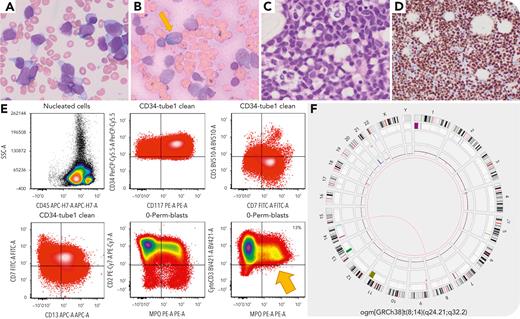
A 66-year-old man presented with abnormal complete blood count (white blood cell count 28.4 × 109/L, hemoglobin 6.9 g/dL, platelets 54 × 109/L) with 81% blasts. The bone marrow (BM) aspirate showed 79% blasts (panel A, Wright-Giemsa stain, 100× objective), with a subset positive for myeloperoxidase (panel B, 100× objective). The BM biopsy showed sheets of blasts (panel C, hematoxylin and eosin stain, 100× objective). Blasts had a T/myeloid phenotype (panel E, flow cytometry) positive for CD2, cCD3, CD7, CD13, CD33, CD34, CD117, CD123, CD133, HLA-DR, MPO (13%), TDT, and negative for CD1a, sCD3, CD5, CD8. This prompted additional immunohistochemical staining for BCL11B, which was diffusely positive (panel D, 40× objective). Sequencing identified FLT3 internal tandem duplication and TET2 (R1465∗) and RAD21 (L193fs) mutations. Optical genome mapping showed t(8;14)(q24.21;q32.2) with CCDC26::SET3 putative fusion gene (panel F), previously shown to lead to BCL11B deregulation due to enhancer hijacking.
Acute leukemias (ALs) with BCL11B deregulation can manifest with various phenotypes including undifferentiated myeloid, T/myeloid, early T precursor (ETP), or near-ETP AL. BCL11B deregulation is driven by 14q32 rearrangements juxtaposing BCL11B to superenhancers or amplifications generating superenhancers from noncoding elements distal to BCL11B. These frequently co-occur with FLT3, WT1, and epigenetic mutations and are important to recognize as they may be sensitive to FLT3 or JAK/STAT inhibition. This patient’s neoplasm would be classified as AL of ambiguous lineage with BCL11B rearrangement or mixed phenotype AL with BCL11B activation in the World Health Organization, 5th Edition and International Consensus Classification, respectively.
For additional images, visit the ASH Image Bank, a reference and teaching tool that is continually updated with new atlas and case study images. For more information, visit https://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal