Background and Significance: Glucocorticoids have been a mainstay in virtually all therapy regimens for B-lymphoid malignancies since the 1950s. Glucocorticoids selectively kill B-cell leukemia and lymphoma cells but have very limited effects on myeloid and epithelial cells. Likewise, inhibitors of B-cell signaling (e.g. ibrutinib) and selective depletion of B-cells (e.g. rituximab, CD19 CAR-T cells) have achieved important progress and are well tolerated.
Drug discovery tool for selective targeting of B-cell malignancies: For these reasons, we developed an interactive computational tool ( lymphoblasts.org) to identify novel B-cell selective vulnerabilities based on integrated genetic and pharmacological compound screens ( Figure 1). Compound screening data from CTD and GDSC databases were reprocessed by fitting 4-parameter log-logistic dose response curves. Differential gene-dependency was also tested using DEMETER2 and Chronos scores, from Project Achilles RNAi and CRISPR datasets, respectively. To prioritize cell-type specific targets, compounds were ranked by average differential rank percentile of both compound-sensitivity and target-gene dependency.
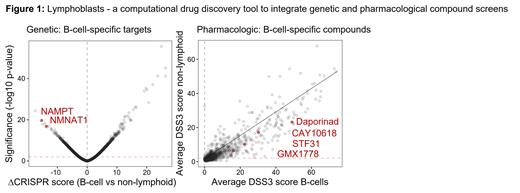
NAD+ biosynthesis as unique B-lymphoid vulnerability: Integration of genetic RNAi and CRISPR-based sensitivity data identified NMNAT1 and NAMPT as top-ranking B-cell selective targets that regulate NAD+ salvage and biosynthesis in the nucleus ( Figure 1). NAMPT, was previously identified as “pre-B-cell colony enhancing factor” and functions as rate-limiting enzyme in the salvage biosynthesis pathway of NAD+, which is critical for cellular energy metabolism. These genetic results were further corroborated by the identification of four small molecule inhibitors of NAMPT, with a striking B-cell-selective sensitivity profile. Compared to a panel of 1,078 myeloid leukemia and solid tumor cell lines, B-cell leukemia and lymphoma cell lines (n=98) were dramatically more sensitive to NAMPT inhibitors, including STF31, CAY10618, GMX1778 and Daporinad ( Figure 1). To verify these results based on the DepMap dataset and our drug discovery tool (lymphoblasts.org), we studied the effects of seven NAMPT inhibitors (CAY10618, FK866, GMX1778, OT82, STF118804, STF31 and KPT9274) on a panel of myeloid leukemia and solid tumor cell lines as well as PDX from patients with B-ALL and mantle cell lymphoma. Interestingly, three of these NAMPT inhibitors have demonstrated favorable PK/PD and safety profiles (NCT04281420, NCT04914845, NCT03921879).
Genetic modeling of NAMPT-deficiency in B-cell malignancies. To model consequences of Nampt deletion in B-ALL, we transformed pre-B cells from Namptfl/fl mice with BCR-ABL1 and NRAS G12D oncogenes. Deletion of Nampt subverted competitive fitness of B-ALL cells, loss of colony-forming ability and cell cycle arrest. Additionally, we developed a conditional mouse model ( Namptfl/fl x Mb1-Cre) for B-cell-specific Nampt deletion, which resulted near-complete ablation of B-lymphopoiesis. Moreover, in vitro inducible deletion of Nampt caused rapid depletion and reduced colony formation in cultured bone marrow pre-B cells. Deletion of Nampt profoundly affected B-cell energy metabolism, significantly reduced FCCP-stimulated OCR and resulted in cell death owing to energy stress. Metabolomic studies of genetic Nampt-deletion using mass spectrometry revealed depletion of metabolites involved in amino acid metabolism, purine/pyrimidine metabolism, and the TCA cycle. Notably, phosphoserine was among the top-ranked downregulated metabolites, suggesting the involvement of one carbon pathway disruption in mediating energy crisis upon Nampt-deletion in B-ALL cells.
Conclusions: These findings highlight that the classical ‘glucocorticoid paradigm’ can be extended to discover novel vulnerabilities, including NAD+ biosynthesis. Computational integration of genetic and pharmacological compound screens was particularly powerful in identifying previously unrecognized opportunities to leverage selective vulnerabilities of B-lymphoid leukemia and lymphoma. Our genetic studies corroborate the unique role of NAMPT and NMNAT1 in B-cell development and metabolic regulation. Further studies will focus on efforts to repurpose three clinically approved NAMPT small molecule inhibitors for the treatment of refractory B-cell malignancies.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal