry (FD-NIRS) measures oxygen saturation and cerebral blood volume. Because these optical techniques are noninvasive and have high throughput, they could more readily be used to trend changes to medication in an outpatient environment. They also can be performed in children of all ages, without the use of anesthesia.
The data from Brothers et al are particularly pertinent because the developing brain has higher metabolic demands for oxygen, peaking in the school-age years. Hemoglobin affinity modulators, like voxelotor, are inherently double-edged swords. Their primary benefit is improved red blood cell survival, which lessens the primary cerebrovascular stressors (anemia and hemolysis). However, higher-affinity hemoglobins have downsides as well. If the partial pressure of oxygen at 50% hemoglobin saturation (p50) of hemoglobin is too low, such as hemoglobin H in α-thalassemia, oxygen is never released to the capillary bed, rendering it functionally useless. To maintain adequate tissue perfusion and cerebral protection, pretransfusion hemoglobin thresholds in α-thalassemia have to be based entirely on the normal-affinity fraction.5 Hebbel and Hedlund raised the concern that the 30% of voxelotor-bound hemoglobin could be similarly nonfunctional, based on their biophysical models of oxygen transport.6 If so, gains in hemoglobin from improved red blood cell survival would be more than trumped by the loss of functional hemoglobin.
Fortunately, there are several lines of evidence that this may not be occurring. First, erythropoietin and reticulocyte levels stay the same or decrease in voxelotor-treated subjects, unlike in α-thalassemia.5 Erythropoetin levels are governed by hypoxia-inducing factor and would almost certainly increase if there was a net loss in effective oxygen delivery to the kidneys. Second, direct partial pressure of oxygen (pO2) electrode recordings from brains of sickle cell mice do not demonstrate cerebral hypoxia with GBT1118, a more potent version of voxelotor under development by Global Blood Therapeutics.7 Last, CBF declined with voxelotor treatment in the present study. CBF is regulated by pO2,8 not by oxygen saturation, so a decline in CBF is reassuring that brain arterioles are perceiving an adequate pO2.
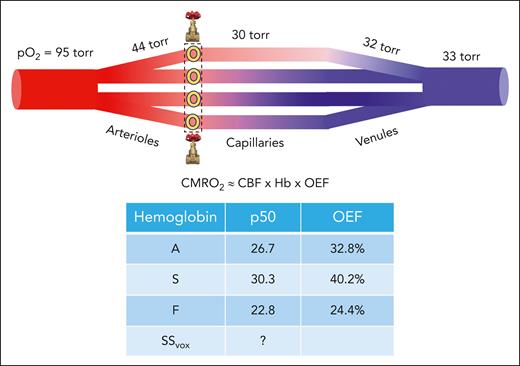
In contrast to changes in brain blood flow, a reduction in OEF cannot be interpreted as an improvement in cerebrovascular stress, because it is a direct consequence of increasing oxygen affinity. A putative cerebrovascular bed with arterial and venous pO2 values of 95 and 33 torr, respectively, is illustrated (see figure). Oxygen exchange begins at the arteriolar level, accounting for approximately one-third of oxygen exchange.9 Terminal arterioles sense pO2 and increase or decrease flow to maintain a set pO2 level.8 Oxygen tension reaches a nadir in the capillary bed before increasing slightly in collecting venules and veins.9 OEF reflects the arteriovenous difference in oxygen saturation, normalized to the arterial saturation. Because the brain vasculature regulates tissue pO2, not oxygen saturation, the expected OEF will depend on hemoglobin’s oxygen affinity. The table in the figure illustrates the range in OEFs predicted for different hemoglobins using the pO2 values from the figure; OEF values for S and F hemoglobin are ≈25% higher or lower than for A hemoglobin, respectively. Voxelotor’s hemoglobin binding and impact on the hemoglobin dissociation curve when administered at doses targeting 30% occupancy are complicated, and not well described by a simple p506,10; however, OEF will certainly be lower than for A hemoglobin.
(Top) Plot depicting brain microvasculature, consisting of conduit arteries, arterioles, capillaries, venules, and collecting veins. Partial pressures of oxygen for each segment are assumed for illustration purposes. Blood flow across the capillary network is nonuniform and regulated by upstream dissolve oxygen sensors in muscular sphincters located in the terminal arterioles (depicted by the yellow circles in the dashed box).8 Oxygen saturation in the collecting vein is the flow-weighted summation of the upstream oxygen saturations; high-flow segments skew the downstream collecting vein oxygen saturation to higher values.9 Brain OEF is calculated as the difference between the saturation in the collecting vein and the arterial oxygen saturation, divided by the arterial saturation. (Middle) The equation represents the balance between CBF, hemoglobin (Hb), and OEF in calculating the cerebral metabolic rate of oxygen (CMRO2), neglecting arterial saturation because it is nearly unity. (Bottom) Table depicting the expected OEF values for 3 different hemoglobin species, assuming an upstream pO2 of 95 torr and a downstream pO2 of 33 torr as well as pH and partial pressure of carbon dioxide under standard conditions (7.40 and 40 torr, respectively).
(Top) Plot depicting brain microvasculature, consisting of conduit arteries, arterioles, capillaries, venules, and collecting veins. Partial pressures of oxygen for each segment are assumed for illustration purposes. Blood flow across the capillary network is nonuniform and regulated by upstream dissolve oxygen sensors in muscular sphincters located in the terminal arterioles (depicted by the yellow circles in the dashed box).8 Oxygen saturation in the collecting vein is the flow-weighted summation of the upstream oxygen saturations; high-flow segments skew the downstream collecting vein oxygen saturation to higher values.9 Brain OEF is calculated as the difference between the saturation in the collecting vein and the arterial oxygen saturation, divided by the arterial saturation. (Middle) The equation represents the balance between CBF, hemoglobin (Hb), and OEF in calculating the cerebral metabolic rate of oxygen (CMRO2), neglecting arterial saturation because it is nearly unity. (Bottom) Table depicting the expected OEF values for 3 different hemoglobin species, assuming an upstream pO2 of 95 torr and a downstream pO2 of 33 torr as well as pH and partial pressure of carbon dioxide under standard conditions (7.40 and 40 torr, respectively).
Is a lowering OEF beneficial to the brain? The metabolic rate of the brain for oxygen, or CMRO2, is approximately proportional to the product of CBF, hemoglobin level, and OEF (neglecting arterial oxygen saturation, which is close to unity for most patients). By itself, a decrease in OEF will decrease CMRO2, unless CBF or hemoglobin increase to compensate for the decreased oxygen unloading. In the present study, the decrease in OEF (−13%) was matched by an increase in hemoglobin (13%). According to simple oxygen transport considerations, one would not have expected any reduction in CBF. Unfortunately, this is where the pilot nature of the study becomes limiting. The diffuse correlation spectroscopy flow index used in the article correlates coarsely with arterial spin labeling, and the broad distribution of CBF indexes observed at baseline (varying over a fivefold range) does not inspire confidence that its quantitation is robust. Furthermore, although techniques, like FD-NIRs and asymmetric spin-echo,4 offer important insights into microvascular health, OEFs calculated by these methods cannot be used to infer CMRO2 because they do not account for nonuniform microvascular flow rates.9 Despite these limitations, the changes observed in CBF index with voxelotor treatment is first-in-human evidence that brain pO2 is at least stable in this cohort of well-treated children with sickle cell disease, which is reassuring. However, it is critical to continue monitoring the oxygen tug-of-war between the blood and the brain using all the tools available (optical, magnetic resonance imaging, and positron emission tomography) in more diseased populations and with subsequent hemoglobin-affinity agents.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal