We identified 7 new genetic regions for factor VIII levels, 1 for von Willebrand factor levels, and 3 in a combined analysis.
Silencing B3GNT2 and CD36 reduced factor VIII release in vitro. Silencing B3GNT2, CD36, and PDIA3 reduced von Willebrand factor release.
Visual Abstract
Coagulation factor VIII (FVIII) and its carrier protein von Willebrand factor (VWF) are critical to coagulation and platelet aggregation. We leveraged whole-genome sequence data from the Trans-Omics for Precision Medicine (TOPMed) program along with TOPMed-based imputation of genotypes in additional samples to identify genetic associations with circulating FVIII and VWF levels in a single-variant meta-analysis, including up to 45 289 participants. Gene-based aggregate tests were implemented in TOPMed. We identified 3 candidate causal genes and tested their functional effect on FVIII release from human liver endothelial cells (HLECs) and VWF release from human umbilical vein endothelial cells. Mendelian randomization was also performed to provide evidence for causal associations of FVIII and VWF with thrombotic outcomes. We identified associations (P < 5 × 10−9) at 7 new loci for FVIII (ST3GAL4, CLEC4M, B3GNT2, ASGR1, F12, KNG1, and TREM1/NCR2) and 1 for VWF (B3GNT2). VWF, ABO, and STAB2 were associated with FVIII and VWF in gene-based analyses. Multiphenotype analysis of FVIII and VWF identified another 3 new loci, including PDIA3. Silencing of B3GNT2 and the previously reported CD36 gene decreased release of FVIII by HLECs, whereas silencing of B3GNT2, CD36, and PDIA3 decreased release of VWF by HVECs. Mendelian randomization supports causal association of higher FVIII and VWF with increased risk of thrombotic outcomes. Seven new loci were identified for FVIII and 1 for VWF, with evidence supporting causal associations of FVIII and VWF with thrombotic outcomes. B3GNT2, CD36, and PDIA3 modulate the release of FVIII and/or VWF in vitro.
Introduction
Coagulation factor VIII (FVIII) and its carrier protein von Willebrand factor (VWF) are essential hemostasis proteins that play important roles in bleeding and thrombosis.1 FVIII markedly enhances FIX–catalyzed FX activation and ultimately fibrin clot formation, through amplification of the intrinsic coagulation pathway.2 VWF promotes thrombosis by mediating platelet adhesion and aggregation.1 VWF also binds to and stabilizes FVIII, transports FVIII in the blood, limits proteolysis of FVIII, and delivers FVIII to local sites of vascular injury,3 resulting in a high phenotypic and genetic correlation between plasma levels of these 2 factors.4,5
FVIII and VWF levels are quantitative traits with a substantial genetic component as estimates of their heritabilities range from 60% to 75%.6,7 Prior genome-wide association studies (GWAS) identified 22 loci associated with FVIII or VWF levels,8-11 of which 9 were identified for both FVIII and VWF levels. An additional 2 loci were identified through bivariate GWAS of FVIII and VWF levels.8 Mendelian randomization studies using genetic variants at these loci as instrumental variables provide evidence suggestive of a causal association of FVIII and/or VWF levels on venous thromboembolism (VTE), coronary artery disease (CAD), ischemic stroke, and peripheral artery disease (PAD).8,12,13
Whole-genome sequencing enables the simultaneous interrogation of rare and population-specific genetic variants that were poorly covered or absent in previous GWAS. Furthermore, using Trans-Omics for Precision Medicine (TOPMed) as a reference panel for genotype imputation considerably improves imputation quality for rare and population-specific variants, expanding coverage of many of these variants to studies that lack whole-genome sequencing data.14,15 To more comprehensively evaluate the genetic architecture of FVIII and VWF, we performed a genomic analysis of FVIII and VWF levels based on whole-genome sequencing data from the TOPMed program in combination with TOPMed-imputed genotype data from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium.16
Methods
Study population
The study population included 42 125 participants with measured FVIII levels and 45 289 participants with measured VWF levels. These participants represent 10 TOPMed studies (supplemental Table 1, available on the Blood website) and 20 CHARGE studies (supplemental Table 2).14,16 Several new studies were added compared with our previous GWAS of FVIII and VWF,8 including CaPS, FHS OMNI, Inter99, LBC1921, LBC1936, NEO, PREVEND, retrove, SAFS, and WHI. At the same time, several studies that contributed to the previous GWAS did not contribute to this study, including AGES, B58C, MEGA, and ORCADES. In TOPMed studies, participants were included if they had freeze 6 whole-genome sequencing data and measures of FVIII or VWF levels. CHARGE studies included participants with imputed genotype data and measures of FVIII or VWF levels. Because some studies were analyzed both as a part of TOPMed and a part of CHARGE, overlapping participants were removed from CHARGE analyses. Summaries of the study design of each of the included studies can be found in the supplemental Methods. The total study population across TOPMed and CHARGE was diverse, with reported membership of race or ethnicity population groups as follows: Asian (NFVIII = 609; NVWF = 210), Black (NFVIII = 7970; NVWF = 4445), Hispanic/Latino (NFVIII = 1311; NVWF = 656), White (NFVIII = 32 092; NVWF = 39 617), and other or unknown (NFVIII = 143; NVWF = 151). All studies were approved by their respective institutional review committees, and all included participants gave written informed consent.
Measurement of FVIII and VWF
Circulating FVIII activity or antigen levels and VWF antigen levels were measured in plasma of TOPMed and CHARGE participants and reported in international unit (IU) per dL. All studies except the Jackson Heart Study measured FVIII activity, whereas the Jackson Heart Study measured FVIII antigen. Further study-specific details on these measurements are provided in the supplemental Methods. Within each TOPMed study and each population group, FVIII and VWF levels were adjusted for age, sex, and study-specific covariates, and the residuals were rank normalized and rescaled by multiplying by the original standard deviation, so that the transformed phenotype data have the same variances as on the original scale. Each CHARGE study prepared harmonized phenotypes using a similar strategy as described above, with 2 key differences: (1) CHARGE studies stratified their phenotype harmonization by population group and (2) only CHARGE studies with family structure adjusted for the corresponding genetic relatedness matrix.
Whole-genome sequencing and genotyping
Whole-genome sequencing in TOPMed studies was conducted at a mean depth of >30× using Illumina HiSeq X Ten instruments at 6 sequencing centers. Variant and sample-level QC are described in the supplemental Methods.
Genetic data in CHARGE studies was based on genotyping arrays and imputation. The studies imputed genotypes using TOPMed Freeze 6 as the reference panel,14 except for the Rotterdam Study and Inter99, which were imputed to the Haplotype Reference Consortium (HRC) panel.17 Because TOPMed Freeze 6 used genome build 38, whereas the HRC panel used genome build 37, genomic positions from studies with HRC imputation were converted to build 38 using liftOver. EasyQC was used to harmonize data across studies and perform standard quality control.18 We calculated the effective minor allele count of each variant by multiplying the minor allele count by the imputation quality. Variants with imputation quality <0.3 or effective minor allele count ≤5 were excluded.
Genetic analyses
We used 2 approaches to analyze associations with genetic variants: single-variant analyses and gene-based aggregation analyses. Single-variant analyses were carried out across TOPMed and CHARGE, whereas gene-based aggregation analyses were restricted to the whole-genome sequencing data from TOPMed to ensure accurate coverage of rare variants.
Single-variant analysis
The transformed phenotype data from TOPMed were pooled together into a single analysis, using a heteroscedastic linear-mixed model allowing for different residual variances in each study/population group,19 adjusting for age, sex, study, population group, sequence center, and top 10 ancestry-informative principal components as fixed-effects covariates,20 and including a genetic relatedness matrix calculated by mixed-model analysis for pedigrees and populations to model the random effects for relatedness. Association analyses in TOPMed were performed on the Analysis Commons using variant set mixed model association tests (SMMAT) for all variants with MAC >5.21,22 In CHARGE, variants with MAC >5 were analyzed separately in each study, stratified by population group. METAL was used to perform inverse-variance weighted fixed-effects meta-analysis across summary statistics from the TOPMed analyses and each of the CHARGE studies.23 Genomic control corrections were applied to each set of summary statistics before meta-analysis. Variants with total minor allele count <20 in the meta-analysis were excluded. We used a genome-wide significance threshold of 5 × 10−9, as suggested for whole-genome sequencing data.24 Variants within 1 Mb of each index variants were considered to belong to the same locus.
To assess how significant loci affect FVIII and VWF levels differently, for each index variant we calculated the ratio between the β for FVIII and the β for VWF. We used the Genotype-Tissue Expression project version 8 to explore whether index variants at loci significantly associated with FVIII and/or VWF levels were associated with expression levels of genes across a wide range of tissues.25 Additional post-GWAS analyses based on single-variant summary statistics are described in the supplemental Methods, including multiphenotype analysis, conditional analysis, transcriptome-wide association analysis. The transcriptome-wide association analyses were limited to White participants, because these analyses require the use of a population-specific reference panel.
Gene-based aggregate analysis in TOPMed
We used gene-based aggregate analyses to increase the power to detect associations with low-frequency and rare variants. These analyses were also implemented using SMMAT on the Analysis Commons.21,22 We used 3 complementary strategies for variant selection: (1) loss-of-function variants of all frequencies, (2) loss-of-function and deleterious missense variants with minor allele frequency <5%, and (3) coding, enhancer, and promoter variants with minor allele frequency <5%. Further methods are provided in the supplemental Methods.
Evaluation of associations with VWF propeptide to VWF ratio
We examined whether variants associated with FVIII and/or VWF levels, including those from the multiphenotype analysis, were also associated with VWF propeptide to VWF ratio. This phenotype reflects clearance of VWF from the circulation,26 and was available in 3238 participants from 2 studies: Genes and Blood Clotting Study and Trinity Student Study. Results from these studies were meta-analyzed using METAL as described above.23 The significance threshold was adjusted using the Bonferroni correction, dividing .05 by the number of tested genetic variants.
Mendelian randomization
We performed 2-sample summary statistics-based Mendelian randomization to assess the association of genetically determined levels of FVIII and VWF with the risk of several thrombotic outcomes, including VTE, CAD, myocardial infarction, PAD, ischemic stroke, and ischemic stroke subtypes (large artery stroke, cardioembolic stroke, and small vessel stroke). Summary statistics for these outcomes were obtained from large-scale published GWAS.27-29 Mendelian randomization analyses are described in the supplemental Methods.
Functional validation of identified loci
We previously used human umbilical vein endothelial cells (HUVECs) as gene silencing models for FVIII and VWF.8 Although these cells released abundant VWF into the media, not enough FVIII was released to be detected in vitro.8 Thus, we identified and used human liver endothelial cells (HLECs, also known as liver sinusoidal endothelial cells) as an alternative cell model to study release of FVIII. Methods for the gene silencing experiments in primary HUVECs and HLECs are provided in the supplemental Methods.
Results
Baseline characteristics
Baseline characteristics of the study participants are shown in supplemental Table 1 for TOPMed studies and supplemental Table 2 for CHARGE studies. The mean age ranged from 21.2 to 75.4 years across the included studies. In total, 54.7% of participants were female for FVIII and 46.1% were female for VWF. Mean FVIII levels ranged from 68.2 to 231.4 IU/dL across the studies, whereas mean VWF antigen levels ranged from 57.4 to 200.7 IU/dL.
Single-variant analysis
A total of 34 234 667 genetic variants were included in the cross-population single-variant analyses for FVIII, and 35 749 360 were included for VWF. Quantile-quantile plots are shown in supplemental Figure 1. The genomic inflation factors that were adjusted for were <1.1 for all contributing studies (supplemental Table 3). The genomic inflation factors of the cross-population meta-analyses were 1.07 for FVIII and 1.02 for VWF. A total of 2543 variants at 17 loci were significantly associated with FVIII (supplemental Table 4), whereas 2824 variants at 15 loci were significantly associated with VWF (supplemental Table 5). Of these loci, 11 were genome-wide significant (P < 5 × 10−9) for both FVIII and VWF. Manhattan plots are shown in supplemental Figure 2, and regional plots are shown in supplemental Figures 3 and 4. Among the 17 significant FVIII loci, 11 were reported by previous GWAS,8,11 whereas 6 were newly identified associated loci, including: ST3GAL4, CLEC4M, B3GNT2, ASGR1, F12, and KNG1 (Table 1). KNG1 has previously been reported in a multiphenotype GWAS of FVIII and VTE,5 but not in a GWAS of FVIII alone. All 15 loci associated with VWF have been reported by previous GWAS. Analyses restricted to White participants identified an additional association for FVIII at the TREM1/NCR2 locus that had not been identified in previous studies and an additional association for VWF at B3GNT2 (Table 1), whereas analyses restricted to Black participants did not identify additional new loci (supplemental Table 6). Conditional analysis, based on summary statistics using GCTA, indicates that there are multiple independently associated variants at ABO, VWF, STAB2, SCARA5, and F8 (supplemental Table 7). The independently associated variants identified by GCTA across all significant loci explained 19.6% of the variance in FVIII in White participants and 24.8% in Black participants, as well as 19.8% of the variance in VWF in White participants and 28.9% in Black participants.
Index variants at novel loci discovered in the cross-population, population-specific, and multiphenotype analyses
| rsID . | Chr:pos . | Gene . | Alleles . | FVIII levels . | VWF levels . | Multiphenotype . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freq, % . | Beta . | SE . | P value . | Freq, % . | Beta . | SE . | P value . | P value . | ||||
| FVIII levels (cross-population) | ||||||||||||
| rs75077631 | 5:177413083 | GRK6/F12 | G/GC | 37.2 | −2.56 | 0.36 | 1.8E−12 | 31.2 | −0.44 | 0.40 | 2.8E−01 | 6.0E−13 |
| rs62061426 | 17:7170806 | ASGR1 | A/G | 18.8 | 2.42 | 0.38 | 1.3E−10 | 19.0 | 1.37 | 0.41 | 7.9E−04 | 1.4E−09 |
| rs710446 | 3:186742138 | KNG1 | T/C | 57.0 | −1.85 | 0.29 | 1.4E−10 | 57.7 | −0.55 | 0.32 | 8.1E−02 | 9.1E−10 |
| rs6727115 | 2:62389406 | B3GNT2 | T/C | 76.4 | −2.13 | 0.35 | 1.3E−09 | 78.0 | −2.00 | 0.39 | 2.4E−07 | 6.9E−11 |
| rs35257264 | 11:126426921 | ST3GAL4/KIRREL3 | T/C | 2.2 | 7.45 | 0.98 | 2.5E−14 | 2.4 | 6.67 | 1.07 | 3.7E−10 | 8.1E−16 |
| rs868875 | 19:7766280 | CLEC4M | A/G | 72.0 | 2.10 | 0.32 | 6.0E−11 | 70.9 | 2.36 | 0.35 | 1.7E−11 | 2.3E−15 |
| FVIII levels (White participants) | ||||||||||||
| rs35206772 | 6:41325017 | TREM1/NCR2 | A/G | 67.6 | −2.02 | 0.33 | 9.3E−10 | 67.8 | −0.72 | 0.43 | 9.0E−02 | 2.3E−07 |
| VWF levels (White participants) | ||||||||||||
| rs61558368 | 2:62414160 | B3GNT2 | T/C | 34.9 | 1.24 | 0.33 | 1.9E−04 | 35.6 | 2.09 | 0.35 | 2.0E−09 | 1.2E−08 |
| Multiphenotype analysis of FVIII levels and VWF levels (cross-population) | ||||||||||||
| rs12979891 | 19:48723999 | RASIP1/FUT1/FUT2 | T/C | 44.2 | 1.37 | 0.30 | 3.9E−06 | 49.0 | 1.83 | 0.32 | 1.8E−08 | 2.6E−09 |
| rs145633869 | 15:43719006 | CKMT1A/PDIA3 | CAGAG/C | 98.3 | 6.55 | 1.22 | 8.0E−08 | 98.1 | 6.15 | 1.26 | 1.1E−06 | 4.1E−09 |
| rs184336448 | 2:198846960 | PLCL1/SATB2 | A/T | 0.43 | 11.01 | 2.53 | 1.3E−05 | 0.53 | 14.61 | 2.59 | 1.6E−08 | 4.4E−09 |
| rsID . | Chr:pos . | Gene . | Alleles . | FVIII levels . | VWF levels . | Multiphenotype . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freq, % . | Beta . | SE . | P value . | Freq, % . | Beta . | SE . | P value . | P value . | ||||
| FVIII levels (cross-population) | ||||||||||||
| rs75077631 | 5:177413083 | GRK6/F12 | G/GC | 37.2 | −2.56 | 0.36 | 1.8E−12 | 31.2 | −0.44 | 0.40 | 2.8E−01 | 6.0E−13 |
| rs62061426 | 17:7170806 | ASGR1 | A/G | 18.8 | 2.42 | 0.38 | 1.3E−10 | 19.0 | 1.37 | 0.41 | 7.9E−04 | 1.4E−09 |
| rs710446 | 3:186742138 | KNG1 | T/C | 57.0 | −1.85 | 0.29 | 1.4E−10 | 57.7 | −0.55 | 0.32 | 8.1E−02 | 9.1E−10 |
| rs6727115 | 2:62389406 | B3GNT2 | T/C | 76.4 | −2.13 | 0.35 | 1.3E−09 | 78.0 | −2.00 | 0.39 | 2.4E−07 | 6.9E−11 |
| rs35257264 | 11:126426921 | ST3GAL4/KIRREL3 | T/C | 2.2 | 7.45 | 0.98 | 2.5E−14 | 2.4 | 6.67 | 1.07 | 3.7E−10 | 8.1E−16 |
| rs868875 | 19:7766280 | CLEC4M | A/G | 72.0 | 2.10 | 0.32 | 6.0E−11 | 70.9 | 2.36 | 0.35 | 1.7E−11 | 2.3E−15 |
| FVIII levels (White participants) | ||||||||||||
| rs35206772 | 6:41325017 | TREM1/NCR2 | A/G | 67.6 | −2.02 | 0.33 | 9.3E−10 | 67.8 | −0.72 | 0.43 | 9.0E−02 | 2.3E−07 |
| VWF levels (White participants) | ||||||||||||
| rs61558368 | 2:62414160 | B3GNT2 | T/C | 34.9 | 1.24 | 0.33 | 1.9E−04 | 35.6 | 2.09 | 0.35 | 2.0E−09 | 1.2E−08 |
| Multiphenotype analysis of FVIII levels and VWF levels (cross-population) | ||||||||||||
| rs12979891 | 19:48723999 | RASIP1/FUT1/FUT2 | T/C | 44.2 | 1.37 | 0.30 | 3.9E−06 | 49.0 | 1.83 | 0.32 | 1.8E−08 | 2.6E−09 |
| rs145633869 | 15:43719006 | CKMT1A/PDIA3 | CAGAG/C | 98.3 | 6.55 | 1.22 | 8.0E−08 | 98.1 | 6.15 | 1.26 | 1.1E−06 | 4.1E−09 |
| rs184336448 | 2:198846960 | PLCL1/SATB2 | A/T | 0.43 | 11.01 | 2.53 | 1.3E−05 | 0.53 | 14.61 | 2.59 | 1.6E−08 | 4.4E−09 |
Gene shows the closest genes at each locus; alleles show the coded/noncoded alleles; frequency (Freq.) and beta apply to the coded alleles. SE refers to the standard error of the beta.
A previous study found that each 1% increase in VWF levels results in a 0.54% increase in FVIII activity.30 For each of the index variants at the FVIII-associated loci we calculated the ratio between the β for FVIII levels and the β for VWF levels (supplemental Table 4). Except for the F8 locus (β ratio = −1.5), all values for this ratio were positive and ranged from 0.62 to 5.87, indicating that the index variants at most significant loci share the same effect direction for FVIII as VWF, and that many loci have direct effects on both FVIII and VWF as opposed to only affecting FVIII indirectly through VWF. Index variants at ABO, SCARA5, and VWF were also associated with VWF propeptide to VWF ratio (P < 8.5 × 10−4, adjusting for 59 tested variants), suggesting that the mechanism at these loci may involve clearance of VWF (supplemental Table 8). Association of the index variants with gene expression across tissues and cell types are shown in supplemental Table 9.
Multiphenotype analysis
Multiphenotype analysis of FVIII and VWF levels using metaUSAT identified an additional 2 previously reported loci and 3 novel loci: RASIP1/FUT1/FUT2, CKMT1A/PDIA3, and PLCL1/SATB2 (supplemental Table 10). All 3 of these new loci had similar effect sizes for FVIII and VWF and were suggestively associated with both phenotypes (Table 1).
Gene-based rare variant analysis
Across the 3 aggregation strategies and 2 phenotypes, 178 012 gene-based aggregation units were analyzed using 2 statistical tests, resulting in a P value threshold of .05/(2 × 178 012) = 1.4 × 10−7. As shown in Table 2, gene-based rare variant analyses identified associations of FVIII and VWF levels with genes at 3 loci (ABO, STAB2, and VWF). Results for aggregate tests involving genes annotated to single-variant associations are shown in supplemental Table 11. Supplemental Figures 5 and 6 respectively show leave-one-out analyses for gene-based aggregation units associated with FVIII and VWF, to identify the individual rare variants driving the gene-based associations. Some aggregation units appear to be driven by a subset of ≥1 driver variants, for example, the aggregate test that included loss-of-function and deleterious missense VWF variants with FVIII and VWF levels is markedly attenuated when excluding rs1800386 (12:6018667:T:C). This variant was also independently associated with FVIII and VWF in the conditional analysis with GCTA (supplemental Table 7). In STAB2, rs149382223 (12:103695776:T:C) appears to be an important driving variant for both FVIII and VWF.
Significantly associated gene-based aggregation units for circulating FVIII and VWF levels based on whole-genome sequencing data from the TOPMed program
| Aggregation strategy . | Gene . | nVar . | MAC . | Pburden . | PSMMAT . |
|---|---|---|---|---|---|
| FVIII levels | |||||
| Coding, promoter, and enhancer variants | STAB2 | 61 | 315 | 2.71E−06 | 2.85E−10 |
| LOF and deleterious missense variants | STAB2 | 158 | 611 | 1.97E−16 | 4.93E−19 |
| LOF and deleterious missense variants | VWF | 86 | 353 | 8.62E−18 | 1.23E−18 |
| LOF variants | STAB2 | 53 | 297 | 2.44E−06 | 1.92E−11 |
| LOF variants | ABO | 17 | 16763 | 3.36E−11 | 7.29E−11 |
| VWF levels | |||||
| Coding, promoter, and enhancer variants | STAB2 | 36 | 123 | 3.64E−08 | 1.08E−08 |
| LOF and deleterious missense variants | VWF | 76 | 222 | 3.34E−15 | 8.83E−16 |
| LOF and deleterious missense variants | STAB2 | 98 | 307 | 9.50E−15 | 8.76E−15 |
| LOF variants | VWF | 22 | 44 | 5.30E−07 | 5.30E−10 |
| LOF variants | STAB2 | 29 | 114 | 7.64E−09 | 6.14E−10 |
| LOF variants | ABO | 15 | 12438 | 2.67E−08 | 9.39E−08 |
| Aggregation strategy . | Gene . | nVar . | MAC . | Pburden . | PSMMAT . |
|---|---|---|---|---|---|
| FVIII levels | |||||
| Coding, promoter, and enhancer variants | STAB2 | 61 | 315 | 2.71E−06 | 2.85E−10 |
| LOF and deleterious missense variants | STAB2 | 158 | 611 | 1.97E−16 | 4.93E−19 |
| LOF and deleterious missense variants | VWF | 86 | 353 | 8.62E−18 | 1.23E−18 |
| LOF variants | STAB2 | 53 | 297 | 2.44E−06 | 1.92E−11 |
| LOF variants | ABO | 17 | 16763 | 3.36E−11 | 7.29E−11 |
| VWF levels | |||||
| Coding, promoter, and enhancer variants | STAB2 | 36 | 123 | 3.64E−08 | 1.08E−08 |
| LOF and deleterious missense variants | VWF | 76 | 222 | 3.34E−15 | 8.83E−16 |
| LOF and deleterious missense variants | STAB2 | 98 | 307 | 9.50E−15 | 8.76E−15 |
| LOF variants | VWF | 22 | 44 | 5.30E−07 | 5.30E−10 |
| LOF variants | STAB2 | 29 | 114 | 7.64E−09 | 6.14E−10 |
| LOF variants | ABO | 15 | 12438 | 2.67E−08 | 9.39E−08 |
LOF, loss-of-function; MAC, cumulative minor allele count of the included variants; nVar, number of genetic variants included in the aggregation test.
Transcriptome-wide association study
A total of 72 086 transcript-tissue pairs across 4 tissues were included in transcriptome-wide association analyses for FVIII and VWF levels, which aggregates results across tissues. After Bonferroni correction (P value threshold = .05/72 086 = 6.94 × 10−7), genetically determined expression of 83 transcript-tissue pairs was significantly associated with FVIII, involving 27 unique genes at 10 loci (supplemental Table 13). Among these 10 loci, 6 were not reported in the single-variant analysis, including ADSSL1, GALC, ZNF337, SHANK3, PDHB, and HLA-C. Similarly, 109 transcript-tissue pairs were associated with VWF, involving 36 unique genes in 13 loci, all of which were reported in the single-variant analysis (supplemental Table 13). Genes that were prioritized using FOCUS for fine mapping are shown in supplemental Table 14.
Mendelian randomization
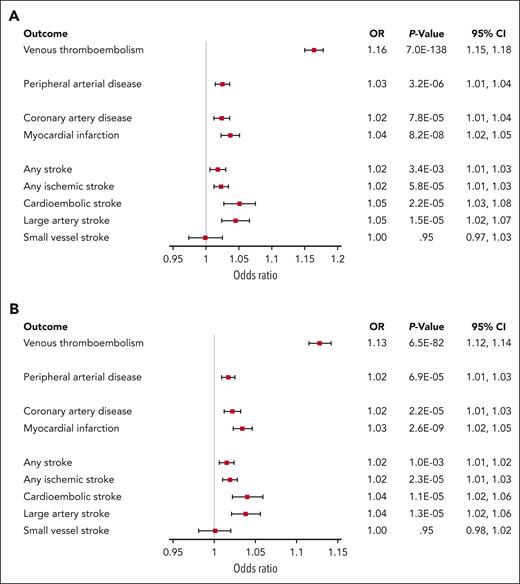
Up to 22 genetic instruments were used for FVIII levels and up to 19 were used for VWF levels. Forest plots that list the included genetic instruments and show each of their respective causal association estimates are provided in supplemental Figures 7 and 8. When causal estimates were meta-analyzed across these genetic instruments, genetically determined circulating FVIII and VWF levels were associated with increased risk of VTE, PAD, CAD, myocardial infarction, ischemic stroke, cardioembolic stroke, large artery stroke, but no significant association was found with small vessel stroke (Figure 1). These associations were generally consistent across sensitivity analyses (supplemental Figure 9), including the exclusion of genetic instruments pertaining to the ABO locus. When the ABO variant was excluded from the analyses, confidence intervals widened for all outcomes.
Results of the inverse-variance weighted Mendelian randomization analyses of FVIII and VWF levels on a range of thrombotic outcomes. Odds ratios are shown per 10 IU/dL change in FVIII and VWF levels. (A) FVIII levels. (B) VWF levels.
Results of the inverse-variance weighted Mendelian randomization analyses of FVIII and VWF levels on a range of thrombotic outcomes. Odds ratios are shown per 10 IU/dL change in FVIII and VWF levels. (A) FVIII levels. (B) VWF levels.
Functional validation of newly identified loci
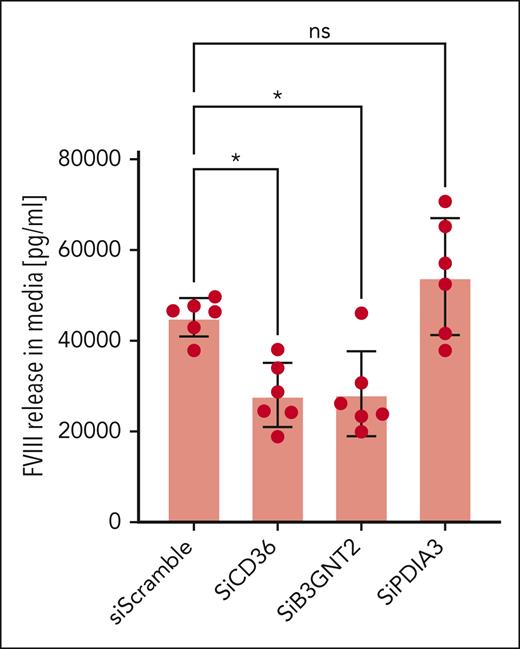
FVIII messenger RNA expression was not detected in HUVECs, but it was detected in the HLECs (supplemental Figure 10A). We tested CD36, B3GNT2, and PDIA3, three genes that (1) were expressed in HLECs (supplemental Figure 10B-D), (2) had not been reported in association with FVIII or VWF levels at the time of these experiments, and (3) did not clearly relate to a known biological mechanism. An association of CD36 with FVIII was reported by Pankratz et al after the experiments had been conducted.11 The 3 genes were successfully silenced in HLECs (supplemental Figure 11). Silencing of CD36 and B3GNT2 decreased FVIII release into the media, whereas silencing of PDIA3 did not (Figure 2).
Candidate genes identified by GWAS regulate FVIII release from HLECs. HLECs were transfected with small interfering RNA (siRNA), including control siRNA (Scramble Ct), siRNA directed against CD36 (siCD36), siRNA directed against B3GNT2 (siB3GNT2), and siRNA directed against PDIA3 (siPDIA3). The cells were washed and fed with fresh media, cultured for 24 hours, and the media was collected. FVIII concentrations in the cell culture media were measured by enzyme-link immunosorbent assay (n = 6 ± SD; ∗P < .05 compared with Scramble Ct). SD, standard deviation.
Candidate genes identified by GWAS regulate FVIII release from HLECs. HLECs were transfected with small interfering RNA (siRNA), including control siRNA (Scramble Ct), siRNA directed against CD36 (siCD36), siRNA directed against B3GNT2 (siB3GNT2), and siRNA directed against PDIA3 (siPDIA3). The cells were washed and fed with fresh media, cultured for 24 hours, and the media was collected. FVIII concentrations in the cell culture media were measured by enzyme-link immunosorbent assay (n = 6 ± SD; ∗P < .05 compared with Scramble Ct). SD, standard deviation.
We then tested the effect of CD36, B3GNT2, and PDIA3 silencing in HUVECs on VWF release into the media, with and without stimulation with histamine. Silencing of the 3 genes in HUVECs was successful (supplemental Figure 12). Silencing of CD36 only decreased VWF levels in media under basal conditions, but not after histamine stimulation (supplemental Figure 13A). Silencing of B3GNT2 decreased VWF release with and without histamine stimulation (supplemental Figure 13B). The direction of the effects of CD36 and B3GNT2 silencing in HUVECs on VWF release were thus consistent with the direction of the effects in HLECs on FVIII release. In contrast, although PDIA3 silencing in HLECs did not significantly affect FVIII release, PDIA3 silencing in HUVECs decreased VWF release (supplemental Figure 13C).
Discussion
We used whole-genome sequencing data from the TOPMed program, along with TOPMed-imputed genotype data from the CHARGE consortium, to perform genetic association studies of FVIII and VWF levels. We identified 7 novel and 11 known loci for FVIII levels, 1 novel and 15 known loci for VWF levels, and 3 novel and 2 known loci in a multiphenotype analysis of FVIII and VWF levels. Of the loci discovered for FVIII and VWF, 11 overlapped at genome-wide significance in the cross-population meta-analysis. All VWF loci were associated with FVIII levels at nominal significance (P < .05), however 3 FVIII loci were not associated with VWF (P > .05), including F12, RP1, and KNG1.
B3GNT2 is a newly identified locus for FVIII and VWF levels. It encodes β-1,3-N-acetylglucosaminyltransferase 2, which is involved in the synthesis of N-acetyllactosamine chains. Specifically, B3GNT2 catalyzes the addition of N-acetylglucosamine to N-acetyllactosamine.31 Variants at this locus were associated with the levels of both FVIII and VWF. FVIII and VWF are both heavily glycosylated, and there is evidence that VWF carries N-acetyllactosamine chains.32 However, it is unclear from these associations whether only VWF or both FVIII and VWF are specific substrates of B3GNT2. Our gene silencing experiments show that B3GNT2 silencing in HLECs decreases the release of FVIII, and B3GNT2 silencing in HUVECs decreases the release of VWF. These results suggest that both FVIII and VWF are glycosylated by B3GNT2 and that this glycosylation promotes the production and/or secretion of these proteins, and/or decreases the clearance of these proteins from the medium.
PDIA3 was identified as a novel locus in the multiphenotype analysis, with the same effect direction for FVIII and VWF. We identified PDIA3 as a candidate causal gene at this locus because it regulates the folding of glycoproteins by catalyzing the formation of disulfide bonds in the endoplasmic reticulum,33 and both FVIII and VWF rely on multiple disulfide bonds established in the endoplasmic reticulum to dimerize.34,35 To test whether PDIA3 regulates FVIII, VWF, or both, we performed silencing of PDIA3 in HLECs and HUVECs. We did not find evidence of an effect of PDIA3 silencing on FVIII release from HLECs, but we did identify a decrease in VWF release from HUVECs. This is consistent with a previous report that indicated that VWF dimerization is likely to be performed by either PDI or PDIA3.36
We identified several loci for FVIII levels that have not been reported in previous GWAS and relate to mechanisms that are known to interact with or regulate FVIII. ASGR1 encodes the Ashwell receptor, which is involved in catabolism of circulating glycoproteins, including VWF and FVIII.37,38F12 and KNG1 were associated only with FVIII levels and respectively encode FXII and high molecular weight kininogen, 2 contact pathway proteins that activate coagulation via the intrinsic pathway. They may involve differences in the rate of contact factor activation and hence on the time for initial fibrin clot formation via the intrinsic and common coagulation pathways. Indeed, variants in F12 and KNG1 respectively explain 12% and 6% of the variance in activated partial thromboplastin time,39 a global test of coagulation potential of the intrinsic and common pathways. Variants in F12 and KNG1 are the most significant genetic contributors to this commonly used hemostatic measure. Importantly, FVIII activity is measured using an activated partial thromboplastin time-based clotting time assay. It is therefore unclear whether the identified variants in F12 and KNG1 affect circulating FVIII levels in vivo or they only influence the laboratory measurement of FVIII levels.
In the multiphenotype analysis of FVIII and VWF we identified an association at the locus harboring the FUT1 and FUT2 genes. FUT1 encodes the H antigen that is expressed in individuals with ABO blood group O and upon which the A and/or B antigens are attached in those with blood groups A, B, or AB. ABO blood group is the most important genetic determinant of FVIII and VWF levels, explaining as much as 13% to 16% of their variance.8FUT2 encodes a protein that is required for the secretion of A, B, and H antigens into plasma. Secretor status based on FUT2 alleles has been associated with VWF levels,40 and rare variants in FUT1 and FUT2 have been linked to markedly lower VWF levels.41
Our study also provides new insights into the CD36 locus, for which we recently reported an association with FVIII levels in an exome-wide association study.11 Although loss-of-function variant rs3211938 in CD36 reached genome-wide significance for FVIII levels, it was also suggestively associated with VWF levels (P = .0037). The associated loss-of-function variant has also been reported to be under selective pressure and associated with multiple hematological and cardiometabolic traits.42-47 This variant is largely restricted to populations with African ancestry, and our study population included considerably more Black participants for FVIII (N = 7448) than for VWF (N = 4445). It is thus possible that CD36 is associated with both FVIII and VWF, but that there was less power to detect this association for VWF. The CD36 receptor is a glycoprotein on the surface of endothelial cells, platelets, and monocytes. Although there are no prior reports that CD36 regulates FVIII or VWF levels, our gene knockdown experiments show that silencing CD36 directly decreased FVIII levels in HLECs and VWF levels in HUVECs. This is consistent with evidence from our GWAS in which the loss-of-function allele for rs3211938 was associated with lower FVIII levels. It remains unclear how FVIII and VWF are regulated by CD36, and whether this involves an effect of CD36 on the synthesis, secretion, or clearance of these proteins.
Several of the new loci identified for FVIII levels have been previously described in association with VWF levels, such as CLEC4M, ST3GAL4, and PDHB.8,9 CLEC4M appears to affect FVIII not only indirectly through clearance of VWF,48 but also directly by interacting with mannose-exposed glycans on FVIII to promote its clearance, both in the presence of absence of VWF.49 Although ST3GAL4 has not been reported in association with FVIII levels by any previous GWAS, it was associated with FVIII in a candidate gene study.50 In contrast to CLEC4M, ST3GAL4 appears to prevent clearance of VWF.51 Whether or not ST3GAL4 also targets FVIII directly has not been studied. The mechanism linking PDHB to VWF and FVIII is unclear, but we previously showed that PDHB silencing in HUVECs increased VWF release into media.8
A potential benefit of using whole-genome sequencing data is the ability to study rare variants. In our study, we identified several gene-based associations that included rare variants, but all of these mapped to loci that were also found by previous studies as well as in our single-variant analysis, including ABO, VWF, and STAB2.10,11 In our conditional analysis we identified an association of VWF with rs1800386 (Tyr1584Cys), a rare variant of uncertain clinical significance that may contribute to von Willebrand disease.52 All in all, our results highlight the ability of whole-genome sequencing to provide insights into the role of rare variants, but at the same time suggest that these insights may largely be limited to loci already known to harbor associations with common variants.
Mendelian randomization analyses using improved genetic instruments as a result of the genetic discovery in this study confirm previous reports that genetically determined FVIII and VWF levels are associated with the risk of a range of thrombotic outcomes.8,13 However, because of the extensive genetic overlap between FVIII and VWF, it is unclear whether the effector underlying these associations is FVIII or VWF, or both. It may be possible to determine the causal relationships by relying on FVIII-specific and/or VWF-specific loci. The identification of several FVIII-specific loci in this study is therefore important, but additional FVIII-specific and/or VWF-specific loci will need to be identified in order to distinguish between the effect of FVIII and VWF in future Mendelian randomization studies.
Our study had several limitations. First, although our study represents the most diverse genomic analysis of FVIII and VWF levels to date, many population groups remain underrepresented. Second, because our aggregated rare variant analyses were restricted to only participants with whole-genome sequencing data, the sample size, and power for these analyses was lower than for the single-variant analysis. Third, although sample overlap between each of the exposure and outcome GWAS used in the Mendelian randomization analyses was low, we cannot rule out that sample overlap has influenced the results of the Mendelian randomization analysis.53 Fourth, because of the modest effect size of the genetic variants used as instruments in the Mendelian randomization analyses, it is not clear whether the effect sizes of FVIII and VWF on thrombotic outcomes can be extrapolated to the full range of FVIII and VWF values, including extremely low or high values.
In conclusion, we identified 7 novel loci associated with FVIII levels, 1 with VWF levels, and 3 with both FVIII and VWF in multiphenotype analysis. For the newly identified B3GNT2 locus, as well as the recently reported CD36 locus, we show that short interfering RNA–mediated gene silencing in HLECs led to reduced levels of FVIII. Many of the newly identified variants are located in or near plausible candidate causal genes, some of which relate to known mechanisms, while others, such as B3GNT2, may represent new mechanisms that should be explored further by in vitro and in vivo studies. Our findings have several translational implications. The novel loci we identified can contribute to more accurate genetic risk scores that may be used to predict risk of bleeding or thrombosis. Additionally, these newly identified loci may represent biological pathways that improve our understanding of new potential therapeutic targets. Finally, because some of the variants are associated with FVIII levels, but not VWF levels, our study emphasizes that VWF stability is not the only determinant of FVIII levels.
Acknowledgments
The authors thank the CARDIoGRAMplusC4D and MEGASTROKE consortium investigators for making their summary statistics publicly available, as well as the MVP study investigators for making their summary statistics available through dbGaP (database of Genotypes and Phenotypes). The authors also acknowledge the participants and staff of the contributing studies.
This work and the CHARGE-TOPMed (Cohorts for Heart and Aging Research in Genomic Epidemiology-Trans-Omics for Precision Medicine) Hemostasis Working Group was funded in part by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) grant numbers R01 HL134894 and R01 HL139553. Infrastructure for the CHARGE consortium was supported by NIH/NHLBI grant number R01HL105756. The Analysis Commons was funded by NIH/NHBLI grant number R01 HL131136. PR was supported by the American Heart Association and the DC Women's Board (23POST1021300). C.J.L. was supported by NIH/NHLBI grant number R33 HL141791 and the Michel Mirowski M.D. Professorship in Cardiology. C.H. and J.F.W. were supported by the UK Medical Research Council grant U. MC_UU_00007/10. G.T.-S. is supported by the Pla Estratègic de Recerca i Innovació en Salut grant from the Catalan Department of Health (SLT017/20/000100). M.S.-L is supported by a Miguel Servet contract from the Instituto de Salud Carlos III Spanish Health Institute (CPII22/00007) and cofinanced by the European Social Fund. L.M.R. was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant KL2TR002490. Molecular data for the TOPMed program was supported by the NIH/NHLBI. Core support including centralized genomic read mapping and genotype calling, along with variant quality metrics and filtering were provided by the TOPMed Informatics Research Center (3R01HL-117626-02S1; contract HHSN268201800002I). Core support including phenotype harmonization, data management, sample-identity QC, and general program coordination were provided by the TOPMed Data Coordinating Center (R01HL-120393; U01HL-120393; contract HHSN268201800001I). The study-specific omics support information is provided in the TOPMed Omics Support Table (supplemental Table 15). Further study-specific funding and acknowledgments are detailed in the supplemental Methods.
The views expressed in this article are those of the authors and do not necessarily represent the views of the NHLBI, the National Institutes of Health, or the US Department of Health and Human Services.
Authorship
Contribution: A.C.M., A.P.R., A.L., A. Beswick, A.D.J., A.v.H.V., B.D.M., B.M.P., C.H., D.J.S., D.-A.T., D.O.M.-K., F.R.R., H.C., I.R., J.W.J., J.F.W., J.-F.D., J.I.R., J.E.C., J.B., J.M.S., J.P.L., J.C.S., K.C.D., K.D.T., L.M.R., L.A., L. C. Becker, L.A.L., L. C. Brody, L.E., M.F.D., M.A.I., M.-H.C., M.F., N.F., N.L.S., O.P., P.-E.M., R.A.M., S.R.C., S.S.R., T.E.H., W.M., and Y.B.-S. contributed to the design and/or funding of the included studies from the TOPMed program and/or CHARGE consortium; A.C.M., A.P.R., A.L., A. Beswick, A.D.J., A.M.-P., A.v.H.V., A.B.O., B.A.T.R., B.D.M., B.M.P., C.H., D.J.S., D.-A.T., D.J., D.O.M.-K., F.v.R., F.R.R., G.D., G.D.C., I.R., I.K., J.W.J., J.F.W., J.-F.D., J.R.O., J.E.H., J.E.C., J.B., J.P.L., J.C.S., K.C.D., K.A.R., K.D.T., L.A., L. C. Brody, L.R.Y., M.E.K., M.S.-L., M.R.B., M.-H.C., M.P.M.d.M., M.F., N.P., N.F., N.-Q.L., O.P., P.S.d.V., P.S., P.-E.M., P.v.d.H., R.A.M., R.L.G., S.E.H., S.R.C., S.T., T.H.E., T.H., T.O.K., and Y.B.-S. contributed to the acquisition of genotype and/or phenotype data; A.S.H., A.C.M., A. Bebo, A.D.J., A.M.-P., A.P.M., A.B.O., B.A.T.R., B.D.M., C.H., D.-A.T., D.J., F.T., G.D., G.T.S., G.D.C., G.E.D., J.F.D., J.R.O.C., J.E.H., J.A.B., J.Y., J.P.L., K.C.D., K.A.R., L.M.R., L.R.Y., L.K., M.E.K., M.S.-L., M.R.B., M.-H.C., M.G., N.-Q.L., N.L.S., P.S.d.V., P.K.J., R.A.M., R.N., R.E.M., R.L.G., S.T., T.M.B., and X.G. contributed to the genetic epidemiology analyses and interpretation; C.J.L., M.A., P.R., and W.O.O. contributed to the design of the gene silencing experiments; P.R. performed the gene silencing experiments; P.R. and C.J.L. contributed to data analysis and interpretation of the gene silencing experiments; A.S.H., A.C.M., C.J.L., M.R.B., M.S.-L., N.L.S., P.R., and P.S.d.V. drafted the initial manuscript; and all coauthors participated in critical review of the manuscript.
Conflict-of-interest disclosure: C.J.L. received funding in 2020 from Novartis for a research grant. B.M.P. serves on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. L.M.R. is a consultant for the TOPMed Administrative Coordinating Center through WeStat. R.L.-G. is a part-time consultant for Metabolon, Inc. The remaining authors declare no competing financial interests.
Complete lists of the members of TOPMed and INVENT appear in the supplemental Appendix.
Correspondence: Paul S. de Vries, Department of Epidemiology, Human Genetics, and Environmental Sciences, Human Genetics Center, School of Public Health, The University of Texas Health Science Center at Houston, 1200 Pressler St, Ste E-429, Houston, TX 77030; email: paul.s.devries@uth.tmc.edu; and Alanna C. Morrison, Department of Epidemiology, Human Genetics, and Environmental Sciences, Human Genetics Center, School of Public Health, The University of Texas Health Science Center at Houston, 1200 Pressler St, Ste E-429, Houston, TX 77030; email: alanna.c.morrison@uth.tmc.edu.
References
Author notes
P.S.d.V. and P.R. contributed equally to this study as junior authors.
M.S.-L., C.J.L., N.L.S., and A.C.M contributed equally to this work as senior authors.
Whole-genome sequencing data from the Trans-Omics for Precision Medicine program are available on dbGap: the study-specific accession numbers are listed in supplemental Table 14. Summary statistics from genome-wide association analyses will be made available on dbGaP, accession number phs001974.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal