In this issue of Blood, Ghorashian et al report results from a phase 1 clinical trial, in which they investigated a novel CD19/CD22-bispecific chimeric antigen receptor (CAR) T-cell product in a cohort of 12 patients with relapsed/refractory (r/r) acute lymphoblastic leukemia (ALL), and they report no antigen-negative relapses.1
CD19-CAR T-cell therapy has transformed the treatment of pediatric patients with r/r ALL. However, despite the impressive initial remission rates of >80% across multiple studies using Food and Drug Administration–approved or investigational CD19-CAR T-cell products, 36% to 57% of patients who achieve complete remission (CR) relapse within 1 year, and durable remission occurs in only approximately 40% to 50% of patients.2 Outcomes post-CAR T-cell relapse remain dismal, and relapse due to emergence of CD19− leukemia, in particular, is associated with extremely poor prognosis.3 Indeed, the emergence of CD19− disease and limited CAR T-cell persistence are the major limitations of current CD19-CAR T-cell approaches.
The incidence of CD19− ALL relapse post-CD19-CAR T cell varies and has been reported to be up to 57%.4 Several mechanisms for this immune escape have been described including (1) mutations in CD19 that lead to shedding of the extracellular domain, (2) selection of a preexisting CD19− clone, and (3) lineage switch with the recurring leukemia having an AML phenotype.5,6 The logical approach to overcome immune escape is to target additional antigens. This is an area of active research, and most efforts are focused on targeting CD22 based on the encouraging results of CD22-CAR T-cell therapy for pediatric ALL.
The most commonly employed CD19/22 dual targeting strategies can be summarized under 3 broad approaches: (1) sequential or coadministration of CD19 and CD22 CAR T-cell products, (2) engineering T cells to express CD19-CAR and CD22-CAR using either a single (bicistronic) vector or double (cotransduction) vectors, or (3) engineering T cells to express a single CAR that recognizes both CD19 and CD22 (bispecific).
All 3 approaches have been evaluated in early phase clinical studies with the largest cohort of patients having received 2 CAR T-cell products.7-9 The results of these studies indicate that all 3 approaches are safe. However, relapse rates in most of these studies are >40%, and it remains to be determined which approach is best. Of note, none of the studies targeting CD19/22 have resulted in improved outcomes compared with CD19-CAR T-cell therapy alone, and none, except for the current study, have eliminated antigen-negative relapses.
This is what makes the study by Ghorashian et al unique. It is based on a strategy that was thoughtfully designed, taking into account the limitations associated with targeting CD19 and CD22 in previous studies. The CD19-targeting component utilized a CD19-CAR (AUTO1) with a fast-off rate post CD19 binding, which endows CAR T cells with enhanced effector function and showed efficacy in a previous trial.10 To target CD22, the investigators designed a novel construct. CD22 is known to have low antigen density on ALL blasts, and relapses post CD22-CAR T-cell therapy are associated with further CD22 downregulation. Therefore, the group designed a highly sensitive CD22-CAR that recognized target cells expressing low levels of CD22.
These CD19- and CD22-CAR constructs were used to generate CD19/CD22-CAR T-cell products by cotransducing T cells with separate lentiviral vectors encoding the CD19- or CD22-CAR. The median transduction efficiency was 83.2% (range 60.8%-92.6%) with the majority of T cells expressing CD19- and CD22-CARs and having a predominant central memory T-cell phenotype. There was excellent early expansion of both CD19- and CD22-CAR T cells with high cumulative CAR T-cell exposure post infusion and also balanced engraftment of mono- and bispecific CAR T cells. This is in contrast to previous reports using CD19/22-directed CAR T-cell strategies where skewing of engrafted CAR T-cell populations was observed.
Twelve patients with advanced high-risk B-ALL who were heavily pretreated and had high-risk features often associated with risk of CD19− relapse (CD19− disease, previous receipt of blinatumomab or CD19-CAR T-cell therapy) were infused. Ten of 12 patients achieved measurable residual disease–negative CR at 2 months post infusion including 2 of 3 patients who had had CD19− disease. Of the 10 responding patients, 5 relapsed with CD19+ and CD22+ disease. Relapses were associated with loss of CAR T-cell persistence in 4 of the 5 relapsing patients. It is likely that the additional targeting through the highly sensitive CD22 construct, which could kill CD19− leukemia cell lines in preclinical assays, contributed to prevention of antigen-negative relapse.
It is possible that with larger patient numbers and longer follow-up, antigen-negative relapses could occur. However, the data here contrast with the prior experience of the same group with AUTO1 CD19-CAR T-cell therapy and other groups using CD19/CD22-CAR T cells in which antigen-negative relapses occurred in the same time frame.
There was no significant toxicity; no severe cytokine release syndrome or hemophagocytic lymphohistiocytosis occurred. One patient had possible grade 4 immune effector cell-associated neurotoxicity syndrome, but this could have been due to fludarabine toxicity. These data are promising and suggest that dual targeting using the vectors described is safe and may prevent antigen-negative relapse after CAR T-cell therapy.
The main limitation of the study was lack of CAR T-cell persistence. Multiple studies have highlighted that limited CAR T-cell persistence is most likely multifactorial and includes T-cell intrinsic as well as extrinsic mechanisms. Several approaches are actively being explored to improve persistence by evaluating the benefit of novel CAR designs or other synthetic T-cell receptors, providing cytokine support, or manipulating regulatory circuits in T cells that are associated with T-cell dysfunction and/or exhaustion.
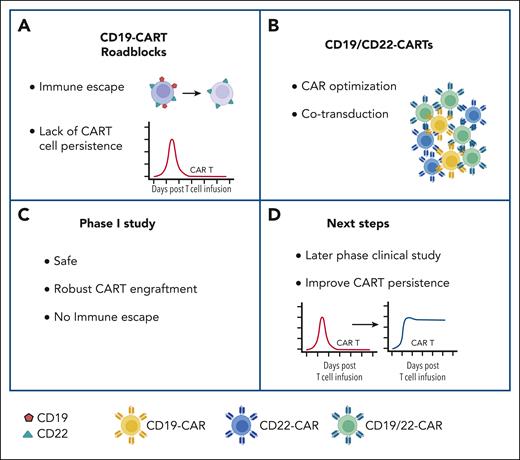
The use of CD19-CAR T-cell therapy has become an integral part of the treatment armamentarium for patients with r/r ALL, but antigen-negative relapses and lack of persistence remain the major challenges. Based on astute observations of leukemia biology and mechanism of antigen escape that led to informed CAR design, the study by Ghorashian et al provides hope that the dilemma of antigen-negative relapse could be overcome. Similar insightful approaches to overcome the lack of persistence would allow for the promise of long-term cures for patients with r/r ALL (see figure).
Novel CD19/CD22-CAR T-cell product overcomes one of the current roadblocks of CD19-CAR T-cell therapy (CART) for ALL. (A) Current roadblocks of CART include immune escape and limited persistence. (B) Novel CD19/CD22-CART product generated by cotransduction with optimized CARs: product contains T cells expressing both CARs (green) or one of the two CARs (blue or yellow). (C) Outcome of phase 1 clinical study. (D) Next steps. For additional details, see text. Figure created with BioRender.com.
Novel CD19/CD22-CAR T-cell product overcomes one of the current roadblocks of CD19-CAR T-cell therapy (CART) for ALL. (A) Current roadblocks of CART include immune escape and limited persistence. (B) Novel CD19/CD22-CART product generated by cotransduction with optimized CARs: product contains T cells expressing both CARs (green) or one of the two CARs (blue or yellow). (C) Outcome of phase 1 clinical study. (D) Next steps. For additional details, see text. Figure created with BioRender.com.
Conflict-of-interest disclosure: S.G. has patents/patent applications in the fields of T-cell and gene therapy for cancer, is a consultant of TESSA Therapeutics, is a member of the Data and Safety Monitoring Board of Immatics, is on the Scientific Advisory Board of Be Biopharma and CARGO, and has received honoraria from Tidal and Sanofi within the last 2 years. S.N. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal