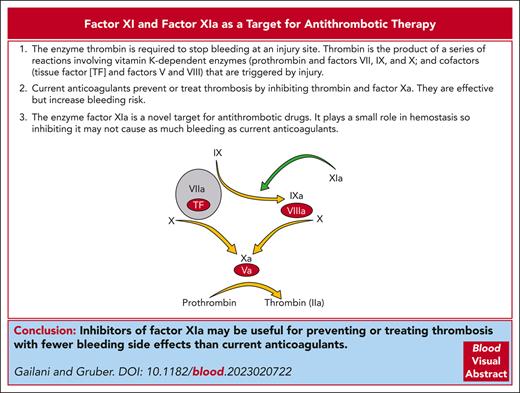
Visual Abstract
Direct oral anticoagulants (DOACs) that inhibit the coagulation proteases thrombin or factor Xa (FXa) have replaced warfarin and other vitamin K antagonists (VKAs) for most indications requiring long-term anticoagulation. In many clinical situations, DOACs are as effective as VKAs, cause less bleeding, and do not require laboratory monitoring. However, because DOACs target proteases that are required for hemostasis, their use increases the risk of serious bleeding. Concerns over therapy-related bleeding undoubtedly contribute to undertreatment of many patients who would benefit from anticoagulation therapy. There is considerable interest in the plasma zymogen factor XI (FXI) and its protease form factor XIa (FXIa) as drug targets for treating and preventing thrombosis. Laboratory and epidemiologic studies support the conclusion that FXI contributes to venous and arterial thrombosis. Based on 70 years of clinical observations of patients lacking FXI, it is anticipated that drugs targeting this protein will cause less severe bleeding than warfarin or DOACs. In phase 2 studies, drugs that inhibit FXI or FXIa prevent venous thromboembolism after total knee arthroplasty as well as, or better than, low molecular weight heparin. Patients with heart disease on FXI or FXIa inhibitors experienced less bleeding than patients taking DOACs. Based on these early results, phase 3 trials have been initiated that compare drugs targeting FXI and FXIa to standard treatments or placebo. Here, we review the contributions of FXI to normal and abnormal coagulation and discuss results from preclinical, nonclinical, and clinical studies of FXI and FXIa inhibitors.
Introduction
In 1977, in an edition of the British Medical Bulletin devoted to the pathology of blood coagulation, the Oxford University researcher R. Gwynn Macfarlane described thrombosis as “hemostasis in the wrong place.”1 Macfarlane, who was first to propose that the coagulation protease thrombin (factor IIa [FIIa]) is formed by a cascading sequence of plasma enzymatic reactions,2 suggested that “the key to the problem of thrombosis lies in a deeper understanding of the negative aspects of hemostasis.”1 A conclusion readily drawn from this intuitive premise is that thrombosis can be treated or prevented by inhibiting processes required for the normal response to injury. This principle has guided development of anticoagulation therapy with remarkable success. However, bleeding associated with vitamin K antagonists (VKAs) and, more recently, with direct oral anticoagulants (DOACs), seems inevitable with this approach. Anticoagulation-related bleeding causes significant morbidity and mortality, limits the intensity of therapy that can be applied, and places restrictions on who can receive treatment. This reality is the driving force behind efforts to develop drugs that separate antithrombotic activities from deleterious effects on hemostasis.3,4 Here, we discuss the rationale for inhibiting the plasma protein factor (F) XI or its protease form FXIa to improve the safety of antithrombotic therapy.
Thrombin generation and anticoagulation
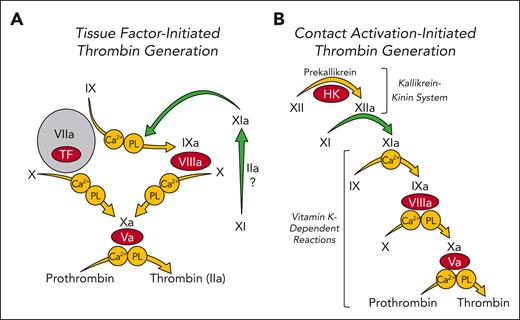
The protease thrombin is central to the host response to injury, catalyzing fibrinogen conversion to fibrin, stimulating platelets and cells by cleavage of protease activated receptors, and activating clotting factors.5,6 In current models of plasma coagulation, thrombin is the product of a set of reactions involving vitamin K–dependent proteases and their cofactors (indicated by yellow arrows in Figure 1A) that are initiated when FVIIa forms a complex with the cofactor tissue factor, which is expressed on surfaces of cells at an injury site.7-10 Studies with genetically altered mice indicate that absence of any component of this core system is either incompatible with life (prothrombin, FV, FVII, FX, and tissue factor), or causes severe bleeding (FVIII and FIX).11 For the past 80 years, components of this vital mechanism have been the primary targets of anticoagulation therapy.
Models of thrombin generation. Shown are major enzymatic reactions (represented by arrows) during plasma coagulation. In each reaction a plasma zymogen (black Roman numerals) is converted to an active protease (Roman numerals followed by “a”). Coagulation cofactors are shown as red ovals. Requirements for calcium ions (Ca2+) or phospholipids (PLs) for specific reactions are indicated within circles. Green arrows indicate reactions involving factor XI (XI) or factor XIa (XIa). (A) Tissue factor (TF)-initiated thrombin generation. Coagulation is initiated when FVIIa binds to TF that is exposed to blood at a wound site (gray circle). The FVIIa-TF complex converts FX to FXa leading to initial thrombin generation. FVIIa-TF also converts FIX to FIXa, which sustains thrombin generation by activating additional FX. In this scheme FXIa serves a limited role, generating some FIXa. FXI may be activated by thrombin or other unknown proteases (?) in this system. Its activation is not dependent on FXII. (B) Contact activation–initiated thrombin generation. The process is initiated by exposure of plasma to a surface (typically carrying a negative charge), which triggers FXII activation by a process called contact activation. This sets off a series (cascade) of enzymatic reactions culminating in conversion of prothrombin to thrombin. Here, FXI is a component of the contact activation process that triggers coagulation and is a major contributor to thrombin generation.
Models of thrombin generation. Shown are major enzymatic reactions (represented by arrows) during plasma coagulation. In each reaction a plasma zymogen (black Roman numerals) is converted to an active protease (Roman numerals followed by “a”). Coagulation cofactors are shown as red ovals. Requirements for calcium ions (Ca2+) or phospholipids (PLs) for specific reactions are indicated within circles. Green arrows indicate reactions involving factor XI (XI) or factor XIa (XIa). (A) Tissue factor (TF)-initiated thrombin generation. Coagulation is initiated when FVIIa binds to TF that is exposed to blood at a wound site (gray circle). The FVIIa-TF complex converts FX to FXa leading to initial thrombin generation. FVIIa-TF also converts FIX to FIXa, which sustains thrombin generation by activating additional FX. In this scheme FXIa serves a limited role, generating some FIXa. FXI may be activated by thrombin or other unknown proteases (?) in this system. Its activation is not dependent on FXII. (B) Contact activation–initiated thrombin generation. The process is initiated by exposure of plasma to a surface (typically carrying a negative charge), which triggers FXII activation by a process called contact activation. This sets off a series (cascade) of enzymatic reactions culminating in conversion of prothrombin to thrombin. Here, FXI is a component of the contact activation process that triggers coagulation and is a major contributor to thrombin generation.
Current anticoagulants inhibit the activity of thrombin (dabigatran, bivalirudin, and argatroban), FXa (rivaroxaban, apixaban, edoxaban, and fondaparinux), or both proteases (unfractionated heparin, low molecular weight heparin, and danaparoid) or reduce plasma concentrations of their precursors (VKAs). Because these proteases are required for hemostasis, drugs targeting them can cause bleeding that increases with anticoagulation intensity. From the standpoint of avoiding bleeding, DOACs (which target 1 protease) are superior to warfarin (which reduces FII, FVII, FIX, and FX plasma concentrations).12-14 However, the annual risk of serious bleeding in patients with atrial fibrillation on DOACs to prevent stroke is as high as 3%, with intracranial bleeding occurring in 0.3% to 0.5%.12 Bleeding concerns likely contribute to one-third of patients with atrial fibrillation not receiving anticoagulation and to underanticoagulation in many who are treated.15-17 According to a meta-analysis by Khan et al of patients receiving DOACs for venous thromboembolism (VTE), the rate of major bleeding events was 1.12 (confidence interval [CI], 0.72-1.62) per 100 patient years, with a case-fatality rate of 9.7% (CI, 3.3%-19.2%).18 Rates were even higher for patients with reduced kidney function, with a bleeding history, on antiplatelet agents, or aged >65 years.18 In addition, the safety advantage that DOACs have over VKAs may partly reflect less intense anticoagulation and, thus, a weaker antithrombotic effect. This may partly explain why DOACs are less effective than warfarin for patients with mechanical heart valves,19,20 antiphospholipid antibody syndrome,21,22 and atrial fibrillation caused by rheumatic heart disease.23
FXI in thrombin generation and hemostasis
FXI is the zymogen of the protease FXIa.24 Its properties are discussed in the accompanying paper by Moellmer et al.25 FXI was first identified as a constituent of plasma missing in individuals with mild bleeding symptoms but with a defect in in vitro clotting similar to the one in the severe bleeding disorders hemophilia A and B (deficiency of FVIII or FIX, respectively).26 In the activated partial thromboplastin time (aPTT) assay, thrombin generation is initiated when FXII is converted to the protease FXIIa by a process called contact activation (Figure 1B).27,28 FXIIa converts FXI to FXIa which then activates FIX. Consequently, the aPTT is prolonged in plasma lacking FXI.28 Indeed, FXI-deficient plasma usually has a longer aPTT than plasma lacking FVIII or FIX. Despite this, bleeding is milder in patients who are FXI deficient than in those with hemophilia and follows a different pattern (refer to the accompanying paper by Barg et al).29-31 Plasma FXI levels correlate poorly with bleeding propensity,28,30,32,33 and many individuals with FXI deficiency do not experience abnormal hemostasis. Furthermore, persons lacking FXII, the activator of FXI in the aPTT, do not have a hemostatic defect.28 From this, it is reasonable to conclude that the role of FXI in hemostasis differs from its role in the aPTT assay.
In contrast to its major contribution in the aPTT, FXI serves a minor role in tissue factor–initiated models of thrombin generation, in which it supplements FIXa produced by the FVIIa-tissue factor complex (Figure 1A).6,8,10 Clinical observations suggest that FXIa activity is most important for hemostasis when injury involves tissues with robust fibrinolytic activity, such as the nasopharynx, mouth, and urinary tract.28-33 Because FXII deficiency does not cause abnormal bleeding, it is likely that FXI is activated by proteases other than FXIIa during hemostasis. For example, thrombin converts FXI to FXIa (Figure 1A).24,25,34-36 Other proteases may activate it as well.
Evidence from animal studies implicating FXI in thrombosis
The limited role of FXI in hemostasis might suggest that its impact on thrombosis would also be small. However, data from animal models indicate that FXI is an important contributor to thrombosis. In 2003, Gruber and Hanson described the effects of a polyclonal anti-FXI immunoglobulin G (IgG) on experimentally induced thrombosis in baboons.37 Although the antibody did not prevent clots from forming on surfaces of Dacron or tissue factor–coated Teflon grafts inserted into arteriovenous shunts, it greatly reduced clot growth into the graft lumen. Intraluminal growth is necessary for a thrombus to occlude a vessel but is likely less important for hemostasis, during which clots form primarily within walls of damaged vessels and in extravascular spaces. In this study, the antithrombotic effect of FXI inhibition was comparable with that of unfractionated heparin. However, unlike heparin, FXI inhibition did not prolong the bleeding time. The authors concluded that targeting FXI could be antithrombotic without the profound impairment of hemostasis caused by anticoagulants targeting other coagulation factors.37 Subsequent studies with monoclonal IgGs supported the premise that thrombus growth requires FXI in the vascular graft model38,39 and suggest that as a thrombus expands away from the vessel wall containing the stimulus for clotting, FXI sustains thrombin generation to drive clot expansion.
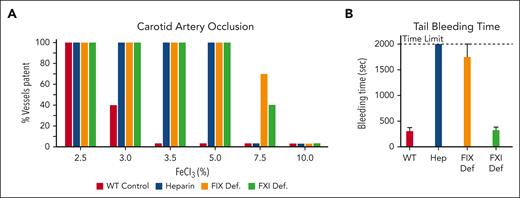
FXI also supports thrombosis in rodents.40-44 In mice in which carotid artery occlusion is induced by injuring the vessel with ferric chloride (FeCl3), an infusion of unfractionated heparin produces an antithrombotic effect that is reflected in the higher FeCl3 concentration needed to occlude the vessel (Figure 2A, compare red and blue bars).41 Comparable resistance to thrombosis occurs in mice lacking FIX, a model of hemophilia B (Figure 2A, orange bars).41,45 For both heparin infusion and FIX deficiency, the antithrombotic effect is accompanied by severe bleeding after tail transection (Figure 2B), consistent with disrupted hemostasis. FXI-deficient mice are as resistant to FeCl3-induced carotid artery occlusion as FIX-deficient mice (Figure 2A, compare orange and green bars), but their tail bleeding times are comparable with those of normal mice (Figure 2B).41 Indeed, FXI deficiency or FXIa inhibition has not caused major hemostatic abnormalities in any nonhuman species in which it has been tested.
Separating an antithrombotic effect from an antihemostatic effect. (A) Ferric chloride–induced carotid artery occlusion model. The carotid artery is briefly exposed to a pad saturated with FeCl3, and blood flow through the vessel is monitored for 30 minutes. Each bar represents a group of 10 mice, and the bar height indicates the percent of each group with patent blood vessels at the end of the experiment. Note that all wild-type mice (red bars) have occluded arteries with 3.5% FeCl3, whereas mice treated with heparin (blue bars), or mice deficient in FIX (orange bars) or FXI (green bars) require higher concentrations of FeCl3 to induce vessel occlusion. (B) Tail bleeding times. Wild-type mice treated with vehicle (red bar) or heparin (blue bar), or mice lacking FIX (orange bar), or FXI (green bar) underwent removal of the tail tip. The bleeding tail was immersed in normal saline at 37°C, and time to cessation of bleeding was recorded. Results are the mean bleeding times for groups of 10 mice ± 1 standard deviation. If bleeding did not stop by the 2000-second time point, the tail was cauterized.
Separating an antithrombotic effect from an antihemostatic effect. (A) Ferric chloride–induced carotid artery occlusion model. The carotid artery is briefly exposed to a pad saturated with FeCl3, and blood flow through the vessel is monitored for 30 minutes. Each bar represents a group of 10 mice, and the bar height indicates the percent of each group with patent blood vessels at the end of the experiment. Note that all wild-type mice (red bars) have occluded arteries with 3.5% FeCl3, whereas mice treated with heparin (blue bars), or mice deficient in FIX (orange bars) or FXI (green bars) require higher concentrations of FeCl3 to induce vessel occlusion. (B) Tail bleeding times. Wild-type mice treated with vehicle (red bar) or heparin (blue bar), or mice lacking FIX (orange bar), or FXI (green bar) underwent removal of the tail tip. The bleeding tail was immersed in normal saline at 37°C, and time to cessation of bleeding was recorded. Results are the mean bleeding times for groups of 10 mice ± 1 standard deviation. If bleeding did not stop by the 2000-second time point, the tail was cauterized.
Inhibition or deficiency of FXII also reduces thrombus formation in animal models. The effect is most striking in mice46-48 but is observed in primates.49 Because FXII deficiency does not cause abnormal bleeding, FXII inhibition could be an even safer antithrombotic strategy than FXI inhibition. However, the acute vessel injuries used to induce thrombosis in healthy animals may not mirror the biology of disorders, such as VTE, myocardial infarction (MI), or ischemic stroke, in which clots form in the setting of chronic vascular disease. Data from population studies have been important for assessing the relative contributions of FXI and FXII to thrombosis in humans.
Data implicating FXI in thrombosis in humans
In 2000, Meijers et al described an association between plasma FXI and VTE risk in enrollees in the Leiden Thrombophilia Study.50 Participants with FXI antigen concentrations >90th percentile had a more than twofold increased risk compared with the rest of the group, independent of other known genetic risk factors. Subsequent studies reported correlations between plasma FXI levels and incidences of VTE,51-55 MI54,56-58, and ischemic stroke.54,58-60 VTE and ischemic stroke are also less frequent in individuals with FXI deficiency than in the general population.61,62 FXI deficiency is particularly prevalent in people of Ashkenazi Jewish ancestry.28-30 In 2017, Preis et al presented results from a historical cohort study of >10 000 patients in a health care system in Israel: 690 with mild FXI deficiency (activity 30%-50% of normal) and 542 with moderate-to-severe deficiency (≤30% of normal).54 The adjusted hazard ratio of VTE for those with FXI deficiency was 0.26 (95% CI, 0.08-0.84). Arterial events (MI, stroke, and transient ischemic attacks) were also lower in patients with mild (0.52; 95%, CI 0.31-0.87) or moderate-to-severe (0.57; 95% CI, 0.35-0.93) FXI deficiency than for the general population.
FXI vs FXII as an antithrombotic target
Over the past decade, efforts to develop safer antithrombotic strategies have focused more on FXI than on FXII for 2 reasons. First, in contrast to the epidemiologic data for FXI, evidence that FXII influences VTE, MI, or stroke risk in humans is weak. Most recent studies show a negative correlation,55,57,63,64 and 2 reported inverse relationships for MI and cardiovascular death.57,64 Second, FXIIa is probably not the only FXI activator in vivo. The FVIIa-tissue factor complex is an important trigger for venous and arterial thrombus formation,9 and FXI is activated independently of FXIIa during tissue factor–initiated coagulation (Figure 1A).34-36,65 Based on these considerations, drugs targeting FXI or FXIa (hereafter referred to collectively as FXI/XIa inhibitors) may have advantages over drugs directed at FXII in some clinical situations.
This is not meant to imply that FXII inhibition is not a useful antithrombotic strategy. FXII triggers surface-mediated thrombin generation, which contributes to thrombosis when blood is exposed to medical devices during renal dialysis, cardiopulmonary bypass, or extracorporeal membrane oxygenation.66-68 Artificial heart valves, ventricular assist devices, and venous catheters are also prothrombotic, at least partly through induction of contact activation.67,69,70 Inhibiting FXIIa could reduce surface-induced thrombus formation with little, if any, impact on hemostasis.
FXI inhibition and bleeding
Because FXI deficiency causes a mild bleeding diathesis, it is anticipated that FXIa inhibitors will cause less major bleeding than DOACs. However, FXI deficiency can compromise hemostasis, and we should anticipate treatment-related bleeding in some patients on these drugs. Excessive bleeding after trauma, particularly to the nose, mouth, or genitourinary tract, may occur. Although unprovoked bleeding is likely to be rare, heavy menstrual bleeding may occur. A recent analysis by de Jong et al of women aged between 18 and 50 years on anticoagulation for acute VTE (87% on DOACs) found that 2 of 3 experienced abnormal uterine bleeding.71 Heavy menstrual bleeding is more common in women who are FXI deficient than in the general population,72 suggesting that it may be induced or aggravated by FXI/XIa inhibitors.
Perhaps most important from the standpoint of comparison to VKAs and DOACs is that intracranial and gastrointestinal bleeding are not prominent features of severe FXI deficiency. Thus, there may be relatively few life-threatening bleeds in patients administered FXI/XIa inhibitors, regardless of the overall impact on bleeding incidence. If this is the case, drugs targeting FXI or FXIa could be used in ways that are not practical with thrombin or FXa inhibitors. For example, drug dose could be pushed to abolish FXI or FXIa activity to maximize an antithrombotic effect. It may also be possible to use agents with long half-lives that can be administered every few weeks.
Drugs targeting FXI and FXIa
Table 1 lists drugs that reduce plasma FXI or inhibit FXI or FXIa that have undergone phase 2 testing. Long-acting agents include DNA antisense oligonucleotides (ASOs) that reduce plasma FXI antigen by promoting degradation of FXI messenger RNA in the liver (Ionis-FXI Rx),73,74 antibodies that bind FXI and interfere with its activation and activity (abelacimab and xisomab [gruticibart]),39,75,76 and antibodies that block the FXIa active site (osocimab).77 The small molecule oral FXIa active site inhibitors milvexian and asundexian have half-lives more comparable with those of current DOACs.78,79 FXI/XIa inhibitors have been tested for efficacy and/or safety as VTE prophylaxis, for secondary stroke prevention, and in patients with heart disease or renal failure. Several excellent reviews of completed and ongoing clinical trials have been published recently.4,80-83 Here, we focus on findings that illustrate potential strengths and weaknesses of FXI/XIa inhibitors.
Drugs targeting FXI and FXIa studied in phase 2 trials
| Drug name . | Drug description . | Mechanism of action . | Administration . | Duration of effect . |
|---|---|---|---|---|
| Ionis FXIRx (BAY-2306001) | DNA antisense oligonucleotide with complementary sequence to FXI messenger RNA | Reduces synthesis of FXI in hepatocytes | Subcutaneous or IV | Effect may persist for weeks to months after discontinuation |
| Osocimab | Monoclonal IgG that binds to the catalytic domain of FXIa | Inhibits FXIa catalytic activity through allosteric effects | Subcutaneous or IV | Days to weeks depending on dose. Mean elimination half-life, 30-44 days. |
| Abelacimab | Monoclonal IgG that binds to the catalytic domain of FXI and FXIa | Binds FXI preventing its activation. It also neutralizes FXIa activity | Subcutaneous or IV | Mean elimination half-life, 25-30 days. The IgG becomes saturated with FXI over time. |
| Xisomab (gruticibart) | Monoclonal IgG that binds to the apple 2 domain of FXI and FXIa | Interferes with FXI activation by FXIIa | Subcutaneous or IV | Half-life, 121.5 h with single dose of 5 mg/kg |
| Milvexian | Small molecule inhibitor of the FXIa catalytic active site | Inhibits FXIa catalytic activity by active site binding | Oral | Half-life, 11.4 to 18.1 h when administered daily |
| Asundexian | Small molecule inhibitor of the FXIa catalytic active site | Inhibits FXIa catalytic activity by active site binding | Oral | Dose dependent terminal half-life of 14.2 to 17.4 h |
| Drug name . | Drug description . | Mechanism of action . | Administration . | Duration of effect . |
|---|---|---|---|---|
| Ionis FXIRx (BAY-2306001) | DNA antisense oligonucleotide with complementary sequence to FXI messenger RNA | Reduces synthesis of FXI in hepatocytes | Subcutaneous or IV | Effect may persist for weeks to months after discontinuation |
| Osocimab | Monoclonal IgG that binds to the catalytic domain of FXIa | Inhibits FXIa catalytic activity through allosteric effects | Subcutaneous or IV | Days to weeks depending on dose. Mean elimination half-life, 30-44 days. |
| Abelacimab | Monoclonal IgG that binds to the catalytic domain of FXI and FXIa | Binds FXI preventing its activation. It also neutralizes FXIa activity | Subcutaneous or IV | Mean elimination half-life, 25-30 days. The IgG becomes saturated with FXI over time. |
| Xisomab (gruticibart) | Monoclonal IgG that binds to the apple 2 domain of FXI and FXIa | Interferes with FXI activation by FXIIa | Subcutaneous or IV | Half-life, 121.5 h with single dose of 5 mg/kg |
| Milvexian | Small molecule inhibitor of the FXIa catalytic active site | Inhibits FXIa catalytic activity by active site binding | Oral | Half-life, 11.4 to 18.1 h when administered daily |
| Asundexian | Small molecule inhibitor of the FXIa catalytic active site | Inhibits FXIa catalytic activity by active site binding | Oral | Dose dependent terminal half-life of 14.2 to 17.4 h |
FXI or FXIa inhibition for VTE prevention
Anticoagulants are often tested first as VTE prophylaxis for patients undergoing total knee arthroplasty (TKA). In the absence of prophylaxis, 40% to 60% of patients undergoing TKA are expected to form clots in the involved leg that are detectable with venography. Results for 4 phase 2 trials of FXI or FXIa reduction or inhibition in patients undergoing TKA are available (Table 2).74,75,77,84 Their effects on clot formation determined by venography 8 to 14 days after surgery were compared with those of enoxaparin (40 mg/d) administered for at least 10 days. Bleeding was the primary safety end point, but the trials were of insufficient size to identify significant differences in bleeding between treatment arms.
Trials of drugs targeting FXI(a) for patients undergoing TKA
| Clinical trial (drug) . | Dosing . | Primary efficacy end point . | Primary safety end point . | Incidence of venous thrombosis . | Incidence of bleeding . |
|---|---|---|---|---|---|
| FXI-ASO TKA (Ionis FXI Rx) | 9 subcutaneous doses starting 36 days before op. Last dose 3 days after op | VTE by bilateral venography 8-12 d after op or symptomatic VTE | Major or clinically relevant nonmajor bleeding out to 100 d after op | Ionis FXIRx 200 mg (27%)∗ Ionis FXIRx 300 mg (4%)† Enoxaparin (30%) | Major or CRNM bleeding: Ionis FXIRx 200 mg (3%) and 300 mg (3%) Enoxaparin (8%) |
| FOXTROT (osocimab) | Single IV dose the day after surgery or just before surgery | VTE by bilateral venography 10-13 d after op or symptomatic VTE | Major or clinically relevant nonmajor bleeding out to 10-13 d after op | Post-op osocimab (mg/kg): 0.3 (23.7%), 0.6 (15.7%)∗, 1.2 (16.5)∗, 1.8 (17.9%)∗ Pre-op osocimab (mg/kg): 0.3 (29.9%) and 1.8 (11.3%)† Enoxaparin (26.3%) Apixaban (14.5%) | Major or CRNM bleeding: Osocimab after op (0%-3%) Osocimab before op (1.9%-5.9%) Enoxaparin (5.9%) Apixaban (2%) |
| ANT-005 TKA (abelacimab) | Single IV dose 4-8 h after op | VTE by venography of involved leg 8-12 d after op or symptomatic VTE | Major or clinically relevant nonmajor bleeding out to 30 d after op | Abelacimab: 30 mg (13%)∗, 75 mg (5%)†, and 150 mg (4%)† Enoxaparin (22%) | Major or CRNM bleeding: Abelacimab 30 mg (2%), 75 mg (2%), and 150 mg (0%) Enoxaparin (0%) |
| AXIOMATIC-TKR (milvexian) | Once-daily or BID oral dosing starting 12-24 h after op, for 10-14 d | VTE by venography of involved leg 10-14 d after op, symptomatic VTE or death | Major, clinically relevant nonmajor, and minimal bleeding out to 6 wks after op | Milvexian BID: 25 mg (21%), 50 mg (11%)‡, 100 mg (9%)‡, and 200 mg (8%)‡ Milvexian once per day: 25 mg (25%), 50 mg (24%), and 200 mg (7%)‡ Enoxaparin (21%) | Major or CRNM bleeding: Milvexian (1%) and enoxaparin (2%) Any bleeding: Milvexian (4%) and enoxaparin (4%) |
| Clinical trial (drug) . | Dosing . | Primary efficacy end point . | Primary safety end point . | Incidence of venous thrombosis . | Incidence of bleeding . |
|---|---|---|---|---|---|
| FXI-ASO TKA (Ionis FXI Rx) | 9 subcutaneous doses starting 36 days before op. Last dose 3 days after op | VTE by bilateral venography 8-12 d after op or symptomatic VTE | Major or clinically relevant nonmajor bleeding out to 100 d after op | Ionis FXIRx 200 mg (27%)∗ Ionis FXIRx 300 mg (4%)† Enoxaparin (30%) | Major or CRNM bleeding: Ionis FXIRx 200 mg (3%) and 300 mg (3%) Enoxaparin (8%) |
| FOXTROT (osocimab) | Single IV dose the day after surgery or just before surgery | VTE by bilateral venography 10-13 d after op or symptomatic VTE | Major or clinically relevant nonmajor bleeding out to 10-13 d after op | Post-op osocimab (mg/kg): 0.3 (23.7%), 0.6 (15.7%)∗, 1.2 (16.5)∗, 1.8 (17.9%)∗ Pre-op osocimab (mg/kg): 0.3 (29.9%) and 1.8 (11.3%)† Enoxaparin (26.3%) Apixaban (14.5%) | Major or CRNM bleeding: Osocimab after op (0%-3%) Osocimab before op (1.9%-5.9%) Enoxaparin (5.9%) Apixaban (2%) |
| ANT-005 TKA (abelacimab) | Single IV dose 4-8 h after op | VTE by venography of involved leg 8-12 d after op or symptomatic VTE | Major or clinically relevant nonmajor bleeding out to 30 d after op | Abelacimab: 30 mg (13%)∗, 75 mg (5%)†, and 150 mg (4%)† Enoxaparin (22%) | Major or CRNM bleeding: Abelacimab 30 mg (2%), 75 mg (2%), and 150 mg (0%) Enoxaparin (0%) |
| AXIOMATIC-TKR (milvexian) | Once-daily or BID oral dosing starting 12-24 h after op, for 10-14 d | VTE by venography of involved leg 10-14 d after op, symptomatic VTE or death | Major, clinically relevant nonmajor, and minimal bleeding out to 6 wks after op | Milvexian BID: 25 mg (21%), 50 mg (11%)‡, 100 mg (9%)‡, and 200 mg (8%)‡ Milvexian once per day: 25 mg (25%), 50 mg (24%), and 200 mg (7%)‡ Enoxaparin (21%) | Major or CRNM bleeding: Milvexian (1%) and enoxaparin (2%) Any bleeding: Milvexian (4%) and enoxaparin (4%) |
BID, twice daily; CRNM, clinically relevant nonmajor bleeding; op, operation.
Noninferior compared with enoxaparin.
Superior compared with enoxaparin.
Significantly lower than with enoxaparin.
Bueller et al investigated the anti-FXI ASO Ionis-FXI Rx (FXI-ASO TKA trial).74 Because this drug takes 3 weeks to achieve stable reduction of plasma FXI, patients started treatment 36 days before surgery and were under the full effect of the drug during surgery. Average plasma FXI levels were reduced to 38% and 20% of normal in patients on 200 or 300 mg doses of ASO, respectively. The incidence of venous clot formation was significantly lower in patients receiving 300 mg ASO (4%) than in those receiving enoxaparin (30%) or 200 mg ASO (27%), indicating a dose response for the ASO, with a near maximal effect at a FXI activity of ∼20% of normal.
In the FOXTROT trial, Weitz et al compared single doses of osocimab to enoxaparin or apixaban (2.5 mg twice daily).77 Osocimab blocks the FXIa active site, and a dose of 1.8 mg/kg increased the aPTT to ∼1.6× normal. This is comparable with the effect of 300-mg Ionis-FXI Rx in the ASO trial.74 Venous clots occurred in 11.3% and 17.9% of patients receiving 1.8 mg/kg osocimab before or after surgery, respectively, in 26.3% with enoxaparin, and in 14.5% with apixaban.
In the ANT-005 TKA trial, Verhamme et al compared single 30, 75, or 150 mg doses of the IgG abelacimab administered within 8 hours after surgery.75 FXI bound to abelacimab is locked in a zymogen conformation. Because the normal plasma FXI concentration is between 15 and 45 nM, each abelacimab dose was sufficient to saturate FXI in most patients. As a result, peak aPTTs for all doses were similar and higher (∼1.9× normal) than those in the ASO or FOXTROT studies. Clots occurred in 13%, 5%, and 4% of the patients on 30, 75, and 150 mg abelacimab, respectively, and in 22% on enoxaparin.
In the AXIOMATIC-TKA trial, Weitz et al studied the oral FXIa inhibitor milvexian administered for 10 to 14 days, starting from 12 to 24 hours after TKA.84 Patients receiving a daily dose of milvexian of ≥100 mg (administered once or twice daily) had significantly fewer clots than patients on enoxaparin (7%-11% vs 21%, respectively). aPTT ratios for patients receiving ≥100 mg milvexian per day were at least twofold above baseline by day 4 of therapy.
These studies showed that reducing FXI or FXIa activity significantly reduces venous clot formation after TKA compared with enoxaparin and support observations that inherited FXI deficiency decreases VTE risk.50-55 Severe bleeding was uncommon (Table 2) despite some patients having low FXI or FXIa activity during surgery (AXIOMATIC-TKA and FOXTROT trials)74,77 or nearly undetectable activity after operation (ANT-005 TKA trial).75 Although severe bleeding is not common after TKA, the results support the impression that FXI or FXIa inhibition is safe in this setting.
Targeting FXIa for secondary prevention of ischemic stroke
Two trials compared the oral FXIa inhibitors asundexian (PACIFIC-STROKE)85 and milvexian (AXIOMATIC-SSP)86 with placebo for patients with recent acute stroke who were on antiplatelet therapy with aspirin and/or clopidogrel (Table 3). The primary efficacy outcomes were recurrent symptomatic ischemic stroke or magnetic resonance imaging (MRI)-detected covert infarct. As with the TKA trials, the stroke prevention trials were not large enough to establish significant differences in bleeding. Shoamanesh et al reported results for the PACIFIC-STROKE trial, in which patients were randomized to receive asundexian (10, 20, or 50 mg/d) or placebo.85 Over 26 weeks, the primary efficacy outcome occurred at similar rates in the asundexian (19%-22%) and placebo (19%) arms. International Society on Thrombosis and Haemostasis major or clinically relevant nonmajor bleeding occurred in 3% to 4% of patients administered asundexian and 2% on placebo. In the AXIOMATIC-SSP trial, Sharma et al assigned patients to milvexian (25-200 mg, once or twice daily) or placebo for 90 days.86 The primary safety end point was major bleeding (Bleeding Academic Research Consortium–modified type 3 and 5 bleeding). There were no differences between treatment arms for the primary efficacy or safety endpoints (Table 3).
Trials of drugs targeting FXIa for secondary prevention of ischemic stroke
| Clinical trial . | Study drug . | Patients . | Primary end point . | Patient population . | Incidence of bleeding . |
|---|---|---|---|---|---|
| PACIFIC-STROKE | Asundexian (10, 20, or 50 mg/d) compared with placebo | More than 45 y with acute noncardioembolic stroke | Symptomatic ischemic stroke or MRI-detected covert Infarct at 26 wks | Asundexian 10 mg (19%)∗ Asundexian 20 mg (22%)∗ Asundexian 50 mg (20%)∗ Placebo (19%) | Major or CRNM bleeding (ISTH) Asundexian 10 mg (4%) Asundexian 20 mg (3%) Asundexian 50 mg (4%) Placebo (2%) |
| AXIOMATIC-SSP | Milvexian (25 to 200 mg once or twice daily) compared with placebo | More than 40 y with acute ischemic stroke (<48 h) or high-risk TIA | Symptomatic ischemic stroke or MRI-detected covert Infarct at 90 weeks | Twice daily dosing: Milvexian 25 mg (18.5%)∗ Milvexian 50 mg (14.1%)∗ Milvexian 100 mg (14.8%)∗ Milvexian 200 mg (16.4%)∗ Placebo (16.1%) | BARC-modified 3 or 5 bleeding Milvexian 25 mg (0.6%) Milvexian 50 mg (1.5%) Milvexian 100 mg (1.6%) Milvexian 200 mg (1.5%) Placebo (1%) |
| Clinical trial . | Study drug . | Patients . | Primary end point . | Patient population . | Incidence of bleeding . |
|---|---|---|---|---|---|
| PACIFIC-STROKE | Asundexian (10, 20, or 50 mg/d) compared with placebo | More than 45 y with acute noncardioembolic stroke | Symptomatic ischemic stroke or MRI-detected covert Infarct at 26 wks | Asundexian 10 mg (19%)∗ Asundexian 20 mg (22%)∗ Asundexian 50 mg (20%)∗ Placebo (19%) | Major or CRNM bleeding (ISTH) Asundexian 10 mg (4%) Asundexian 20 mg (3%) Asundexian 50 mg (4%) Placebo (2%) |
| AXIOMATIC-SSP | Milvexian (25 to 200 mg once or twice daily) compared with placebo | More than 40 y with acute ischemic stroke (<48 h) or high-risk TIA | Symptomatic ischemic stroke or MRI-detected covert Infarct at 90 weeks | Twice daily dosing: Milvexian 25 mg (18.5%)∗ Milvexian 50 mg (14.1%)∗ Milvexian 100 mg (14.8%)∗ Milvexian 200 mg (16.4%)∗ Placebo (16.1%) | BARC-modified 3 or 5 bleeding Milvexian 25 mg (0.6%) Milvexian 50 mg (1.5%) Milvexian 100 mg (1.6%) Milvexian 200 mg (1.5%) Placebo (1%) |
BARC, Bleeding Academic Research Consortium; CRNM, clinically relevant nonmajor bleeding; TIA, transient ischemic attack
Not significantly different from results with placebo.
There are several issues to consider regarding the negative results of these trials. Obviously, although FXI deficiency appears to reduce risk of initial stroke in population studies,54,58-60 this does not mean that FXIa inhibition will reduce stroke recurrence. Also, FXIa inhibition may not add to the protective effect provided by dual antiplatelet therapy. Issues around study design also need consideration. The doses of FXIa inhibitors might have been insufficient to affect stroke recurrence. Although the higher doses of milvexian used in the AXIOMATIC-SSP trial reduced VTE in the AXIOMATIC-TKA trial,84 the doses of asundexian used in the PACIFIC-STROKE trial have not been shown to reduce thrombus formation in humans. Regarding the primary efficacy end points, approximately two-thirds of events in both trials were asymptomatic lacunar infarcts detected by MRI. FXI or FXIa inhibition may not prevent these common poststroke events. In a post-hoc analysis, symptomatic recurrent ischemic strokes were reduced in patients receiving the FXIa inhibitor in the PACIFIC-STROKE and AXIOMATIC-SSP trials. The OCEANIC-STROKE and LIBREXIA-STROKE trials (Table 4) will continue the evaluation of FXIa inhibition in preventing recurrent stroke.
Phase 3 studies involving asundexian, milvexian, and abelacimab
| Study drug . | Trial . | Comparator . | Estimated enrollment . | Patients and primary outcome . |
|---|---|---|---|---|
| Asundexian | OCEANIC-STROKE | Placebo | 9 300 | Patients: acute noncardioembolic ischemic stroke or high-risk transient ischemic attack Efficacy: time to first occurrence of ischemic stroke Safety: time to first occurrence of ISTH major bleeding |
| OCEANIC-AF | Apixaban | 18 000 | Patients: atrial fibrillation at risk of stroke Efficacy: time to first occurrence of stroke or systemic embolism Safety: time to first occurrence of ISTH major bleeding | |
| Milvexian | LIBREXIA-ACS | Placebo | 16 000 | Patients: acute coronary syndrome, with percutaneous intervention or conservative treatment, on antiplatelet agents Efficacy: time to occurrence of ischemic stroke |
| LIBREXIA-STROKE | Placebo | 15 000 | Patients: acute stroke or high-risk transient ischemic attack on antiplatelet therapy Efficacy: time to occurrence of major adverse cardiovascular event (death, MI, or stroke) | |
| LIBREXIA-AF | Apixaban | 15 500 | Patients: atrial fibrillation Efficacy: time to first occurrence of stroke or non–central nervous system embolization | |
| Abelacimab | LILAC-TIMI 76 | Placebo | 1 900 | Patients: patients who are at high risk with atrial fibrillation deemed unsuitable for oral anticoagulation Efficacy: time to first ischemic stroke or systemic symbolism Safety: time to first occurrence of BARC type 3c/5 bleeding |
| ASTER | Apixaban | 1 655 | Patients: patients with cancer with venous thromboembolism Efficacy: time to VTE recurrence (new deep vein thrombosis, new pulmonary embolism, or fatal pulmonary embolism) | |
| MAGNOLIA | Dalteparin | 1 020 | Patients: gastrointestinal and genitourinary cancers with venous thromboembolism Efficacy: time to VTE recurrence (new deep vein thrombosis, new pulmonary embolism, or fatal pulmonary embolism) |
| Study drug . | Trial . | Comparator . | Estimated enrollment . | Patients and primary outcome . |
|---|---|---|---|---|
| Asundexian | OCEANIC-STROKE | Placebo | 9 300 | Patients: acute noncardioembolic ischemic stroke or high-risk transient ischemic attack Efficacy: time to first occurrence of ischemic stroke Safety: time to first occurrence of ISTH major bleeding |
| OCEANIC-AF | Apixaban | 18 000 | Patients: atrial fibrillation at risk of stroke Efficacy: time to first occurrence of stroke or systemic embolism Safety: time to first occurrence of ISTH major bleeding | |
| Milvexian | LIBREXIA-ACS | Placebo | 16 000 | Patients: acute coronary syndrome, with percutaneous intervention or conservative treatment, on antiplatelet agents Efficacy: time to occurrence of ischemic stroke |
| LIBREXIA-STROKE | Placebo | 15 000 | Patients: acute stroke or high-risk transient ischemic attack on antiplatelet therapy Efficacy: time to occurrence of major adverse cardiovascular event (death, MI, or stroke) | |
| LIBREXIA-AF | Apixaban | 15 500 | Patients: atrial fibrillation Efficacy: time to first occurrence of stroke or non–central nervous system embolization | |
| Abelacimab | LILAC-TIMI 76 | Placebo | 1 900 | Patients: patients who are at high risk with atrial fibrillation deemed unsuitable for oral anticoagulation Efficacy: time to first ischemic stroke or systemic symbolism Safety: time to first occurrence of BARC type 3c/5 bleeding |
| ASTER | Apixaban | 1 655 | Patients: patients with cancer with venous thromboembolism Efficacy: time to VTE recurrence (new deep vein thrombosis, new pulmonary embolism, or fatal pulmonary embolism) | |
| MAGNOLIA | Dalteparin | 1 020 | Patients: gastrointestinal and genitourinary cancers with venous thromboembolism Efficacy: time to VTE recurrence (new deep vein thrombosis, new pulmonary embolism, or fatal pulmonary embolism) |
Bleeding in patients with heart disease on FXI/XIa inhibitors
Three phase 2 trials were designed to address the impact of FXI or FXIa inhibition on hemostasis in patients with heart disease (Table 5). In two, the drug targeting FXI or FXIa was compared with a DOAC. In the PACIFIC-AF trial, Piccini et al compared asundexian (20 or 50 mg/d) with twice daily apixaban for patients ≥45 years of age with nonvalvular atrial fibrillation and at least 1 bleeding risk factor (bleeding within past 12 months, glomerular filtration rate 30-50 mL/min, or current indication for aspirin).87 Ratios of incidence for the primary end point, major or clinically relevant nonmajor (CRNM) bleeding over 12 weeks, were significantly lower in patients on asundexian 20 mg (0.50; 90% CI, 0.14-1.68) or 50 mg (0.16; 90% CI, 0.01-0.99) than in those on apixaban. PACIFIC-AF was the first trial to show that patients on a FXIa inhibitor bleed less than those on a DOAC. The study had limitations, including a brief follow-up period (12 weeks), a small number of bleeding events (10 episodes across all arms), and no major bleeding (all events were CRNM bleeds).
Clinical trials of drugs targeting FXI or FXIa for patients with heart disease
| Clinical trial . | Study drug . | Comparator . | Primary end point . | Patient population . | Incidence of bleeding . |
|---|---|---|---|---|---|
| PACIFIC-AF | Asundexian | Apixaban | Major or CNMR bleeding (ISTH) out to 12 wk | At least 45 y nonvalvular atrial fibrillation, CHAD2DS2-VASc score ≥ 2 if male and ≥ 3 if female. | Asundexian 20 mg (1.2%)∗ Asundexian 50 mg (0.4%)∗ Apixaban 5 mg BID (2.4%) |
| PACIFIC-AMI | Asundexian | Placebo | BARC level 2, 3, or 5 bleeding out to 6 mo | At least 45 y, acute MI by clinical symptoms and elevated myocardial necrosis markers. | Asundexian 10 mg (7.6%)† Asundexian 20 mg (8.1%)† Asundexian 50 mg (10.5%)† Placebo (9.0%) |
| AZALEA-TIMI 71 | Abelacimab | Rivaroxaban | Major or CNMR bleeding (ISTH) (average follow-up, 21 mo) | At least 55 y, nonvalvular atrial fibrillation, and a moderate-to-high risk of embolic stroke. | Abelacimab 90 mg (1.9/100 patient years)‡ Abelacimab 150 mg (2.7/100 patient years)‡ Rivaroxaban 20 mg/d (8.1/100 patient years) |
| Clinical trial . | Study drug . | Comparator . | Primary end point . | Patient population . | Incidence of bleeding . |
|---|---|---|---|---|---|
| PACIFIC-AF | Asundexian | Apixaban | Major or CNMR bleeding (ISTH) out to 12 wk | At least 45 y nonvalvular atrial fibrillation, CHAD2DS2-VASc score ≥ 2 if male and ≥ 3 if female. | Asundexian 20 mg (1.2%)∗ Asundexian 50 mg (0.4%)∗ Apixaban 5 mg BID (2.4%) |
| PACIFIC-AMI | Asundexian | Placebo | BARC level 2, 3, or 5 bleeding out to 6 mo | At least 45 y, acute MI by clinical symptoms and elevated myocardial necrosis markers. | Asundexian 10 mg (7.6%)† Asundexian 20 mg (8.1%)† Asundexian 50 mg (10.5%)† Placebo (9.0%) |
| AZALEA-TIMI 71 | Abelacimab | Rivaroxaban | Major or CNMR bleeding (ISTH) (average follow-up, 21 mo) | At least 55 y, nonvalvular atrial fibrillation, and a moderate-to-high risk of embolic stroke. | Abelacimab 90 mg (1.9/100 patient years)‡ Abelacimab 150 mg (2.7/100 patient years)‡ Rivaroxaban 20 mg/d (8.1/100 patient years) |
BARC, Bleeding Academic Research Consortium; BID, twice daily; CHAD2DS2-VASc, a scoring system that stratifies patients with stroke risk and atrial fibrillation. The acronym stands for congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke (doubled), vascular disease, age between 65 and 74 years, and sex (female); ISTH, International Society on Thrombosis and Haemostasis.
Significantly lower than apixaban 5 mg twice a day.
Not significantly different from placebo.
Significantly lower than rivaroxaban 20 mg/d (P < .0001).
The AZALEA-TIMI 71 (ANT-006) trial compared patients treated with the anti-FXI IgG abelacimab (90 or 150 mg subcutaneously monthly) with those treated with rivaroxaban (20 mg/d; 15 mg if GFR ≤ 50 mL/min).88 Patients were followed for ∼2 years, providing information on long-term treatment. Enrollees were aged ≥55 years with atrial fibrillation at moderate-to-high risk for embolic stroke. The primary end point was International Society on Thrombosis and Haemostasis major or CRNM bleeding. Median free FXI antigen was reduced by 97% (interquartile range [IQR], 50%-99%) and 99% (IQR, 98%-99%) over the dosing interval in patients on 90 and 150 mg abelacimab, respectively, indicating a prolonged state of severe FXI deficiency in most patients.
AZALEA-TIMI 71 was stopped early because reductions in bleeding for patients on abelacimab, compared with rivaroxaban, substantially exceeded expectation. Incidences for the primary end point in patients on 90 and 150 mg abelacimab were 1.9 and 2.7 cases per 100 patient years, respectively, compared with 8.1 per 100 patient years with rivaroxaban. Over a median follow-up of 20.7 months, the primary end point was reduced by 67% (hazard ratio [HR], 0.33; 95% CI, 0.19-0.55; P < .0001) in patients on 150 mg abelacimab compared with those on rivaroxaban, with a 74% reduction in major bleeding (HR 0.26; 95% CI, 0.11-0.61; P < .002), and a 93% reduction in gastrointestinal bleeding (HR 0.07;95% CI, 0.01-0.50; P < .008). Intracranial bleeds were rare and comparable for 150 mg abelacimab and rivaroxaban (0.3 and 0.6 cases per 100 patient years, respectively). AZALEA-TIMI 71 is the first study to show reduced major bleeding with a FXI/XIa inhibitor compared with a DOAC, using drug doses previously shown to reduce VTE in patients undergoing TKA.75
In the PACIFIC-AMI trial, Rao et al compared asundexian (10, 20, or 50 mg/d) with placebo for patients with acute MI on antiplatelet therapy.89 The primary outcome (Bleeding Academic Research Consortium level 2, 3, or 5 bleeding) occurred at similar rates in patients on asundexian (7.6% to 10.5%) and placebo (9.0%) over a median follow-up of 368 days. Data from the PACIFIC-STROKE and AXIOMATIC-SSP trials had suggested that superimposing FXIa inhibition on antiplatelet therapy was safe,85,86 and the PACIFIC-AMI trial supports this impression with data collected over a longer period.
FXI or FXIa inhibition in patients with renal failure
FXI/XIa inhibitors are undergoing testing for patients at increased risk of thromboembolism who are not good candidates for treatment with a DOAC or VKA. Patients with chronic renal failure are not only at increased risk of MI, stroke, and VTE90,91 but also at high risk of bleeding.90,92 Lorentz et al compared single doses of the anti-FXI IgG xisomab (0.25 or 0.50 mg/kg) with placebo for patients undergoing heparin-free hemodialysis.76 Xisomab reduced occlusive events requiring circuit dialysis exchange by ∼60% compared with occlusion rates in the same patients before receiving xisomab. There was no evidence of compromised hemostasis. Xisomab also lowered plasma C-reactive protein and thrombin-antithrombin complex levels. Phase 2 trials assessing the safety of Ionis-FXI Rx (EMERALD), the new generation ASO BAY2976217 (Re-THINC), and osocimab (CONVERT) in adults with end-stage renal disease on hemodialysis are underway.90
Summary of clinical studies
Phase 2 studies of FXI/XIa inhibitors provide preliminary indications of efficacy and safety. At this point, evidence for efficacy is limited to demonstrations that FXI or FXIa inhibition prevents VTE in patients undergoing TKA74,75,77,84 and reduces clotting in hemodialysis circuits.76 Superimposing FXIa inhibition on antiplatelet therapy did not reduce recurrent stroke but might have reduced recurrent symptomatic ischemic stroke. The effectiveness of FXI or FXIa inhibition relative to DOACs remains to be established. The phase 3 OCEANIC-AF, LIBREXIA-AF, and ASTER trials (Table 4) are designed to address this issue. On 19 November 2023, the OCEANIC-AF trial comparing asundexian with apixaban for prevention of stroke or embolization in patients with atrial fibrillation was stopped because data surveillance indicated inferior efficacy for asundexian.93 This raises the possibility that FXI/XIa inhibitors are not as effective as DOACs for reducing thromboembolism associated with atrial fibrillation. Unlike the other drugs listed in table 4, asundexian was not tested as prophylaxis in knee replacement surgery, which would have facilitated comparison to other FXI/XIa targeting drugs. Several doses of asundexian were tested for recurrent stroke prevention,85 but none affected the primary study outcome. At this point, therefore, we lack the information needed to determine if the OCEANIC-AF results reflect a limitation of FXI/XIa inhibitors as a class, or a limitation specific to asundexian at the doses tested.
In head-to-head comparisons in the PACIFIC-AF and AZALEA-TIMI 71 trials, FXI/XIa inhibitors caused less bleeding than DOACs. The results of the AZALEA-TIMI 71 trial are worth emphasizing. In this trial, a high dose of an antibody was used to essentially ablate FXI for >20 months. The profound reduction in overall major and CRNM bleeding when compared with rivaroxaban, and the very low incidence of intracranial and gastrointestinal bleeding supports the premise that FXI or FXIa inhibition will have a significant safety advantage over currently used anticoagulants and that long-term near total reduction of FXI or FXIa activity does not place patients at unacceptable risk of severe bleeding.
Future considerations, FXI or FXIa inhibition and inflammation
The FXI gene arose from a duplication of the gene for prekallikrein, the precursor of the protease plasma kallikrein.94 Plasma kallikrein contributes to inflammation and kinin formation, and FXI retains some of the functional characteristics of its parent molecule.24,25 FXI contributes to the pathology of sepsis in primate and rodent models.95-99 Although this may partly reflect its role in thrombin generation, FXI also supports the systemic inflammatory response to infection. FXI deficiency or treatment with xisomab reduced the early “cytokine storm” in sepsis models. Curiously, although FXI is a downstream substrate for FXIIa during contact activation (Figure 1B), FXI inhibition blunted FXII activation in vivo during sepsis.98,99 These data suggest that FXI, rather than serving as a link in a unidirectional chain of enzymatic reactions that culminates in thrombin generation, resides at a nexus where several host-defense mechanisms converge.
Conclusions
In 2005, Hirsh et al proposed that an ideal anticoagulant should have a high efficacy-to-safety index and a predictable dose response so that laboratory monitoring is not required.100 DOACs are improvements on VKAs in both regards. FXI or FXIa inhibitors may further uncouple antithrombotic effects from adverse effects on hemostasis.3 If phase 3 trials demonstrate that these drugs combine efficacy with improved safety, then antithrombotic therapy may become available for many patients who currently go untreated because of bleeding concerns.
Acknowledgments
The authors acknowledge generous support from the National Institutes of Health, National Heart, Lung, and Blood Institute awards R35 HL140025, HL101972, and HL144113 and the Ernest W. Goodpasture Chair in Experimental Pathology for Translational Research, Vanderbilt University Medical Center.
Authorship
Contribution: D.G. and A.G. contributed to writing the manuscript.
Conflict-of-interest disclosure: D.G. is a consultant for pharmaceutical companies (Anthos Therapeutics; Aronora, Inc; Bayer Pharma; Bristol Myers Squibb; Ionis Pharmaceuticals; and Janssen Pharmaceuticals), with interests in targeting factor XI, factor XII, and prekallikrein for therapeutic purposes. A.G. is an employee of Aronora Inc, with interests in developing safe antithrombotic agents.
Correspondence: David Gailani, Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Room 4918, The Vanderbilt Clinic, 1301 Medical Center Dr, Nashville, TN 37232; email: dave.gailani@vanderbilt.edu; and Andras Gruber, Aronora, Inc, 1818 SW Ave, Suite 102, Portland, OR 97201; email: andras.gruber@aronorabio.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal