Through a large meta-analysis of factor VIII (FVIII) and von Willebrand factor (VWF) levels and selective follow-up in vitro functionalization, de Vries et al identified 7 novel genetic regions associated with FVIII levels, 1 region associated with VWF levels, and 3 regions associated with both FVIII and VWF levels, as described in this issue of Blood.1
The search for genetic regulators of thrombosis and hemostasis has been an ongoing monumental task in the field of hematology, made difficult by the sheer complexity of the underlying biochemical mechanisms of coagulation and bleeding phenotypes and their interactions with other genetic loci and environmental factors. As technology has advanced our capacity for data collection, the need for subsequent analysis of this vast amount of data has further hindered rather than accelerated our understanding of these genetic regulators. As a result, much of this information remains unexplored.
Many studies have understandably focused on the analysis of patients with specific conditions or on highly penetrant mutations, which necessarily limits the sample size.2 Here, by using circulating coagulation factor levels as the phenotype, the authors were able to take a more agnostic approach and utilize large databases with many clinical phenotypes available.
Circulating FVIII and VWF levels have been previously shown to be correlated.3 Both FVIII and VWF proteins are capable of promoting thrombosis at high levels and bleeding at lower levels.4 Thus, focusing on these proteins as linked quantitative traits that affect complex thrombotic phenotypes makes biological sense. Therefore, the authors performed not only single-variant analyses on VWF, FVIII, and the ratio between the β for FVIII and the β for VWF, but also more sophisticated and modern genomic analyses such as multiphenotype analyses, gene-burden analyses, and Mendelian randomization. Supporting the biochemical linkage of these 2 proteins, 11 genetic loci surpassed genome-wide significance for both VWF and FVIII levels individually. Mendelian randomization replicated previous observations that genetically determined FVIII and VWF levels are associated with thrombotic outcomes including venous thromboembolism, coronary artery disease, ischemic stroke, and peripheral artery disease.
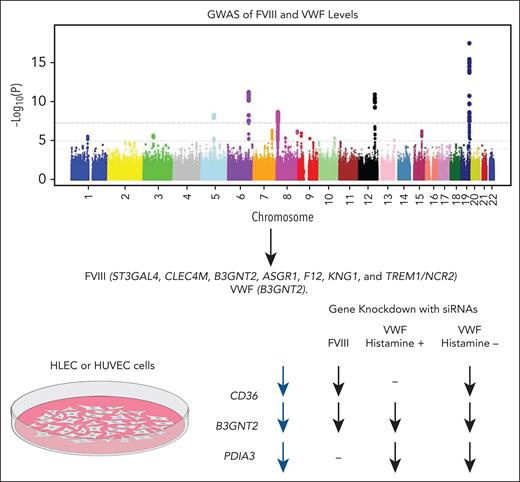
Importantly, the authors not only replicated several previously reported associations,5 but also reported several novel associations, 3 of which they selected for follow-up functionalization: CD36, B3GNT2, and PDIA3. These were selected based on the lack of a previous identification and also for having a potentially unknown biological mechanism.
Since stimulation with histamine is known to cause rapid secretion of VWF multimeric strings from human umbilical vein endothelial cells (HUVECs),6 the effect of silencing each of the 3 genes in HUVECs on VWF release into the media was tested both with and without histamine stimulation. Gene silencing for FVIII was performed in human liver endothelial cells (HLECs), which release more FVIII in vitro than HUVECs. The direction of effect of CD36 and B3GNT2 was the same for VWF and FVIII, and PDIA3 silencing affected VWF and not FVIII, suggesting more evidence for the intertwining of VWF and FVIII (see figure).
Novel genome-wide asociation studies (GWAS) associations for each gene as well as the 3 genes that were identified in a multiphenotype analysis and selected for functionalization in HUVECs (VWF) or HLECs (FVIII). Directions of effect are shown for each, including with and without histamine stimulation. siRNA, small interfering RNA.
Novel genome-wide asociation studies (GWAS) associations for each gene as well as the 3 genes that were identified in a multiphenotype analysis and selected for functionalization in HUVECs (VWF) or HLECs (FVIII). Directions of effect are shown for each, including with and without histamine stimulation. siRNA, small interfering RNA.
Although many of the other variants or genes identified were in regions related to known mechanisms, the mechanism underlying the association with B3GNT2 was not immediately clear. This novel finding of the association of B3GNT2 with VWF and FVIII levels suggests a potential new mechanism for regulation of the levels of these proteins. Beta-1,3-N-acetylglucosaminyltransferases (BGNTs) are Golgi-resident glycosyltransferases that play a crucial role in the synthesis of poly-N-acetyl-lactosamine chains. Their function involves catalyzing the addition of N-acetylglucosamine to the N-acetyl-lactosamine repeat, serving as a pivotal step in the chain elongation process.7 VWF is a carrier of these poly-N-acetyl-lactosamine chains.8 The fact that silencing of this gene in HUVECs decreases the release of VWF and in HLECs decreases the release of FVIII suggests that these proteins are glycosylated by B3GNT2 and that this glycosylation is critical for the proper secretion of VWF.
Most of the N-glycans and O-glycans present on human VWF are terminated with negatively charged sialic acid residues. Recent studies have shown that sialylation plays a crucial role in regulating various aspects of VWF, including its functional activity, susceptibility to proteolysis, and clearance, through distinct pathways. These discoveries hold direct clinical significance, as variations in both the quantity and quality of VWF sialylation have been observed in patients with VWD.9 Therefore, future targeting of these enzymatic pathways could help patients with dysregulated VWF and FVIII. However, functionalization of these enzymes can be difficult. We know that within the Golgi apparatus, the N-linked glycans of VWF undergo a series of modifications, resulting in the formation of complex-type carbohydrate structures.10 Additionally, O-linked glycosylation occurs in this process. As a result, each VWF monomer possesses 12 N-linked and 10 O-linked glycosylation sites. Collectively, these oligosaccharide chains contribute to nearly 20% of the final monomeric mass of VWF.10 The glycosylation of FVIII is similarly complex. There are multiple proteins involved in the proper posttranslational modification of VWF and FVIII, and they are competing with each other in the dynamic glycosylation process.9 Future functionalization efforts are needed to further detangle these confounding mechanisms.
The authors have presented a well-planned and executed study aimed at teasing apart the effects of VWF and FVIII on thrombotic phenotypes. Although they have shown that independent associations do exist, they have simultaneously demonstrated novel ways in which these 2 proteins and their relationship to thrombosis and hemostasis remain exceedingly complex.
Conflict-of-interest disclosure: The author declares no competing financial interest.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal