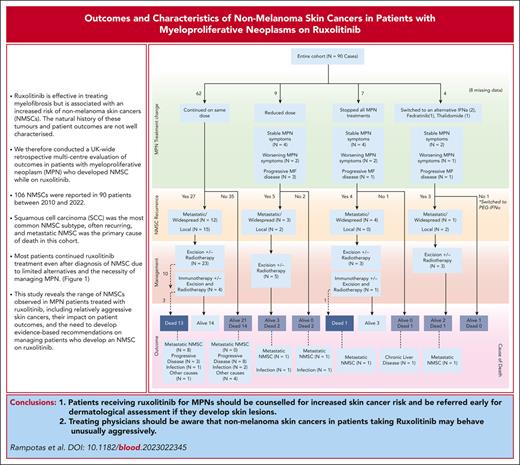
Visual Abstract
Nonmelanoma skin cancers (NMSCs) in ruxolitinib-treated patients with myeloproliferative neoplasms behave aggressively, with adverse features and high recurrence. In our cohort, mortality from metastatic NMSC exceeded that from myelofibrosis. Vigilant skin assessment, counseling on NMSC risks, and prospective ruxolitinib-NMSC studies are crucial.
TO THE EDITOR:
Ruxolitinib was the first Janus kinase inhibitor (JAKi) approved for the treatment of myelofibrosis (MF) and is effective in reducing spleen volume and symptom burden as well as potentially prolonging survival in responding patients. However, benefits need to be balanced against potential toxicities, including an increased risk of nonmelanoma skin cancers (NMSCs), first identified in the pivotal phase 3 clinical trial, Controlled Myelofibrosis Study With Oral JAK Inhibitor Treatment-II. Several subsequent reports confirmed this association.1-4 A warning of a potential increased risk of NMSC was consequently included in the ruxolitinib summary of product characteristics.5 Despite awareness of this association, there are limited data on the histologic spectrum of these cancers, their clinical behavior, and their impact on subsequent myeloproliferative neoplasm (MPN)–directed management.
To address this gap in knowledge, we conducted a real-world retrospective study of patients with MPN from 18 United Kingdom MPN treatment centers who were diagnosed with NMSCs while receiving ruxolitinib therapy. The aim of the study was to evaluate the characteristics of NMSC associated with ruxolitinib, estimate risk of recurrence, and delineate how they were managed. Clinicians, coordinated via the United Kingdom National Cancer Research Institute MPN national research consortium, reviewed the notes of patients with MPN between 2010 and 2022 to identify patients diagnosed with NMSC after starting ruxolitinib. Baseline MPN characteristics, history of skin cancer, histopathologic characteristics of the NMSC, and therapy/outcomes were recorded using standardized pro forma documents. The first NMSC diagnosis was defined as the primary event, and subsequent episodes of the same cancer type were considered recurrences. We further studied those patients who experienced skin cancer recurrence (after first postruxolitinib occurrence) using logistic regression to evaluate the impact of potential prognostic characteristics on the nature of relapse (widespread vs local) with the effect being expressed in terms of odds ratio and 95% confidence interval (CI). The Cox model was used to evaluate the prognostic impact of baseline characteristics on survival with estimation of hazard ratios and 95% CIs; the proportionality assumption for each prognostic variable included in the analysis was graphically assessed with the log-log plot. Significant variables from univariate analysis (at the 10% level) were then included in the multivariable Cox model to test for independent prognostic significance and estimate their impact adjusted for other significant predictors. All patient data were anonymized at source and treated according to the principles of the Declaration of Helsinki and the United Kingdom Data Protection Act (1998).
Ninety patients (median age, 73 years; interquartile range [IQR], 68-78 years) who developed NMSC while receiving ruxolitinib were identified and included. Among them, 71 (78.9%) patients had MF, 17 (18.9%) had polycythemia vera, and 2 had another MPN. Baseline Dynamic International Prognostic Scoring System or Dynamic International Prognostic Scoring System-plus score was available for 58 patients with MF, with most classified as intermediate-2 (72.4%) or high risk (13.8%). Median follow-up was 125.4 (IQR, 78-214) months after MPN diagnosis, and 80.6 (IQR, 54-122) months after commencing ruxolitinib. Median interval between starting ruxolitinib and NMSC diagnosis was 30 months, and the median total daily dose of ruxolitinib at the time of NMSC diagnosis was 22.5 mg. Most patients (n = 60 [68.9%]) had received prior hydroxycarbamide therapy, and 33 (37.9%) had a history of skin cancer before starting ruxolitinib (Table 1).
Baseline characteristics of patients diagnosed with nonmelanoma skin cancer
| Baseline characteristics . | All cases (N = 90) . |
|---|---|
| Age, median (IQR), y (N = 90) | 73 (68-78) |
| Diagnosis | Myelofibrosis: 71/90 (78.9%) Polycythemia vera: 17/90 (18.9%) Essential thrombocythemia/polycythemia vera: 1/90 (1.1%) Other: 1/90 (1.1%) |
| DIPSS | Low: 2/58 (3.4%) Int-1: 6/58 (10.3%) Int-2: 42/58 (72.4%) High: 8/58 (13.8%) Not known: 13/61 Not applicable (nonmyelofibrosis cases): 19/90 |
| No. of prior therapies Not known: 3/90 | 0: 21/87 (24.1%) 1: 43/87 (49.4%) 2: 21/87 (24.1%) 3: 2/87 (2.3%) |
| Prior hydroxycarbamide use Not known: 2/90 | Yes: 60/88 (68.2%) No: 28/88 (31.8%) |
| History of skin cancer Not known: 3/90 Type of prior skin cancer Same cancer as history after ruxolitinib | Yes: 33/87 (37.9%) No: 54/87 (62.1%) |
| Squamous cell carcinoma: 12/33 (36.4%) Basal cell carcinoma: 7/33 (21.2%) Bowen disease: 3/33 (10%) Malignant melanoma: 1/33 (3%) >1 Type of skin cancer: 10/33 (30.3%) | |
| Yes: 15/23 No: 8/23 NA: 10/33 | |
| Additional immunosuppression Not known: 27/90 Types of additional immunosuppression | Yes: 19/63 (30.2%) No: 44/63 (69.8%) |
| Additional MPN treatment (6/19) Navitoclax: 2/19 Pelabresib: 1/19 Prior fedratinib: 1/19 Azacytidine: 2/19 Prior cancer/treatment (5/19) Prior CHOP for non-Hodgkin lymphoma: 1/19 Prior bendamustine and ofatumumab for chronic lymphocytic leukemia: 1/19 Prior allo-HCT and induction chemotherapy with daunorubicin and cytarabine: 1/19 Prior lobectomy for lung adenocarcinoma: 1/19 Prior immunotherapy for skin cancer: 1/19 Autoimmune and rheumatic disease/immunosuppression (5/19) Daily prednisolone: 2/19 Hydroxycarbamide for psoriasis: 1/19 Methotrexate for psoriasis: 1/19 Ulcerative colitis: 1/19 Other (3/19) Chronic kidney disease on hemodialysis: 1/19 Cardiac failure: 1/19 Prior ruxolitinib: 1/19 |
| Baseline characteristics . | All cases (N = 90) . |
|---|---|
| Age, median (IQR), y (N = 90) | 73 (68-78) |
| Diagnosis | Myelofibrosis: 71/90 (78.9%) Polycythemia vera: 17/90 (18.9%) Essential thrombocythemia/polycythemia vera: 1/90 (1.1%) Other: 1/90 (1.1%) |
| DIPSS | Low: 2/58 (3.4%) Int-1: 6/58 (10.3%) Int-2: 42/58 (72.4%) High: 8/58 (13.8%) Not known: 13/61 Not applicable (nonmyelofibrosis cases): 19/90 |
| No. of prior therapies Not known: 3/90 | 0: 21/87 (24.1%) 1: 43/87 (49.4%) 2: 21/87 (24.1%) 3: 2/87 (2.3%) |
| Prior hydroxycarbamide use Not known: 2/90 | Yes: 60/88 (68.2%) No: 28/88 (31.8%) |
| History of skin cancer Not known: 3/90 Type of prior skin cancer Same cancer as history after ruxolitinib | Yes: 33/87 (37.9%) No: 54/87 (62.1%) |
| Squamous cell carcinoma: 12/33 (36.4%) Basal cell carcinoma: 7/33 (21.2%) Bowen disease: 3/33 (10%) Malignant melanoma: 1/33 (3%) >1 Type of skin cancer: 10/33 (30.3%) | |
| Yes: 15/23 No: 8/23 NA: 10/33 | |
| Additional immunosuppression Not known: 27/90 Types of additional immunosuppression | Yes: 19/63 (30.2%) No: 44/63 (69.8%) |
| Additional MPN treatment (6/19) Navitoclax: 2/19 Pelabresib: 1/19 Prior fedratinib: 1/19 Azacytidine: 2/19 Prior cancer/treatment (5/19) Prior CHOP for non-Hodgkin lymphoma: 1/19 Prior bendamustine and ofatumumab for chronic lymphocytic leukemia: 1/19 Prior allo-HCT and induction chemotherapy with daunorubicin and cytarabine: 1/19 Prior lobectomy for lung adenocarcinoma: 1/19 Prior immunotherapy for skin cancer: 1/19 Autoimmune and rheumatic disease/immunosuppression (5/19) Daily prednisolone: 2/19 Hydroxycarbamide for psoriasis: 1/19 Methotrexate for psoriasis: 1/19 Ulcerative colitis: 1/19 Other (3/19) Chronic kidney disease on hemodialysis: 1/19 Cardiac failure: 1/19 Prior ruxolitinib: 1/19 |
allo-HCT, allogeneic haematopoietic stem cell transplant; CHOP, cyclophosphamide, hydroxydaunorubicin, vincristine (Oncovin), prednisolone; DIPSS, Dynamic International Prognostic Scoring System; Int, intermediate; NA, not applicable.
A total of 106 NMSCs were reported, with a median interval of 56 (IQR, 31-104) months between MPN diagnosis and NMSC diagnosis. Squamous cell carcinoma (SCC) was the most common histologic subtype (n = 61 cases), followed by basal cell carcinoma (n = 37 cases), and other NMSCs; Bowen disease (n = 5), pleiomorphic sarcoma (n = 1), sarcomatoid tumor (n = 1), and metastatic sarcomatoid SCC (n = 1) also occurred.
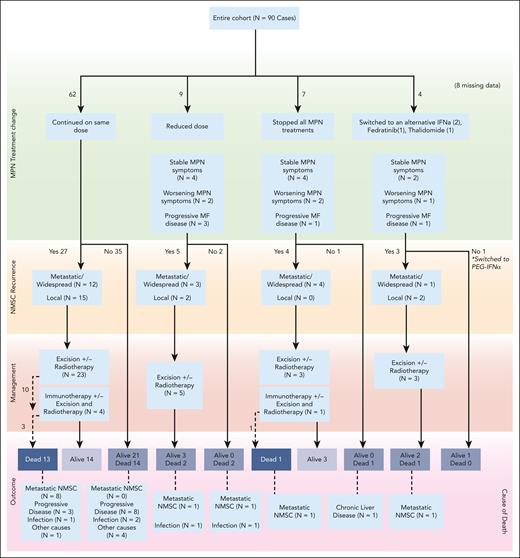
Where reports were available, pathologic and clinical data showed that most SCCs were small in size and of low thickness, but with poor or moderately poor differentiation in most cases (supplemental Table 1 [available on the Blood website]). Although primary excision margins were predominantly clear, SCC recurrence was reported in nearly 60% of patients (n = 34), with some cases of local recurrence (n = 12), but also of widespread/metastatic recurrence (n = 20). Most patients (75.6%) continued ruxolitinib without any dose change after NMSC diagnosis. The outcomes of those who either stopped or changed to an alternative are presented in Figure 1.
Outcomes of patients depending on how their MPN treatment changed after NMSC diagnosis.
Outcomes of patients depending on how their MPN treatment changed after NMSC diagnosis.
Of the 90 patients included, 35 (38.9%) had died at the time of data analysis. The cause of death (CoD) was metastatic NMSC in 12 of 35 cases (34.3%): 9 cases of metastatic SCC, 2 cases of metastatic Merkel cell carcinoma with SCC, and 1 case of metastatic sarcomatoid tumor. The CoD was progressive MF in 11 cases (31.4%; 2/11 transformation to acute myeloid leukemia), infections in 6 cases (17.1%), with 1 case (2.9%) each of cardiac failure, myocardial infarction, liver disease, and gastrointestinal tract bleeding. CoD was unknown in 2 cases (supplemental Table 2). No death was attributed to concurrent immunosuppression therapy.
Descriptive data for the patients with the most aggressive disease who died of metastatic NMSC and those who developed 2 different types of cancers are presented separately (supplemental Table 4). There were 2 patients who died of metastatic Merkel cell carcinoma with SCC component. They had initially been diagnosed with SCC, which reoccurred 2 and 3 times, respectively, and eventually progressed to metastatic/widespread disease. The first patient was treated with excision of the tumor, whereas the second had radiotherapy of the affected areas and immunotherapy with cemiplimab. One patient was receiving daily low-dose prednisolone for polymyalgia rheumatica, whereas the second had received prior chemotherapy for non-Hodgkin lymphoma. They had both received ruxolitinib for >12 months when the skin cancer was diagnosed.
Patients were followed up for a median of 36 months after NMSC diagnosis, and the median overall survival from NMSC diagnosis was 62.9 months. Analysis did not identify significant factors associated with metastatic or widespread recurrence vs local or no recurrence. Prior hydroxycarbamide use appeared to have a protective effect in the SCC subgroup (hazard ratio, 0.34; 95% CI, 0.14-0.79; P = .0088). Multivariable analysis did not reveal any significant factors predictive for overall survival (supplemental Table 3A).
This cohort of 90 patients with MPN who developed NMSC while receiving ruxolitinib therapy represents the largest of its kind yet reported. We highlight the aggressive nature of many of these cancers, with high recurrence, metastatic, and mortality rates. In this cohort with MPN, SCC was more common than basal cell carcinoma, a reversal of the situation seen in the general population where even in SCC cases, poor differentiation is reported only in <10% of cases, whereas metastatic disease and SCC mortality accounts for <5% and 1% to 2%, respectively.6-8 Our findings for ruxolitinib-treated patients with NMSC regarding metastasis frequency and rate of poorly differentiated pathology mirrors the situation described in solid organ recipients receiving immunosuppression.9,10 Metastatic NMSC was the primary cause of death in our cohort, exceeding deaths due to MF progression, emphasizing the aggressive nature of these skin cancers in this setting. Intriguingly, prior hydroxycarbamide use was associated with improved overall survival, possibly skewed by an increased awareness of skin cancer risk and closer monitoring in such patients. The exact mechanisms behind the aggressive behavior of NMSC in ruxolitinib-treated patients with MPN remain unclear, although it may plausibly be related, at least in part, to ruxolitinib’s immunosuppressive activity.
The statistically proven association between NMSC and ruxolitinib is well reported. In a large pharmacovigilance database study of 870 JAKi-related cancers, ruxolitinib was associated with a high skin cancer risk (informational component, 3.29).1 Additional retrospective data demonstrated that ruxolitinib had a hazard ratio of 2.69 for NMSC development.2 Moreover, 3 case reports have also highlighted aggressive NMSC features with ruxolitinib exposure.11-13 Our data add to this evidence by characterising the types of NMSC seen in patients taking ruxolitinib, and their outcomes.14
Following the diagnosis of NMSC, most patients in our cohort continued taking ruxolitinib, most likely in part due to the need for MPN control, a lack of alternatives, and a lack of evidence supporting treatment discontinuation or switching to alternative JAKi or other novel agents. It remains unknown whether the risk of NMSC, in terms of both incidence and outcomes, is different for other JAKis. In the absence of available data, we would recommend counseling all ruxolitinib-treated patients to minimize risk of skin cancer (eg, sun avoidance) and to report new skin lesions promptly. The limitations of our study include its retrospective design and potential selection bias for more aggressive skin cancer cases, as it is possible that small/indolent NMSCs may have been excised in a local hospital and consequently were not recorded at the MPN treatment center.
In conclusion, our study highlights the aggressive nature of NMSCs in ruxolitinib-treated patients with MPN, the importance of counseling patients about the risk of skin cancer before starting ruxolitinib, and a requirement for close dermatological monitoring on treatment. Optimal MF management following diagnosis of NMSC remains uncertain—stopping ruxolitinib may result in MF symptom flare and potentially increase the risk of disease progression, and it is not yet clear whether ruxolitinib cessation (or switching to an alternative JAKi) impacts NMSC outcomes. Consequently, if a patient develops an NMSC while taking ruxolitinib, the risks and benefits of each treatment option need to be carefully weighed and discussed with the patient, acknowledging the uncertainties alluded to above, before deciding whether to change therapy. Larger prospective collaborative studies are needed to better understand NMSC risk and outcomes in ruxolitinib-treated patients with MPN, as are similar evaluations of NMSC risk in patients with MPN treated with other JAKis.
Acknowledgments
Biorender.com software was used to create Figure 1. The study was coordinated via the National Cancer Research Institute United Kingdom network (myeloproliferative neoplasm subgroup).
Authorship
Contribution: J.L. envisaged the idea of the project, supervised the whole project, and had reviewed and edited all aspects of the manuscript; J.F. initiated the project and contributed to data collection; A.R. led the national data collection and coordination of the project as well as performing the initial manuscript write up and descriptive analysis; F.P. performed the statistical analysis; and all authors contributed to data collection and comprehensively reviewed the manuscript and analysis.
Conflict-of-interest disclosure: A.R. reports conference fees from Gilead. T.C.P.S. reports consultancy for AbbVie, Novartis, Bristol Myers Squibb (BMS), and Oryzon Genomics; and research funding from Imago Biosciences and CellCentric Ltd. B.P. reports consultancy, current equity holding, honoraria, other (cofounder), and research funding from Alethiomics; consultancy, honoraria, and speaker’s bureau for Novartis; consultancy for Constellation Therapeutics and Blueprint Therapeutics; and research funding from Galecto and Evotec. C.H. reports membership on an entity's Board of Directors or advisory committees and research funding from Constellation Pharmaceuticals, Inc, a MorphoSys Company, and Celgene/BMS; membership on an entity's Board of Directors or advisory committees for CTI Biopharma, Geron, Galecto, Janssen, AbbVie, Promedior, Gilead, and Shire; other (leadership role) for EHA and MPN voice; honoraria from Sierra; consultancy and membership on an entity’s Board of Directors or advisory committees for Roche, Galecto, and AOP Pharma; consultancy for Keros; speaker’s bureau for Incyte; and membership on an entity's Board of Directors or advisory committees, other (support for attending meetings), and research funding from Novartis. AJ.M. reports consultancy, current equity holding, other (cofounder and equity holder), and research funding from Alethiomics Ltd; consultancy and speaker’s bureau for Gilead, Pfizer, Incyte, Sensyn, Karyopharm, Sierra Oncology, CTI, and AbbVie; consultancy, research funding, and speaker’s bureau for Galecto and Celgene/BMS; and consultancy, honoraria, research funding, and speaker’s bureau for Novartis. M.G. reports consultancy and honoraria from Janssen, Takeda, Novartis, Amgen, BMS, and GSK; consultancy and other (Advisory Board) for Janssen, Amgen, Takeda, and Novartis; and consultancy and speaker’s bureau for Janssen and Amgen. N.M.B. reports honoraria and speaker’s bureau for Novartis. A.J.I. reports speaker’s bureau for Incyte. C.M. reports conference fees from AbbVie and Novartis. A.L.G. reports other (personal fees) from Novartis, Celegene, and AOP Orphan. D.P.M. reports honoraria and speaker’s bureau for JAZZ; research funding and speaker’s bureau for Celgene/BMS; speaker’s bureau for AbbVie; and honoraria, research funding, and speaker’s bureau for Novartis. J.L. reports consultancy and honoraria from Kite; and other (travel and conference fees) from Takeda, Novartis and BMS. The remaining authors declare no competing interests.
Correspondence: Alexandros Rampotas, Paul O'Gorman Bldg, 72 Huntley St, London WC1E 6DD, United Kingdom; email: a.rampotas@nhs.net.
References
Author notes
All data are presented in the main article and in the supplemental Material. Further requests can be satisfied via email correspondence to the corresponding author (a.rampotas@nhs.net).
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal