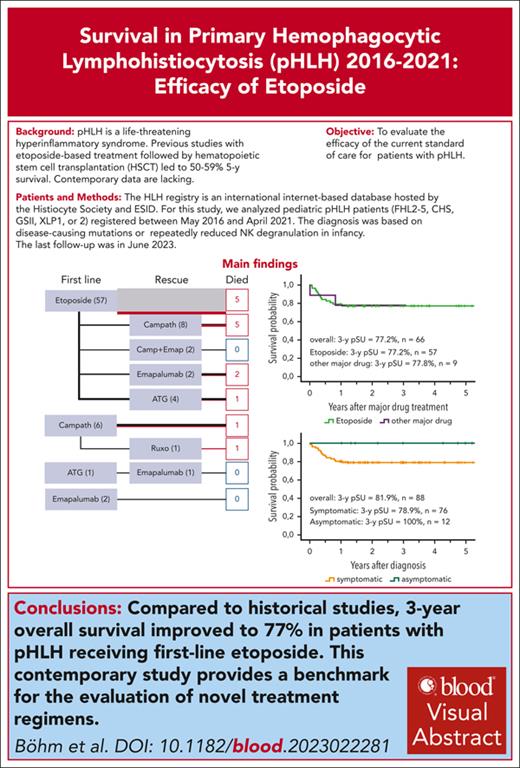
Compared with historical studies, 3-year survival for patients with pHLH improved to 77% for patients receiving first-line etoposide.
Contemporary outcome of patients with pHLH is better than anticipated, providing a benchmark for evaluation of novel treatment regimens.
Visual Abstract
Primary hemophagocytic lymphohistiocytosis (pHLH) is a life-threatening hyperinflammatory syndrome that develops mainly in patients with genetic disorders of lymphocyte cytotoxicity and X-linked lymphoproliferative syndromes. Previous studies with etoposide-based treatment followed by hematopoetic stem cell transplantation (HSCT) resulted in 5-year survival of 50% to 59%. Contemporary data are lacking. We evaluated 88 patients with pHLH documented in the international HLH registry from 2016-2021. In 12 of 88 patients, diagnosis was made without HLH activity, based on siblings or albinism. Major HLH-directed drugs (etoposide, antithymocyte globulin, alemtuzumab, emapalumab, ruxolitinib) were administered to 66 of 76 patients who were symptomatic (86% first-line etoposide); 16 of 57 patients treated with etoposide and 3 of 9 with other first-line treatment received salvage therapy. HSCT was performed in 75 patients; 7 patients died before HSCT. Three-year probability of survival (pSU) was 82% (confidence interval [CI], 72%-88%) for the entire cohort and 77% (CI, 64%-86%) for patients receiving first-line etoposide. Compared with the HLH-2004 study, both pre-HSCT and post-HSCT survival of patients receiving first-line etoposide improved, 83% to 91% and 70% to 88%. Differences to HLH-2004 included preferential use of reduced-toxicity conditioning and reduced time from diagnosis to HSCT (from 148 to 88 days). Three-year pSU was lower with haploidentical (4 of 9 patients [44%]) than with other donors (62 of 66 [94%]; P < .001). Importantly, early HSCT for patients who were asymptomatic resulted in 100% survival, emphasizing the potential benefit of newborn screening. This contemporary standard-of-care study of patients with pHLH reveals that first-line etoposide-based therapy is better than previously reported, providing a benchmark for novel treatment regimes.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a life-threating hyperinflammatory syndrome caused by uncontrolled activation of the immune system. Diagnosis is guided by clinical, laboratory, and cytological criteria (HLH-2004 criteria).1,2 Primary HLH (pHLH) results from an inherited predisposition. This can be caused by loss-of-function mutations in the genes encoding perforin (FHL2) or molecules involved in secretion of perforin-containing cytotoxic granules (FHL3-5).3-5 HLH is also a common manifestation of Griscelli syndrome type 2 (GS2) and Chediak-Higashi syndrome (CHS),3 with a similar pathophysiology, and of X-linked lymphoproliferative syndromes (XLP1 and XLP2), inherited disorders with susceptibility to Epstein-Barr virus–associated HLH.6 The only curative therapy currently available for patients with pHLH is immunosuppression followed by allogeneic hematopoietic stem cell transplantation (HSCT).3,7-9
In the previous HLH-94 and HLH-2004 studies, etoposide-based treatment followed by HSCT led to 5-year overall survival (OS) of 50% and 59%, respectively, in patients with pHLH.10-12 Similar results (OS, 61%) have been achieved when using antithymocyte globulin (ATG) as first-line therapy.13 Recently, several novel agents have been introduced to HLH therapy such as alemtuzumab, emapalumab, or ruxolitinib.14-17 Current standard-of-care for pHLH is still mostly etoposide-based but includes recommendations on salvage therapy using these novel agents in case of refractory or recurrent disease.7,8 Studies evaluating salvage therapy with emapalumab15 or first-line alemtuzumab16 have been performed, and prospective studies on first-line ruxolitinib or etoposide/ruxolitinib in pHLH are ongoing.17,18 However, adequate positioning of these new approaches into HLH treatment recommendations is hampered by lack of contemporary standard-of-care data. Because both immunosuppressive therapy and HSCT affect OS in pHLH, progress in HSCT in the last 20 years is as important as improved treatment of hyperinflammation for better OS of patients with pHLH.19-23
Contemporary natural history and survival data are also essential as a benchmark for evaluating the rationale and feasibility of novel approaches to treat pHLH that may include newborn screening followed by corrective cellular therapies including HSC gene therapy. Case collections have shown that prognosis is better for infants with pHLH diagnosed at birth, but only retrospective24 or prospective OS data from the beginning of the century10 for children born with these conditions are available.
In this study, we evaluated diagnosis, immunosuppressive treatment, HSCT, and outcome of patients with pHLH documented in the HLH registry of the European Society of Immunodeficiencies and the Histiocyte Society between 2016 and 2021. The results show a 3-year OS of 77% for patients who were symptomatic with pHLH receiving first-line etoposide and 82% for all patients with pHLH, including those who were asymptomatic. Thus, contemporary prognosis of patients with pHLH is better than anticipated, providing an important benchmark for evaluation of novel treatment regimes.
Methods
The HLH registry
The HLH registry is an international internet-based database that was implemented by the European Society of Immunodeficiencies and the Histiocyte Society in 2016. The protocol was approved by the Ethics committee of the University of Freiburg (institutional review board approval 493/14 and 610/15) and registered in the German registry for clinical studies (DRKS00010148). All parents of affected children gave written informed consent in accordance with the Declaration of Helsinki. Physicians provided initial documentation, a 3-year follow-up documentation, and survival information at closure of the database (June 2023). Patient data were entered via a standard and secure web tool. Specific queries were issued on uncertain data entries.
Patient cohort and definitions
For this study, we analyzed pediatric patients with pHLH (defined as patients with FHL2-5, CHS, GSII, XLP1, or XLP2) registered between May 2016 and April 2021. Diagnosis was based on disease-causing biallelic or hemizygous mutations, respectively, or repeatedly reduced natural killer cell degranulation in infancy. The last follow-up was in June 2023. Patients with nonprimary HLH, malignancies, or rheumatic diseases and patients from countries with limited access to HSCT were excluded from this analysis. Disease in patients with heterozygous or homozygous PRF1 p.A91V mutations was not considered pHLH. The final data set included 88 patients with pHLH registered from 10 countries: Germany (n = 51), Switzerland (n = 10), United States (9), Israel (n = 6), Denmark (n = 4), Poland (n = 3), Spain (n = 2), Canada (n = 1), Czech Republic (n = 1), and The Netherlands (n = 1). Seventy percent of patients were registered in Germany and Switzerland, where long-standing networks assure unselected capturing of patients with symptomatic and asymptomatic pHLH.
The response to treatment was retrospectively judged by the treating physician based on the 8 HLH criteria and central nervous system (CNS) manifestations, immediately before HSCT. Complete remission was defined as normal values for all assessed HLH parameters. Partial remission was defined as normalization of at least 3 HLH parameters. Other constellations were defined as active disease. To analyze event-free survival (EFS), events were defined as reactivation of primary disease, death from any cause, and second HSCT.
Statistical analysis
The statistical analyses were performed using SPSS (Statistical Package for Social Sciences, version 29; IBM SPSS) for Windows (IBM, Chicago, IL). Probability of survival (pSU) and EFS (event defined as death, reactivation of primary disease, or second HSCT) were estimated using the Kaplan-Meier method. Comparison of survival and comparison of categorical variables were analyzed in a univariate approach with the log-rank test. Two-sided 95% confidence intervals (CIs) were calculated, and statistical tests were performed at the .05 level.
Results
Distribution of genetic diagnoses and clinical presentation
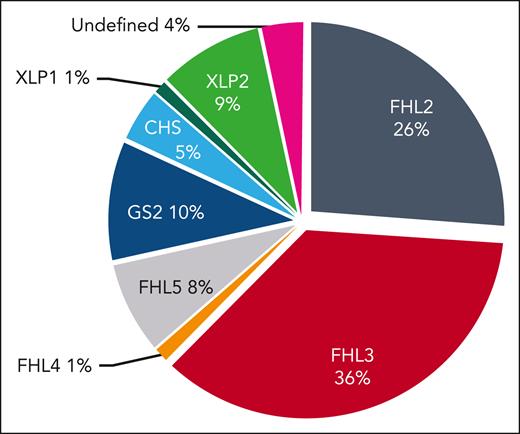
Between May 2016 and April 2021, a total of 88 patients with pHLH were registered in the HLH registry. Of these, 63 patients had a biallelic disease-causing mutation in an FHL gene: 23 FHL2, 32 FHL3, 1 FHL4, and 7 FHL5. Other genetic disorders (hereditary non-FHL) were diagnosed in 25 patients: 9 GS2, 4 CHS, 1 XLP1, and 8 XLP2. Three patients were included based on early (<6 months) onset of HLH and absent natural killer degranulation, which led to HSCT (Figure 1).
Genetic diagnoses. Overview of the genetic diagnoses in the study cohort (n = 88). For the 3 patients without mutation in genes known to be associated with HLH (undefined), absent natural killer (NK) cell degranulation and HLH onset in infancy justified inclusion.
Genetic diagnoses. Overview of the genetic diagnoses in the study cohort (n = 88). For the 3 patients without mutation in genes known to be associated with HLH (undefined), absent natural killer (NK) cell degranulation and HLH onset in infancy justified inclusion.
The cohort included 12 patients who were diagnosed before the onset of symptoms because of an affected sibling with pHLH or in the context of partial albinism, who remained asymptomatic until HSCT: 2 FHL2, 4 FHL3, 1 FHL5, 3 GS2, 1 CHS, and 1 XLP2. Of these 12 patients who were asymptomatic, 8 did not receive HLH-directed treatment, whereas 4 received prophylactic cyclosporine A and/or IV immunoglobulins until HSCT. One patient, diagnosed with FHL3 without symptoms, aged 9 years when she was worked up as a BMT donor for her brother, developed HLH shortly thereafter. We decided to classify this patient as symptomatic.
In 76 patients who were symptomatic, 71 (93%) fulfilled HLH-2004 criteria, 3 presented with isolated CNS-HLH, and 2 patients had CNS-HLH with partial systemic activity. The median age at onset of first HLH symptoms was 5.6 (range, 0-208) months. In 17 of 76 patients (22%), a viral infection (8 Epstein-Barr virus, 5 cytomegalovirus, 1 human herpesvirus 6, 1 human herpesvirus 7, 1 non–severe acute respiratory syndrome coronavirus, 1 rotavirus, and 1 adenovirus); in 2, a bacterial infection with Staphylococcus; and in 1 patient, Toxoplasma were documented as HLH triggers. In 56 of 76 patients (74%), no trigger was detected. CNS involvement (neurological manifestations, cerebrospinal fluid leakage, or imaging abnormalities) was documented in 27 patients (38%) before initiation of treatment (data missing for 5 patients).
First-line therapy of patients who were symptomatic
Out of 76 patients who were symptomatic, 10 did not receive major HLH-directed treatment; they were treated with IV immunoglobulin/steroids/cyclosporine A (n = 8; 3 FHL5, 1 CHS, 3 XLP2, and 1 undefined) or infliximab only (1 XLP2) or had spontaneous resolution without HLH-directed treatment (1 XLP2; supplemental Table 2, available on the Blood website). Among these 10 patients (median age at onset 4.7 years; range 0-16.3 years), 1 (XLP-2) developed severe bleeding, 1 (undefined mutation) developed liver and pulmonary failure, and 1 (FHL-5) developed active CNS-HLH before HSCT that eventually resolved without sequelae.
Major HLH-directed drugs (etoposide, alemtuzumab, ATG, emapalumab, and ruxolitinib) were administered to the remaining patients who were symptomatic (66 of 76 [87%]) (Figure 2). The majority of these patients received etoposide (57 of 66 [86%]), whereas a minority of patients received alemtuzumab (n = 7 [11%]) or emapalumab (n = 2 [3%]) as first-line major treatment. In the group of patients receiving first-line etoposide, 49 of 57 patients (86%) received the recommended 150 mg/m2 for the entire treatment period (range, 138-169 mg/m2), whereas it was transiently reduced to ∼100 mg/m2 (range, 95-130 mg/m2) for 4 patients and to ∼75 mg/m2 (range, 70-83 mg/m2) for another 4 patients (supplemental Figure 1). The median number of etoposide doses administered was 9 (first quartile, 1-4 doses; second and third quartiles, 5-11 doses; and fourth quartile, 12-15 doses; 1 outlier, 23 doses). Reasons for adapting etoposide dosing were anticipated side effects due to the condition of the patient before the start of therapy, actual side effects after initial full dose administration, or good response. Alemtuzumab as first-line therapy was administered in a cumulative dose between 2.4 and 3.0 mg/kg (median, 2.5 mg/kg) over 3 consecutive days. Emapalumab was administered at a dose of 5.7 and 10 mg/kg. Eighteen of 27 children with CNS involvement received intrathecal methotrexate as part of their remission-inducing therapy. A summary of the key diagnosis and treatment data for each individual patient in the cohort are provided in supplemental Table 1.
Treatment regimes. Major drugs used as first-line or salvage therapy (n = 66). One deceased patient with CHS is missing here because he did not receive any major drug before HSCT. Line thickness is proportional to the number of patients receiving the indicated treatment. Red lines and boxes represent deceased patients.
Treatment regimes. Major drugs used as first-line or salvage therapy (n = 66). One deceased patient with CHS is missing here because he did not receive any major drug before HSCT. Line thickness is proportional to the number of patients receiving the indicated treatment. Red lines and boxes represent deceased patients.
Salvage therapy, remission, and mortality before HSCT in patients who were symptomatic
Because of inadequate treatment response or HLH reactivation, 19 of 66 patients (29%) receiving major HLH-directed drugs also received salvage therapy, defined as the application of at least 1 additional major drug. Salvage therapy was administered to 16 of 57 patients (28%) with first-line etoposide, 2 of 7 (29%) with first-line alemtuzumab, and 1 of 2 patients with first-line emapalumab (Figure 2). Survival of all patients who were symptomatic until HSCT (or last follow-up in case of decision against HSCT, n = 4, including 3 XIAP and 1 FHL5) was 69 of 76 (91%), which included survival of 52 of 57 (91%) with first-line etoposide. Among the 7 patients who died before HSCT, 5 had etoposide and 2 had alemtuzumab as first-line drug. Five of these patients, 4 after etoposide and 1 after alemtuzumab as first-line treatment, had also received salvage therapy (3 alemtuzumab, 1 ruxolitinib, and 1 ATG; Figure 2). The causes of death before HSCT were persistent HLH activity (n = 5), infection (n = 1), and 1 answer missing. The time from diagnosis to death for these patients was 2.2 months (range, 0.1-9.8 months). Among 65 of 76 patients who were symptomatic and underwent HSCT, 41 (65%) were in complete remission, 18 (29%) in partial remission, and 4 (6%) with active disease; 2 answers were missing.
HSCT
HSCT was performed in 75 of 88 patients (85%; 10 asymptomatic and 65 symptomatic). Among the patients who were symptomatic, 58 of 65 (89%) and among patients who were asymptomatic, 6 of 10 (60%) of transplantations were performed in the first year after diagnosis. Four patients who were asymptomatic (1 FHL3, 1 CHS, and 2 GS2) underwent transplantations later, and 2 (1 XLP2 and 1 GS2) had not undergone transplantation until last follow-up. Median time from diagnosis to HSCT was 97 days (range, 34-1154 days) for the whole study group, 88 days for patients who were symptomatic, and 246 days for patients who were asymptomatic.
Conditioning regimens were considered fully myeloablative for 1 patient (fludarabine, total body irradiation, and posttransplant cyclosphosphamide), reduced-toxicity myeloablative for 67 patients (busulfan based for 24, treosulfan based for 35, and melphalan based for 8), and reduced intensity (treosulfan and fludarabine) for 6 patients, with 1 answer missing. HSCT donors were matched-related (MRD; n = 12), matched-unrelated (MUD; n = 40), mismatched unrelated (MMUD; ie, ≤9/10 HLA-identical; n = 14), and haploidentical (n = 9). The grafts were bone marrow (n = 48), PBSC (n = 25), cord blood (n = 1), and 1 answer missing. The manipulation of grafts from haploidentical donors included 5 posttransplant cyclophosphamide, 1 CD34 selection, 1 CD3-depletion, 1 alpha/beta T-cell depletion, and 1 answer missing. Individual information on HSCT is provided in supplemental Table 1.
Adverse events after HSCT
The median time from disease onset to last follow-up for surviving patients was 5.39 years (range, 2.81-9.06 years). At least 3-year follow-up data after disease onset were available for 70 of 72 surviving patients, and 2 patients had a follow-up between 2.7 and 2.8 years. Median follow-up after HSCT was 4.83 years (range, 2.35-7.30 years). Three-year follow-up data were available for 60 of 66 surviving patients, whereas the remaining 6 patients had follow-up between 2.35 and 2.8 years. Adverse events after HSCT occurred in 33 of 75 patients (45%; multiple answers possible and 1 answer missing): 7 (9%) patients had veno-occlusive disease, 7 (9%) had thrombotic microangiopathy (TMA), and 7 (9%) had graft loss or rejection. The cumulative incidence of acute graft-versus-host disease (GVHD) grade 3 or 4 was 13.3% (10 of 75) and of chronic GVHD was 18.7% (14 of 75), including 9 limited and 5 extensive. In 3 cases of acute GVHD, patients developed an extensive chronic GVHD over time. At 1 year follow-up, 22 patients (29%) had mixed donor chimerism chimerism (8×, <25%; 9×, 25%-75%; and 5×, 76%-95%). Among 21 patients with this information available, 6 were administered with donor lymphocyte infusions (2-10 doses), 2 received a stem cell boost, and 4 received a second transplant. All patients with a mixed chimerism were alive and well at last follow-up.
OS and EFS
The overall 3-year pSU after diagnosis in the entire cohort of 88 patients with genetic HLH was 81.8% (CI, 72%-88%; Figure 3A), without significant difference between the FHL (80.9%; CI, 69%-89%; n = 63) and non-FHL (84.0%; CI, 63%-94%; n = 25) subgroups. Patients who were symptomatic and had received first-line etoposide had a 3-year OS after initiation of major drug treatment of 77.2% (CI, 64%-86%; n = 57; Figure 3B). The 3-year EFS after diagnosis in the entire cohort was 75.8% (CI, 65%-84%; n = 88), without significant difference between FHL (n = 63 [74.2%]; CI, 61%-83%) and non-FHL (n = 25 [80.0%]; CI, 58%-91%) subgroups (Figure 3C). For the cohort of patients who received etoposide as first-line treatment, the 3-year EFS after initiation of major drug treatment was 70.1% (CI, 56%-80%; n = 57; Figure 3D). All 12 patients who were asymptomatic were alive at the 3-year follow-up (Figure 3E).
Survival and EFS. (A) OS; 3-year pSU for patients with pHLH in the cohort including patients who were asymptomatic: FHL (n = 63) vs hereditary non-FHL (n = 25). (B) Three-year pSU for patients who were symptomatic and received first-line etoposide (n = 57) or other major drug(s) (n = 9). (C) Three-year EFS for all patients with pHLH including patients who were asymptomatic. (D) Three-year EFS for patients who were symptomatic and received etoposide or other major drug(s). In panels A and C, the blue line represents patients with FHL; red line represents patients with hereditary non-FHL. In panels B and D, the green line represents first-line treatment with etoposide and the purple line represents first-line treatment with other major drugs. (E) Overall 3-year pSU of patients who were symptomatic (n = 76; orange line) vs asymptomatic (n = 12; dark turquoise line) at the time of diagnosis. (F) Post-HSCT 3-year pSU of patients who were symptomatic treated with first-line etoposide (n = 52; green line).
Survival and EFS. (A) OS; 3-year pSU for patients with pHLH in the cohort including patients who were asymptomatic: FHL (n = 63) vs hereditary non-FHL (n = 25). (B) Three-year pSU for patients who were symptomatic and received first-line etoposide (n = 57) or other major drug(s) (n = 9). (C) Three-year EFS for all patients with pHLH including patients who were asymptomatic. (D) Three-year EFS for patients who were symptomatic and received etoposide or other major drug(s). In panels A and C, the blue line represents patients with FHL; red line represents patients with hereditary non-FHL. In panels B and D, the green line represents first-line treatment with etoposide and the purple line represents first-line treatment with other major drugs. (E) Overall 3-year pSU of patients who were symptomatic (n = 76; orange line) vs asymptomatic (n = 12; dark turquoise line) at the time of diagnosis. (F) Post-HSCT 3-year pSU of patients who were symptomatic treated with first-line etoposide (n = 52; green line).
Survival after HSCT
Among 75 patients undergoing HSCT, 9 died after the procedure (Table 1); all were patients who were symptomatic. Of these, 8 had received first-line etoposide therapy and 3 had received additional salvage therapy before HSCT (2 emapalumab and 1 alemtuzumab). One patient (CHS) had not received any major drug before HSCT. The causes of death after HSCT were persistent HLH activity (n = 3), infection (n = 4), and vascular event (n = 1), with 1 answer missing. The median time from diagnosis to death was 6.3 months (range, 3.4-12.3 months), whereas the median time from HSCT to death was 3.7 months (range, 0.5-10.0 months), and 2 answers were missing. Deaths occurred in 5 of 9 patients who underwent transplantation from haploidentical donors, 2 of 14 from mismatched unrelated donor, 1 of 40 from MUD, and 1 of 12 from MRD (Table 1).
Summary of patients who died after HSCT
| Patient ID . | Disease . | Remission at HSCT . | Donor . | Conditioning . | Onset to HSCT (d) . | Cause of death . |
|---|---|---|---|---|---|---|
| 07-01-001 | FHL3 | Complete | MUD | RT-MAC (Mel, dose na) | 108 | Reactivated HLH |
| 22-08-001 | FHL2 | No | MRD | RT-MAC (Treo 39 g/m2) | 115 | Infection |
| 22-09-002 | CHS | Complete | Haplo (post-Cy) | RT-MAC (Bu AUC 72 mg × h/L) | 45 | Infection |
| 22-16-001 | FHL2 | Complete | Haplo (CD3 depl) | RT-MAC (Treo 40 g/m2) | 95 | HSCT related |
| 22-22-003 | GS-2 | Complete | Haplo (αβ T depl) | RT-MAC (Treo 37 g/m2) | 95 | HSCT related |
| 22-28-001 | FHL3 | Complete | MMUD | RT-MAC (Treo 45 g/m2) | 131 | Infection |
| 22-35-001 | FHL3 | Partial | MMUD | RT-MAC (Treo 38 g/m2) | 60 | Persistent HLH |
| 26-03-008 | Undefined | na | Haplo (post-Cy) | RT-MAC (Bu, dose na) | na | Persistent HLH |
| 26-03-009 | FHL3 | na | Haplo (na) | na | na | na |
| Patient ID . | Disease . | Remission at HSCT . | Donor . | Conditioning . | Onset to HSCT (d) . | Cause of death . |
|---|---|---|---|---|---|---|
| 07-01-001 | FHL3 | Complete | MUD | RT-MAC (Mel, dose na) | 108 | Reactivated HLH |
| 22-08-001 | FHL2 | No | MRD | RT-MAC (Treo 39 g/m2) | 115 | Infection |
| 22-09-002 | CHS | Complete | Haplo (post-Cy) | RT-MAC (Bu AUC 72 mg × h/L) | 45 | Infection |
| 22-16-001 | FHL2 | Complete | Haplo (CD3 depl) | RT-MAC (Treo 40 g/m2) | 95 | HSCT related |
| 22-22-003 | GS-2 | Complete | Haplo (αβ T depl) | RT-MAC (Treo 37 g/m2) | 95 | HSCT related |
| 22-28-001 | FHL3 | Complete | MMUD | RT-MAC (Treo 45 g/m2) | 131 | Infection |
| 22-35-001 | FHL3 | Partial | MMUD | RT-MAC (Treo 38 g/m2) | 60 | Persistent HLH |
| 26-03-008 | Undefined | na | Haplo (post-Cy) | RT-MAC (Bu, dose na) | na | Persistent HLH |
| 26-03-009 | FHL3 | na | Haplo (na) | na | na | na |
AUC, area under the curve; Bu, busulfan; GS-2, Griscelli syndrome type 2; haplo, haploidentical; MMUD, mismatched unrelated donor; Mel, melphalan; MRD, matched related donor; MUD, matched unrelated donor; na, not available; RT-MAC, reduced-toxicity myeloablative conditioning; Treo, treosulfan.
OS of all patients who underwent transplantation until 3 years after HSCT was 88% (CI, 78%-94%; n = 75; Table 2). Patients in complete remission at HSCT had a similar 3-year pSU (90%; n = 51) as patients in partial or nonremission (91%; n = 22; P = .914, with 2 answers missing). Survival was significantly inferior with a haploidentical donor (3-year pSU, 44%; n = 9) compared with all other types of donors (3-year pSU, 94%; n = 66; P < .001) or mismatched unrelated donors alone (3-year pSU, 86%; n = 14; P = .041). The time from diagnosis to HSCT was longer for patients with haploidentical donors than those with all other types of donors (median, 103 vs 80 days). Outcome after busulfan-based regimens (3-year pSU, 91%; n = 24) was similar compared with outcome after treosulfan-based regimens (3-year pSU, 88%; n = 41; P = .591). Survival among the 22 patients with incomplete donor cell chimerism was 100%. Among the 27 patients with CNS involvement, 21 survived and 20 survived event-free. OS of patients who were symptomatic, underwent transplantation, and were treated with etoposide until 3 years after HSCT was 84.6% (n = 52; CI, 72%-92%; Figure 3F), whereas none of the patients with other first-line therapies who survived until transplantation (5 of 7 with alemtuzumab; and 2 of 2 with amapalumab) died after HSCT. In patients who were symptomatic, the time between diagnosis and HSCT did not significantly influence survival (<3 months after diagnosis: 3-year pSU, 79%; CI, 62%-90%; n = 34; and >3 months after diagnosis: 3-year pSU, 94%; CI, 77%-98%; n = 31; P = .105).
Univariate analysis of factors influencing OS after HSCT
| . | All patients who underwent transplantation . | Symptomatic patients . | Patients with first-line etoposide . | |||
|---|---|---|---|---|---|---|
| n . | OS (CI) . | n . | OS (CI) . | n . | OS (CI) . | |
| 3-y post-HSCT survival∗ | 75 | 88% (78%-94%) | 65 | 86% (75%-93%) | 52 | 85% (72%-92%) |
| Genetic diagnosis | ||||||
| FHL | 56 | 89% (78%-95%) | 49 | 88% (75%-94%) | 41 | 85% (70%-93%) |
| Hereditary non-FHL | 19 | 84% (58%-95%) | 16 | 81% (52%-94%) | 11 | 81% (45%-95%) |
| Remission† | ||||||
| Full | 51 | 90% (78%-96%) | 41 | 88% (73%-95%) | 33 | 88% (71%-95%) |
| Partial or no | 22 | 91% (68%-98%) | 22 | 91% (68%-98%) | 17 | 88% (61%-97%) |
| Time diagnosis to HSCT | ||||||
| <3 mo | 36 | 80% (63%-90%) | 34 | 79% (62%-90%) | 26 | 77% (56%-89%) |
| >3 mo | 39 | 95% (81%-99%) | 31 | 94% (77%-98%) | 26 | 92% (73%-98%) |
| Conditioning‡ | ||||||
| Busulfan based | 24 | 91% (71%-98%) | 18 | 89% (62%-97%) | 13 | 88% (57%-99%) |
| Treosulfan based | 41 | 88% (73%-95%) | 38 | 87% (71%-94%) | 30 | 83% (64%-93%) |
| Donor | ||||||
| Haploidentical | 9 | 44% (14%-72%)∗ | 9 | 44% (14%-72%) | 7 | 43% (10%-73%) |
| MMUD | 14 | 86% (54%-96%) | 13 | 85% (51%-96%) | 9 | 78% (36%-94%) |
| MRD and MUD | 52 | 96% (85%-99%) | 43 | 95% (83%-99%) | 36 | 94% (80%-99%) |
| . | All patients who underwent transplantation . | Symptomatic patients . | Patients with first-line etoposide . | |||
|---|---|---|---|---|---|---|
| n . | OS (CI) . | n . | OS (CI) . | n . | OS (CI) . | |
| 3-y post-HSCT survival∗ | 75 | 88% (78%-94%) | 65 | 86% (75%-93%) | 52 | 85% (72%-92%) |
| Genetic diagnosis | ||||||
| FHL | 56 | 89% (78%-95%) | 49 | 88% (75%-94%) | 41 | 85% (70%-93%) |
| Hereditary non-FHL | 19 | 84% (58%-95%) | 16 | 81% (52%-94%) | 11 | 81% (45%-95%) |
| Remission† | ||||||
| Full | 51 | 90% (78%-96%) | 41 | 88% (73%-95%) | 33 | 88% (71%-95%) |
| Partial or no | 22 | 91% (68%-98%) | 22 | 91% (68%-98%) | 17 | 88% (61%-97%) |
| Time diagnosis to HSCT | ||||||
| <3 mo | 36 | 80% (63%-90%) | 34 | 79% (62%-90%) | 26 | 77% (56%-89%) |
| >3 mo | 39 | 95% (81%-99%) | 31 | 94% (77%-98%) | 26 | 92% (73%-98%) |
| Conditioning‡ | ||||||
| Busulfan based | 24 | 91% (71%-98%) | 18 | 89% (62%-97%) | 13 | 88% (57%-99%) |
| Treosulfan based | 41 | 88% (73%-95%) | 38 | 87% (71%-94%) | 30 | 83% (64%-93%) |
| Donor | ||||||
| Haploidentical | 9 | 44% (14%-72%)∗ | 9 | 44% (14%-72%) | 7 | 43% (10%-73%) |
| MMUD | 14 | 86% (54%-96%) | 13 | 85% (51%-96%) | 9 | 78% (36%-94%) |
| MRD and MUD | 52 | 96% (85%-99%) | 43 | 95% (83%-99%) | 36 | 94% (80%-99%) |
A log-rank test was performed for evaluation of statistical differences between groups for all parameters.
TBI, total body irradiation.
∗Significant haploidentical donor vs nonhaploidentical (P < .001) and MMUD alone (P = .041).
6 patients are included with follow-up time after HSCT between 2.35 and 2.80 years.
2 answers of remission status missing, both patients died within 1 year after HSCT.
10 patients with other conditioning regimes (eg, melphalan-based and TBI).
Discussion
This international registry study provides benchmark data on contemporary (2016-2021) outcome for patients with pHLH treated with standard-of-care protocols in a multicenter setting. Both pre-HSCT and HSCT outcomes were analyzed, allowing for comparison of these 2 important treatment phases with those of other studies. Moreover, to provide outcome data relevant for all patients born with pHLH, we analyzed outcomes of patients who were symptomatic and patients who were asymptomatic at diagnosis. The main finding of the study is that with 83% OS, current prognosis for patients with a diagnosis of pHLH is better than anticipated. In particular, comparison of patients who were symptomatic receiving etoposide-based treatment with the previous HLH94 (1994-1998) and HLH-2004 (2004-2011) studies indicates substantial improvements (Table 3).10-12 Thus, restriction of the analysis to patients who were symptomatic and receiving first-line etoposide yielded a pre-HSCT survival of 92% with an OS of 77% in this study, compared with a pre-HSCT survival and OS of 73% and 50% in HLH94 and 83% and 59% in HLH-2004, respectively.
Comparison of key outcome parameters between the HLH94 and HLH-2004 study cohorts and the current cohort
| . | HLH9411,12 . | HLH-200410 . | Current study (active disease) . | Current study (asymptomatic) . |
|---|---|---|---|---|
| pHLH definition | Affected sibling | Genetics (94%) affected sibling (6%) | Genetics (96%) function (4%) | Genetics 100% |
| Patient number | 60 | 168 FHL and 29 non-FHL (15%) | 56 FHL and 20 non-FHL (26%) | 7 FHL and 5 non-FHL (42%) |
| Median age at onset | 2 mo | 3.4 mo | 5.6 mo | na |
| Treatment (first-line) | HLH94 | HLH-2004 | No major drug 10 (13%) Etoposide 57 (75%) Alemtuzumab 7 (9%) Emapalumab 2 (3%) | 4 Cyclosporin A/IVIG 8 none |
| Median time diagnosis to SCT | 183 d | 148 d | 88 d | 246 d (birth to SCT) |
| Survival before SCT | 44 of 60 (73%) | 140 of 168 (83%) | 69 of 76 (91%) etoposide 52/ of (91%) | 12 of 12 (100%) |
| Survival after SCT | 29 of 44 (66%) | 94 of 135 (70%) | 56 of 65 (86%) etoposide 44 of 52 (85%) | 10 of 10 (100%)∗ |
| OS (CI) | 50% (±13%) (5y) | 59% (52%-67%) (5y) | 79% (68%-86%) (3y) etoposide 77% (64%-86%) | 100% (3y) |
| . | HLH9411,12 . | HLH-200410 . | Current study (active disease) . | Current study (asymptomatic) . |
|---|---|---|---|---|
| pHLH definition | Affected sibling | Genetics (94%) affected sibling (6%) | Genetics (96%) function (4%) | Genetics 100% |
| Patient number | 60 | 168 FHL and 29 non-FHL (15%) | 56 FHL and 20 non-FHL (26%) | 7 FHL and 5 non-FHL (42%) |
| Median age at onset | 2 mo | 3.4 mo | 5.6 mo | na |
| Treatment (first-line) | HLH94 | HLH-2004 | No major drug 10 (13%) Etoposide 57 (75%) Alemtuzumab 7 (9%) Emapalumab 2 (3%) | 4 Cyclosporin A/IVIG 8 none |
| Median time diagnosis to SCT | 183 d | 148 d | 88 d | 246 d (birth to SCT) |
| Survival before SCT | 44 of 60 (73%) | 140 of 168 (83%) | 69 of 76 (91%) etoposide 52/ of (91%) | 12 of 12 (100%) |
| Survival after SCT | 29 of 44 (66%) | 94 of 135 (70%) | 56 of 65 (86%) etoposide 44 of 52 (85%) | 10 of 10 (100%)∗ |
| OS (CI) | 50% (±13%) (5y) | 59% (52%-67%) (5y) | 79% (68%-86%) (3y) etoposide 77% (64%-86%) | 100% (3y) |
IVIG, IV immunoglobulin.
6 of 12 siblings who were asymptomatic underwent transplantation within the first year of life. Another 4 underwent transplantation after age 1 year; 1 patient with XLP2 and 1 with GS-2 did not undergo transplantation within the follow-up period.
How can these improvements be explained? To address this question, it is useful to separately consider the 2 treatment phases. Survival until HSCT or last follow-up if no HSCT performed was 91%, both for the entire cohort and for the etoposide-treated subgroup, which is an improvement compared with the 83% achieved in HLH-2004 and 79% in patients treated with first-line ATG13 and similar to the 92% observed in a prospective study on alemtuzumab first-line therapy.16 The improvement compared with the previous etoposide-based studies cannot be explained by differences in the treatment protocol. Treatment including dose adaptations, made in 15% of cases compared with 17% to 28% of cases in the HLH-2004 study,10 essentially followed consensus recommendations for etoposide-based therapy of HLH issued by the HS.8 It is possible that the more widespread use of salvage therapies, including alemtuzumab, emapalumab, and ruxolitinib,25-27 has contributed to the better pre-HSCT outcome. Salvage therapy was given to 16 of 57 patients receiving etoposide but also to 3 of 9 patients receiving alemtuzumab or emapalumab treatment. Overall, 11 of 19 patients (58%) who received variable salvage therapy survived, which is similar to previous reports.25,26 Notably, increased use of salvage therapy did not improve the proportion of patients achieving full remission compared with previous studies. In our study, full remission at start of conditioning was achieved in 65% of patients, whereas 35% had active disease (partial or nonremission), which is comparable with the results reported for HLH94 (64% vs 36%)12 and the ATG study (56% vs 44%).13 A note of caution is justified because definitions vary and registry information on remission may have limited precision. Although difficult to prove statistically, additional explanations for better pre-HSCT survival could be (1) a more rapid diagnosis due to novel diagnostic algorithms,28-30 resulting in earlier pHLH directed therapy; (2) improved control of triggering infections; and (3) shorter time to HSCT (88 vs 148 days in HLH-2004), reducing the risk of disease reactivation and treatment toxicity before HSCT.
More substantial differences were observed in the HSCT phase of treatment compared with previous etoposide-based treatment studies. OS after HSCT was 88% for patients who were symptomatic (85% for etoposide first-line), which favorably compares with historical data of HLH94 (66%),11 HLH-2004 (70%),10 and an Italian study from 2000 to 2014 (71%).31 A key difference in the HSCT phase was that while in HLH-2004 mostly fully myeloablative busulfan-based conditioning was used, the large majority of patients in this study received reduced-toxicity regimes. Among these, targeted busulfan-based and treosulfan-based regimens led to similar survival. These results align with the excellent HSCT outcomes shown in more recent cohorts with reduced-toxicity regimens in pHLH (survival, 75%-100%), and this likely represents a key factor responsible for the improved outcome of etoposide-treated patients in this study.19-23,32,33 The number of patients treated with alemtuzumab as first-line treatment was too small (n = 7) to draw conclusions from the excellent post-HSCT survival of these patients (100%) in this study.
Although based on small numbers, the poor outcome of patients after HSCT from a haploidentical donor with 44% survival is striking. This finding is similar to other studies reporting on HSCT of patients with pHLH using in vitro and in vivo (post-HSCT cyclophosphamide) T-cell depletion approaches (survival, 50%-68%; n = 6-29).32-34 In contrast, complete remission as opposed to partial remission or nonremission at the time point of HSCT was not associated with better outcome. Although in most of the historical studies disease activity was associated with inferior outcome (58%-64%12 vs 72%-83%35), this difference was not observed in the more recent Italian study31 either. This may be related to less toxicity of contemporary conditioning regimens in the context of persistent disease activity.
Although the Italian study reported that transplantation within 6 months after diagnosis resulted in better EFS than if performed later (69% vs 50%), we did not find that HSCT performed within 3 months improved survival compared with later time points.31 There was even a trend to better survival if HSCT was performed >3 months. Such analysis is limited by the fact that in historical studies, late transplants were frequently because of no or late genetic diagnosis or persistent efforts to induce complete remission, whereas more recently, late transplants frequently reflected organizational delays when dealing with patients with well-controlled disease and less aggressive genetic diagnoses.
Our study also confirms the excellent prognosis if pHLH is diagnosed before the onset of symptoms. In a retrospective collection of 22 patients with pHLH who were diagnosed in the absence of symptoms and remained asymptomatic until HSCT, Lucchini et al reported 95% survival vs 45% in 22 symptomatic index family cases.24 In our prospective registry study, all 12 initially asymptomatic patients survived. This reminds of similar results for patients with severe combined immunodeficiency,36 which have laid the ground for the implementation of newborn screening for this condition and calls for the implementation of newborn screening also for hereditary HLH.
Some limitations of this study should be noted. Similar to any registry study, including HLH94 and HLH-2004, we cannot exclude reporting bias. However, 70% of patients were reported from Germany and Switzerland, where well-established referral structures lead to an excellent capture of cases. On the other hand, the broad international patient recruitment in 27 centers from 10 countries reduces the bias introduced by single-center studies. It should be noted that the proportion of patients with non-FHL diseases, which may be less aggressive, was slightly higher in this study than in previous studies (Table 3). Furthermore, survival analyses were limited to 3 years after diagnosis, and mixed chimerism of variable degree was found in 29% of patients. Patients with donor chimerism <20% to 30% are at risk of reactivation. However, many patients with donor chimerism levels in this range fare well for many years without disease activity.37 Furthermore, post-HSCT survival of patients with pHLH in HLH-2004 only dropped from 72% (1 y) to 71% (5 y),35 and in a large study on HSCT in patients with HLH patients, the 2-year and the 5-year EFS were equal when contemporary conditioning regimens (with an elevated risk of mixed chimerism) had been applied.23
Despite the encouraging results on standard-of-care treatment obtained in this study, results are far from perfect. Studies evaluating new drugs to achieve control of hyperinflammation in pHLH that are more targeted to disease pathogenesis such as emapalumab,15 alemtuzumab,16 or ruxolitinib17,18 are, therefore, more than justified. However, it is important that the results obtained in these studies be compared with contemporary and not historical HLH94 or HLH-2004 data, because this can lead to much uncertainty in the patient and physician communities, particularly, in countries with restricted access to novel agents. Our study shows that with all the experience available,8 at present, etoposide is still a valid drug for the firstline treatment of pHLH.
Acknowledgments
The authors thank additional members of the steering committee of the HLH registry (C. Booth, London, United Kingdom; Eichi Ishii, Ehime, Japan; and D. Moshous, Paris, France) for supporting this study, and Sabrina Daniel for excellent data management. This work was generated within the European Reference Network for Rare Immunodeficiency, Autoinflammatory and Autoimmune Diseases.
This study was supported by Deutsche Kinderkrebsstiftung DKS 2016.04 and DKS 2018.11, Histiozytosehilfe, HistioUK/2017/08/01, and the Förderprogramm Klinische Studien by the Medical Faculty, University of Freiburg, Germany; and also by Deutsche Forschungsgemeinschaft SFB1160 (TPA01) (S.E.); and the Swiss National Science Foundation (320030_205097) (J.P.S.).
Authorship
Contribution: S.B. analyzed the data and drafted the manuscript; S.E. and K.L. conceived the study, coordinated the registry, supervised the analysis, and edited the manuscript; J.-I.H. advised on the configuration of registry data fields; and all other authors contributed patients to the registry and are listed in order of the number of patients contributed to this analysis.
Conflict-of-interest disclosures: M. Hines and K.E.N. receive funding from Incyte for an ongoing clinical trial. K.L., C.L., and M.J. are members of an advisory board for SOBI. M.J. has received research support from SOBI. J.-I.H. is a consultant for Swedish Orphan Biovitrum (SOBI). R.B. received speaker’s honories from MEDAC. The remaining authors declare no competing financial interests.
Correspondence: Stephan Ehl, Institute for Immunodeficiency, Medical Center-University of Freiburg, Breisacher Str 115, 79104 Freiburg, Germany; email: stephan.ehl@uniklinik-freiburg.de.
References
Author notes
K.L. and S.E. have contributed equally to this study.
Data are available on request from the corresponding author, Stephan Ehl (stephan.ehl@uniklinik-freiburg.de). Access to registry data will be provided according to the data sharing rules of the European Society for Immunodeficiencies/Histiocyte Society.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal