In this issue of Blood, Tang and colleagues report that the RNA-binding protein fused in sarcoma (FUS) is a newly identified effector of hematopoietic stem cell (HSC) aging. The protein levels of FUS increase in HSCs with aging and FUS forms aberrant condensates. The aberrant FUS condensates induce global chromatin reorganization to create an aged HSC-like transcriptional signature.1
HSCs alter their function during aging, leading to the disruption of hematopoietic homeostasis. The resulting aging phenotype includes myeloid- and platelet-biased differentiation, anemia, perturbed immunity, and increased propensity to hematological malignancies.2 Recent studies that attempted to functionally rejuvenate HSCs in aged mice by various interventions,3 or transplantation into unconditioned young recipient mice,4 have been unsuccessful. In contrast, transplantation of middle-aged donor HSCs into unconditioned young recipient mice could, in part, functionally rejuvenate the HSCs.4 Stimulation of middle-aged HSCs with an activator of mitochondrial membrane potential or with insulinlike growth factor 1 also have some rejuvenating effects,5,6 suggesting that targeting HSCs at middle age may be an opportunity for rejuvenation.2 Therefore, better understanding how HSCs alter their function during aging is of critical importance to prevent or overcome the age-related deleterious outcomes.
Liquid-liquid phase separation (LLPS) is responsible for the formation of biomolecular condensates, cellular compartments that concentrate macromolecules without surrounding membranes. Eukaryotic cells contain such dynamic membrane-less organelles, many of which are enriched in RNA and RNA-binding proteins.7 FUS is an RNA-binding protein that undergoes LLPS. The physiological functions of FUS include transcriptional activation, DNA repair, alternative splicing, RNA export, and localized translation. Mutations in FUS are implicated in a subset of familial amyotrophic lateral sclerosis and cause mislocalization of FUS to the cytoplasm, resulting in loss of its nuclear function and formation of pathological FUS aggregates in the cytoplasm.8
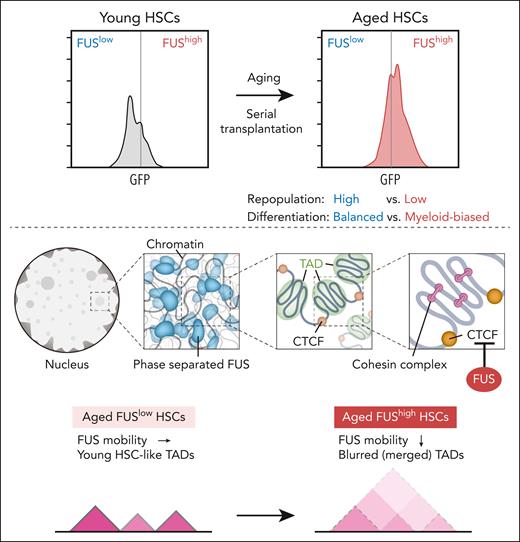
Tang and colleagues now show that FUS protein, but not its mRNA, is significantly increased in aged HSCs. They generated FUS-green fluorescent protein (GFP) fusion in mice, wherein the expression of FUS was monitored by GFP. Using these mice, they demonstrated that FUS is heterogeneously expressed in HSCs, and FUShigh HSCs increase and FUSlow HSCs decrease with aging and during serial transplantation (see figure). Notably, the reconstitution capacity of aged FUShigh HSCs was significantly compromised compared with FUSlow HSCs and showed myeloid-biased differentiation like aged HSCs, and FUSlow aged HSCs exhibited balanced differentiation like young HSCs. Thus, high expression of FUS marks myeloid-biased aged HSCs similar to CD150.9 Transcriptome analysis confirmed that FUShigh and FUSlow HSCs resemble aged and young HSCs, respectively. These findings strongly support the functional heterogeneity of aged HSCs, which had been demonstrated using single HSC transplantation assays.
FUS expression in FUS-GFP mice increases in HSCs with aging and during serial transplantation (upper panels). FUS undergoes LLPS and forms dynamic and reversible condensates. Locally, genomic domains show strong self-interactions and are insulated from nearby regions, forming TADs. In mammals, CTCF-binding sites on chromatin are preferentially found at TAD boundaries. Inside each TAD, cohesin-mediated loop domains facilitate chromatin folding. Once cohesin is loaded onto chromatin, it moves along chromatin and presumably extrudes chromatin loops. Cohesin sliding can be blocked by CTCF, where loop boundaries form (middle panels). Aberrant phase-separated FUS limits the binding of CTCF to its motif, thereby compromising the insulation of TAD by CTCF in aged FUShigh HSCs. Consequently, the boundaries of a large number of TADs in FUShigh HSCs were merged (lower panels).
FUS expression in FUS-GFP mice increases in HSCs with aging and during serial transplantation (upper panels). FUS undergoes LLPS and forms dynamic and reversible condensates. Locally, genomic domains show strong self-interactions and are insulated from nearby regions, forming TADs. In mammals, CTCF-binding sites on chromatin are preferentially found at TAD boundaries. Inside each TAD, cohesin-mediated loop domains facilitate chromatin folding. Once cohesin is loaded onto chromatin, it moves along chromatin and presumably extrudes chromatin loops. Cohesin sliding can be blocked by CTCF, where loop boundaries form (middle panels). Aberrant phase-separated FUS limits the binding of CTCF to its motif, thereby compromising the insulation of TAD by CTCF in aged FUShigh HSCs. Consequently, the boundaries of a large number of TADs in FUShigh HSCs were merged (lower panels).
Since abnormal FUS aggregation has been identified in multiple neurodegenerative diseases,8 Tang et al then evaluated the formation of FUS condensates in HSCs and confirmed that FUS undergoes LLPS and forms dynamic and reversible condensates (see figure). They found that FUS condensate dynamics are drastically reduced in aged FUShigh HSCs, which is rescued by FUS knockdown. Thus, the mobility of FUS in aged FUShigh HSCs is compromised. Enforced expression of wild-type FUS, but not FUS mutants that cannot form condensates, impaired the reconstitution capacity of young HSCs and promoted their myeloid-biased differentiation, suggesting that aberrant phase-separated FUS impairs HSC function.
To identify the underlying molecular mechanism, Tang et al profiled genome-wide chromatin interactions by Hi-C analysis, which revealed that aged FUShigh HSCs exhibit global chromatin organization similar to that of aged bulk HSCs but distinct from that of aged FUSlow HSCs. Topologically associating domains (TADs) are self-interacting genomic regions, in which DNA sequences within a TAD physically interact with each other more frequently than with sequences outside the TAD. The interaction within TADs of aged FUShigh HSCs was compromised, and the interaction between adjacent TADs was strengthened, leading to blurred boundaries of TADs. The authors found that FUS binds to CCCTC-binding factor (CTCF), one of the important insulators that form a TAD boundary. The binding of CTCF was significantly compromised in aged FUShigh HSCs similar to that seen in aged bulk HSCs. Interestingly, as the mobility of FUS decreased, so did the binding of CTCF to its motif. Collectively, these results suggest that aberrant phase-separated FUS limits the binding of CTCF to its motif, thereby compromising the insulation of TAD by CTCF in aged FUShigh HSCs (see figure). Indeed, the boundaries of a large number of TADs in aged FUShigh HSCs were merged due to the compromised CTCF binding, and the transcription of genes within them was not properly regulated, which included aged HSC signature genes.
This study provides the first evidence that implicates aberrant condensate assembly as a driving force behind HSC aging. Logically, the next question is whether we can target FUS to prevent HSC aging. Tang et al demonstrated that although FUS knockdown enhances the reconstitution capacity of aged FUSlow HSCs, it does not rescue the impaired reconstitution capacity or differentiation skewing of aged FUShigh HSCs. These results suggest that the chromatin organization in aged FUShigh HSCs has irreversibly changed, which provides a plausible explanation for why HSCs resist rejuvenation.
Finally, several important questions remain. How does FUS protein accumulate specifically in HSCs and lose its dynamic properties with aging? The authors did not clarify how the FUS condensates change their characteristics, such as their number and size. The composition of FUS condensates may also change with aging. It is possible that such changes stabilize FUS and decrease its dynamics. Characterization of these changes as well as the molecules recruited to the FUS condensates should facilitate our understanding of the role of FUS in HSC aging. How do FUS condensates induce chromatin reorganization? Nuclear condensates, including FUS condensates, have been reported to function as mechano-active chromatin filters that physically pull in targeted genomic loci while mechanically excluding nontargeted chromatin.10 It is possible that the aberrant FUS condensates mechanically restructures chromatin in addition to dysregulating CTCF binding to chromatin. FUS condensates represent just one of the various liquid condensates in HSCs. How these condensates are regulated during aging is another intriguing issue, and further characterization of liquid condensates should create a new field in the research of HSC aging.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal