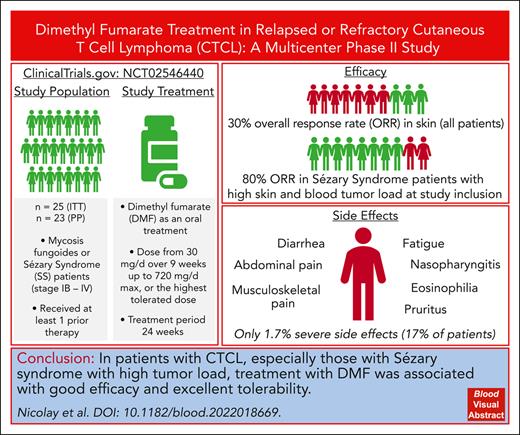
Key Points
DMF treatment shows good efficacy in treatment of patients with CTCL, especially for Sézary syndrome with high tumor load.
The tolerability of DMF in CTCL is excellent compared with other established or experimental CTCL treatments.
Abstract
Targeted therapies for cutaneous T-cell lymphoma (CTCL) are limited and curative approaches are lacking. Furthermore, relapses and drug induced side effects are major challenges in the therapeutic management of patients with CTCL, creating an urgent need for new and effective therapies. Pathologic constitutive NF-κB activity leads to apoptosis resistance in CTCL cells and, thus, represents a promising therapeutic target in CTCL. In a preclinical study we showed the potential of dimethyl fumarate (DMF) to block NF-κB and, specifically, kill CTCL cells. To translate these findings to applications in a clinical setting, we performed a multicentric phase 2 study evaluating oral DMF therapy in 25 patients with CTCL stages Ib to IV over 24 weeks (EudraCT number 2014-000924-11/NCT number NCT02546440). End points were safety and efficacy. We evaluated skin involvement (using a modified severity weighted assessment tool [mSWAT]), pruritus, quality of life, and blood involvement, if applicable, as well as translational data. Upon skin analysis, 7 of 23 (30.4%) patients showed a response with >50% reduction in the mSWAT score. Patients with high tumor burden in the skin and blood responded best to DMF therapy. Although not generally significant, DMF also improved pruritus in several patients. Response in the blood was mixed, but we confirmed the NF-κB–inhibiting mechanism of DMF in the blood. The overall tolerability of the DMF therapy was very favorable, with mostly mild side effects. In conclusion, our study presents DMF as an effective and excellently tolerable therapeutic option in CTCL to be further evaluated in a phase 3 study or real-life patient care as well as in combination therapies. This trial was registered at www.clinicaltrials.gov as #NCT02546440.
Introduction
Cutaneous T-cell lymphomas (CTCLs) include a heterogeneous group of malignant lymphoid neoplasias that primarily affect the skin. Mycosis fungoides (MF) and Sézary syndrome (SS) represent the most common subtypes of CTCL.1 These diseases are characterized by high progression and relapse rates, even upon initially highly effective therapies. To date, besides allogeneic stem cell transplantation, which has been shown to cause long-term complete remissions in a subset of patients, no curative treatment exists.2-5 Especially in advanced-stage MF/SS, this leads to decreased overall survival (OS) and high disease burden6,7 Furthermore, the quality of life is frequently reduced, and in addition to their tumor burden, patients experience symptoms such as pruritus or pain that are often vexing.8,9 Conventional therapies for CTCL, to date, are limited because of the short time to the next treatment, restricted efficacy, or poor tolerability.10 In recent years, new targeted therapies with higher efficacy were established for CTCL, but even they are restricted by either unfavorable side effect profiles or short duration of response and do not achieve response rates >50%.11-13 Therefore, there is a substantial need for the development of novel targeted treatment options for CTCL characterized by both high efficacy, especially in advanced-stage, relapsed or refractory MF/SS; and a good tolerability, reflected by a favorable side effect profile.9,14
CTCL cells are difficult to target therapeutically, because they resemble resting T cells in many aspects and are characterized by cell death resistance rather than hyperproliferation. One factor that substantially contributes to cell death resistance in CTCL cells is the constitutive activation of the transcription factor NF-κB.15 Different cellular and molecular mechanisms and alterations that maintain NF-κB activation in CTCL cells are described at both genetic and functional level and, thus, lead to cell death resistance.16,17 Overcoming cell death resistance by blocking NF-κB, thus, represents an interesting therapeutic approach but is often limited by the toxicity of the respective NF-κB inhibitors.15,18
In 2016, we showed that the small-molecule compound dimethyl fumarate (DMF) very effectively inhibits NF-κB in primary T cells of patients with CTCL, in CTCL cell lines, and in a CTCL xenograft mouse model and, thus, specifically induces cell death in the malignant T cells, sparing benign bystander T cells.19 In addition, it not only presents as a highly effective and well-tolerable monotreatment option in preclinical studies but also as a promising partner for targeted combination therapies in CTCL.20 The exact mechanisms of DMF on NF-κB and its upstream signaling are still subject to intensive research, but several targets and pathways were identified through which DMF influences NF-κB signaling, including thioredoxin-1 and the antioxidative defense.21 DMF is already in safe, clinical use in Germany for the treatment of psoriasis since 1995 and in Europe since 2017.22 Furthermore, its immunomodulatory properties and T-cell targeting led to its approval for therapy of multiple sclerosis in 2012, for which it has since also been used safely and effectively worldwide.23
To date, there is no literature on the clinical use of DMF for treating patients with CTCL, although the preclinical data and immunologic effects would suggest so.19,21 Therefore, to fill this gap and broaden the therapeutic spectrum in the care for patients with CTCL, we performed a phase 2 clinical study to confirm the effects seen ex vivo and in the mouse model in vivo in patients with CTCL. Here, we report the results of a multicenter phase 2 trial performed in the German Dermato-Oncologic Working Group network on DMF in the treatment of patients with relapsed or refractory MF or SS (EudraCT number, 2014-000924-11/NCT number, NCT02546440).
Materials and methods
Study design
This was an open-label, single-arm phase 2 trial performed in 6 centers in Germany. The main objective was to investigate the efficacy and safety of DMF in 25 patients with relapsed or refractory MF or SS (from stages Ib to IV) and at least 1 topical or systemic pretreatment over a 24-week medication phase and subsequently, a 4-week follow-up. The primary end point of the study was tumor response in the skin. Secondary end points included progression -free survival, OS, pruritus measured by a visual analog scale, and quality of life measured using the dermatologic life quality index (DLQI) questionnaire.
The study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice, the Declaration of Helsinki, and the ethical guidelines of the respective study center institutions. All study contents were approved by the German federal authorities (BfArM) as well as the local ethics review boards of each study center.
Patients
In total, 28 patients were screened. The study enrolled 25 patients who were aged ≥18 years with histologically/cytologically confirmed relapsed or refractory MF or SS and had failure with at least 1 prior therapy (completed ≥4 weeks before study entry). Further eligibility criteria were having a Karnofsky index ≥70%24 or Eastern Cooperative Oncology Group performance status <2 and the ability to give consent. Pregnant or lactating women as well as patients without an adequate organ function of liver and kidney or with severe systemic diseases or infections at study entry were not eligible for the study. Moreover, all contraindications for a therapy with DMF were exclusion criteria, as well as elevation of liver enzyme levels >2× normal value, creatinine clearance rate <50 mL/min, leukocyte counts <3000/μL, and lymphocyte counts <700/μL. During the duration of the study, CTCL therapies besides the study drug, with the exception of topical corticosteroids, were not allowed. Simultaneous participation in other clinical studies was excluded. The baseline characteristics of the per-protocol study population are depicted in Table 1 and supplemental Table 1, available on the Blood website.
Baseline characteristics and skin best overall response (mSWAT score decrease of >50%) in patients in the per-protocol cohort (n = 23)
| Characteristics . | n . | % . | Best overall response (skin) . | ||
|---|---|---|---|---|---|
| n . | % . | 95% CI . | |||
| Overall | 23 | 100.0 | 7 | 30.4 | 13.2-52.9 |
| Age | |||||
| <65 y | 8 | 34.8 | 3 | 37.5 | 8.5-75.5 |
| ≥65 y | 15 | 65.2 | 4 | 26.7 | 7.8-55.1 |
| Sex | |||||
| Male | 17 | 73.9 | 6 | 35.3 | 14.2-61.7 |
| Female | 6 | 26.1 | 1 | 16.7 | 0.4-64.1 |
| Disease | |||||
| MF | 11 | 47.8 | 3 | 27.3 | 6.0-61.0 |
| SS | 12 | 52.2 | 4 | 33.3 | 9.9-65.1 |
| Stage | |||||
| IB | 7 | 30.4 | 2 | 28.6 | 3.7-71.0 |
| II/III | 4 | 17.4 | 1 | 25 | 0.6-80.6 |
| IV | 12 | 52.2 | 4 | 33.3 | 9.9-65.1 |
| Superficial radiotherapy before baseline | |||||
| Yes | 6 | 26.1 | 1 | 16.7 | 0.4-64.1 |
| No | 17 | 73.9 | 6 | 35.3 | 14.2-61.7 |
| Photopheresis before baseline | |||||
| Yes | 11 | 47.8 | 4 | 36.4 | 10.9-69.2 |
| No | 12 | 52.2 | 3 | 25 | 5.5-57.2 |
| Local PUVA before baseline | |||||
| Yes | 15 | 65.2 | 4 | 26.7 | 7.8-55.1 |
| No | 7 | 30.4 | 3 | 42.9 | 9.9-81.6 |
| Unknown | 1 | 4.3 | 0 | 0 | 0-97.5 |
| Baseline mSWAT score | |||||
| 1-50 | 9 | 39.1 | 2 | 22.2 | 2.8-60.0 |
| 51-100 | 6 | 26.1 | 1 | 16.7 | 0.4-64.1 |
| 101-150 | 3 | 13.0 | 1 | 33.3 | 0.8-90.6 |
| >150 | 5 | 21.7 | 3 | 60 | 14.7-94.7 |
| Baseline blood Sézary cell count∗ | |||||
| <1000 | 1 | 8.3 | 0 | 0 | 0-97.5 |
| ≥1000 | 6 | 50.0 | 4 | 66.7 | 22.3-95.7 |
| Unknown | 5 | 41.7 | 0 | 0 | 0-52.2 |
| Characteristics . | n . | % . | Best overall response (skin) . | ||
|---|---|---|---|---|---|
| n . | % . | 95% CI . | |||
| Overall | 23 | 100.0 | 7 | 30.4 | 13.2-52.9 |
| Age | |||||
| <65 y | 8 | 34.8 | 3 | 37.5 | 8.5-75.5 |
| ≥65 y | 15 | 65.2 | 4 | 26.7 | 7.8-55.1 |
| Sex | |||||
| Male | 17 | 73.9 | 6 | 35.3 | 14.2-61.7 |
| Female | 6 | 26.1 | 1 | 16.7 | 0.4-64.1 |
| Disease | |||||
| MF | 11 | 47.8 | 3 | 27.3 | 6.0-61.0 |
| SS | 12 | 52.2 | 4 | 33.3 | 9.9-65.1 |
| Stage | |||||
| IB | 7 | 30.4 | 2 | 28.6 | 3.7-71.0 |
| II/III | 4 | 17.4 | 1 | 25 | 0.6-80.6 |
| IV | 12 | 52.2 | 4 | 33.3 | 9.9-65.1 |
| Superficial radiotherapy before baseline | |||||
| Yes | 6 | 26.1 | 1 | 16.7 | 0.4-64.1 |
| No | 17 | 73.9 | 6 | 35.3 | 14.2-61.7 |
| Photopheresis before baseline | |||||
| Yes | 11 | 47.8 | 4 | 36.4 | 10.9-69.2 |
| No | 12 | 52.2 | 3 | 25 | 5.5-57.2 |
| Local PUVA before baseline | |||||
| Yes | 15 | 65.2 | 4 | 26.7 | 7.8-55.1 |
| No | 7 | 30.4 | 3 | 42.9 | 9.9-81.6 |
| Unknown | 1 | 4.3 | 0 | 0 | 0-97.5 |
| Baseline mSWAT score | |||||
| 1-50 | 9 | 39.1 | 2 | 22.2 | 2.8-60.0 |
| 51-100 | 6 | 26.1 | 1 | 16.7 | 0.4-64.1 |
| 101-150 | 3 | 13.0 | 1 | 33.3 | 0.8-90.6 |
| >150 | 5 | 21.7 | 3 | 60 | 14.7-94.7 |
| Baseline blood Sézary cell count∗ | |||||
| <1000 | 1 | 8.3 | 0 | 0 | 0-97.5 |
| ≥1000 | 6 | 50.0 | 4 | 66.7 | 22.3-95.7 |
| Unknown | 5 | 41.7 | 0 | 0 | 0-52.2 |
CI per Clopper-Pearson.
For more detailed information on the study patient population, see supplemental Table 1.
Analyses are restricted to patients with Sézary syndrome.
Study treatment and assessments
Within the trial, patients received DMF as the active component of a mixture of different fumaric acid esters as an oral treatment starting with 30 mg per day. The dose was escalated weekly by 30 mg per day up to 120 mg per day. Then, the DMF treatment was further escalated in steps of 120 mg per day weekly, up to a maximum dose of 720 mg per day after 9 weeks or, if patients had clinically relevant side effects, up to the highest dose tolerated. Subsequently, the treatment was continued up to 24 weeks in total, with a follow-up of 4 weeks (supplemental Figure 1). Study treatment was discontinued earlier in case of unacceptable adverse events (AEs), disease progression, withdrawal of consent, or per the investigator’s decision. Topical steroids were allowed as concomitant treatment but neither systemic steroids nor any other systemic CTCL therapies, topical chemotherapy, nor extracorporeal photopheresis (ECP).
Safety was monitored throughout the study and for 4 weeks after end of treatment. Safety assessments were performed every other week for 16 weeks, then every 4 weeks until the last visit. AEs were assessed in accordance with the National Cancer Institute Common Terminology Criteria for AEs (CTCAEs) version 4.0.
Responses were assessed based on the consensus global response criteria for CTCL,9,25,26 and their values were compared with baseline values. The assessments included different methods, depending on the organs involved. For quantitative assessment of skin disease burden, we used the modified severity weighted assessment tool (mSWAT) in all patients. Possible lymph node or organ involvement were assessed via sonography. In patients with CTCL blood involvement, we used flow cytometry to quantitatively detect the CTCL/Sézary cells in the peripheral blood. The overall global response was calculated by combining the response in the respective compartments, when applicable. Stable disease was defined as the failure to reach complete or partial response with no evidence of progressive disease in any compartment.25 The detailed list of study-specific CTCL disease assessments and DMF therapy surveillance for all visits including baseline is depicted in supplemental Figure 1 and supplemental Table 1.
As additional secondary end points, we assessed the quality of life, measured using the DLQI questionnaire, as well as pruritus and pain using a numeric rating scale (NRS).
We performed translational experiments using blood samples from 3 patients with SS who finished the study. Skin samples of 6 study patients (3 responders and 3 nonresponders) were used for translational experiments.
Skin IHC and CODEX staining
Reagents and T cells
For ex vivo experiments, DMF was purchased from Sigma-Aldrich. Primary CD4+ T cells were isolated from heparinized whole blood, as indicated.19,20 Then, the CD4+ cells were cultured in RPMI medium, with L-glutamine and sodium bicarbonate. Fetal calf serum (10%) (Sigma-Aldrich) and 1% penicillin-streptomycin were added. Cells were cultured at a concentration of 2 × 106/mL, as indicated.
Flow cytometry and cell death assays
The CTCL tumor burden in the peripheral blood was assessed via flow cytometry (fluorescence-activated cell sorter CANTO II, Becton Dickinson), using antibodies directed against CD3, CD4, CD7, CD26, and CD158k. The malignant T-cell population was defined as indicated by typical aberrancies in these markers to determine the patients’ B stage.30
Cell death rates of T or CTCL cells from patients were also measured via flow cytometry. Dead cells were recognized as a distinct population by a lower signal in the forward and side scatter. This equation was used to calculate specific cell death rates: specific cell death % = (% cell death − % spontaneous cell death) ÷ (100% − % spontaneous cell death) × 100.19
NF-κB luciferase assay
To measure NF-κB activity, a luciferase reporter construct containing 4 copies of the NF-κB consensus sequence (GGAAATTCCCC) in the pTATA-Luc vector was used. A Renilla luciferase-expression reporter pRL-TK plasmid (Promega, Mannheim, Germany) was used as the control of transfection efficiency. Patient T cells (1×106 cells) were transfected with 2 μg pRL-TK plasmid and 5 μg luciferase reporter construct via electroporation. After a 72-hour recovery, the cell population was split, and each half was treated with DMF (50 μM) or vehicle (dimethyl sulfoxide) for 24 hours. Luciferase activity was determined as previously described.19,31
Statistical analyses
The complete-analysis cohort included all patients who started the treatment. The per-protocol cohort included all patients of the complete-analysis cohort except those who/whose
entered the study although they did not satisfy the entry criteria,
treatment was not stopped upon progressive disease,
took concomitant medication that affects or violates the inclusion and exclusion criteria, and
response assessment was not performed >12 weeks after start of treatment.
The primary end point of this study was the objective tumor response after an oral DMF treatment period of 24 weeks. Tumor response (improvement) was initially defined by a decrease in mSWAT score of at least 30% and, based on the updated EORTC ISCL recommendations, was later additionally defined as a decrease in mSWAT score of ≥50%.25,26 The response was estimated in all patients of the complete-analysis cohort using relative frequencies. The conditions of patients who did not stop therapy upon progressive disease as well as those of patients who started a new therapy were regarded as failures. The 95% confidence interval (CI) was estimated using the method of Clopper and Pearson. The study should have been stopped for futility if none of the first 18 patients had shown partial response at least.
Best overall response is defined as showing complete response (CR) or partial response (PR) at least once within 28 weeks. As an conservative approach, patients with no mSWAT values after baseline were regarded as having progressed.
Subgroup analyses were conducted for the best overall response and best confirmed overall response in various subgroups (Table 1).
Furthermore, the (best) relative change in mSWAT score within 28 weeks as well as Sézary cell count and CD4:CD8 ratio was analyzed using standard descriptive measures.
The Kaplan-Meier method was used to determine progression-free survival (progression as defined earlier25 or as defined by the physician) and OS. AEs were analyzed descriptively using MedDRA coding, once in total and, in addition, restricted once to serious AEs and once to AEs of grade ≥3 per the CTCAE. Statistical analyses were performed using SAS version 9.4. All aforementioned analyses, except the primary end point, were planned after all data were fully known. The statistical analyses concerning the clinical evaluations and assessments were performed by the Coordination Center for Clinical Studies, Heidelberg.
Results
Disposition
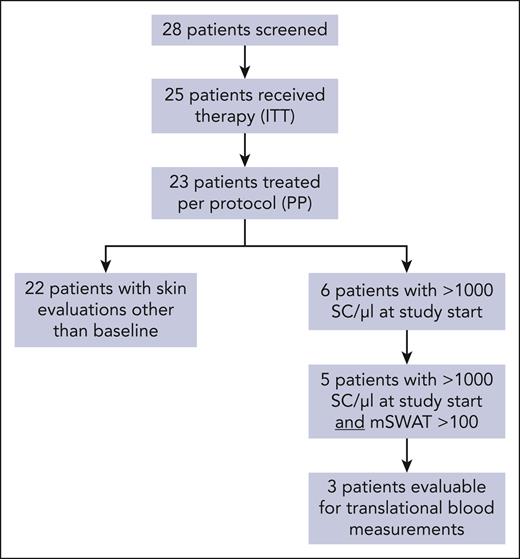
We screened 28 patients, of whom 25 patients with histologically confirmed CTCL were enrolled. Of these, 13 had MF, and 12 had SS without large-cell transformation, although this was not an exclusion criterion. Two protocol deviations reduced the per-protocol cohort of evaluated patients to 23 (11 with MF and 12 with SS; Figure 1). All results are displayed for the per-protocol cohort, except for the primary analysis. Here, the full analysis cohort is used.
In total, 11 patients of the per-protocol cohort discontinued the study prematurely, 5 of whom because of disease progression, 2 because of lack of efficacy, and 1 because of AEs (death not related to study drug). Of the patients in the per-protocol set, 3 patients withdrew their consent during the study. The criterion for study discontinuation because of futility was not fulfilled.
Baseline patient characteristics
Mean age of patients in the per-protocol cohort was 64.43 ± 15.37 years, with an average of 3.26 ± 1.18 different types of prior topical or systemic therapy before the start of the study. The further baseline characteristics of the per-protocol cohort are shown in Table 1 and supplemental Table 1. Twelve patients (52.2%) had stage IV disease, 6 fulfilled the criteria for B2 blood involvement at study entry. 8 patients had an mSWAT score of >100 at screening, 5 of whom with an mSWAT score of >150. Fifteen patients received prior local PUVA treatment, and 11 patients received photopheresis.
Primary end point analysis
A decrease in mSWAT score of at least 30% after 24 weeks, the original primary end point, was achieved in 6 patients in the full analysis cohort (24%; 95% CI, 9.4-45.1).
The primary end point analysis was repeated, regarding a decrease in mSWAT score of at least 50% after 24 weeks per the actualized EORTC ISCL recommendations on study end points.25,26 A decrease in mSWAT score of at least 50% after 24 weeks was shown by 5 patients in the complete-analysis cohort (20%; 95% CI, 6.8-40.7).
Clinical efficacy in the per-protocol population
The median DMF dose for the per-protocol study patients (n = 22) was 402.4 mg per day, which is 56% of the maximum dose approved for psoriasis or multiple sclerosis. In the complete-analysis cohort receiving at least 2 doses of DMF, response in the skin did not correlate to DMF exposure or dosage, with a Spearman correlation coefficient of 0.1, but the study was not powered or designed to investigate such a correlation (supplemental Figure 2). Because the therapeutic effects of DMF in CTCL were expected to be strongest in the skin and the blood and because the regulative authorities and local ethics committee required us to reduce invasive diagnostic measures to a minimum, we mainly assessed responses in the skin and blood, as was applicable.
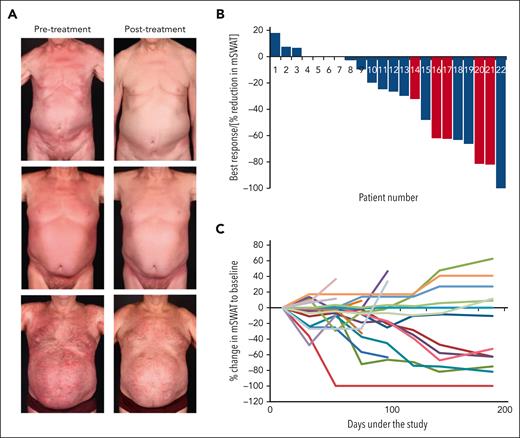
In the per-protocol set, the best overall response rate of the skin was 30.4% (7; 95% CI, 13.2-52.9), with 1 complete global response. Blood stage and high skin tumor burden appeared to correlate with the improvement to therapy. Of the patients with B2 stage and those with an mSWAT score of >100, 4 of 6 (67%) and 4 of 8 (50%), respectively, showed a response in the skin (CR, 1 and PR, 3) to DMF treatment within the study (Figure 2A; Table 1; supplemental Table 3). Most interestingly, of the 5 patients who had Sézary syndrome with high tumor burden in the blood and skin (B2 blood involvement and an mSWAT score >100), 4 (80%) showed a relevant improvement of >50% reduction in mSWAT score as the best response (Figure 2B). The skin was the compartment with the most and deepest responses, whereas only 1 patient (16.7%) with a CR showed responses in the blood (supplemental Figure 3). In most patients with a response, this remained stable over time until the end of the study (Figure 2C). Thirteen (56.5%) patients showed a progression during the study, and 1 patient died (pneumonia at day 13, which was unrelated to thestudy therapy).
Efficacy of DMF treatment in CTCL. (A) Representative clinical pictures of responding patients at the start and the end of study treatment. (B) Waterfall plot of best response in the skin of the patients in the per-protocol cohort, depicted by a reduction in the mSWAT score. Patients with SS with high tumor load (mSWAT score > 100 and SC count > 1000/μL at study start) are depicted in red. (C) Spider plot of response in the skin for all patients in the per-protocol cohort throughout the study. One patient in the per-protocol cohort who died had no mSWAT values after baseline and therefore could not be evaluated for response.
Efficacy of DMF treatment in CTCL. (A) Representative clinical pictures of responding patients at the start and the end of study treatment. (B) Waterfall plot of best response in the skin of the patients in the per-protocol cohort, depicted by a reduction in the mSWAT score. Patients with SS with high tumor load (mSWAT score > 100 and SC count > 1000/μL at study start) are depicted in red. (C) Spider plot of response in the skin for all patients in the per-protocol cohort throughout the study. One patient in the per-protocol cohort who died had no mSWAT values after baseline and therefore could not be evaluated for response.
The median progression-free survival time was 23 weeks (95% CI, 14.00 to not estimable; supplemental Figure 4). The median OS or duration of response were not estimable, but the individual duration of response for respective patients is presented in supplemental Table 4.
Effect on pruritus and the quality of life
In addition, we assessed the effect of the DMF study treatment on pruritus (measured using an NRS) and the quality of life (assessed using the DLQI questionnaire). Relatively, the mean DLQI value did not change, from 21.39 at screening to 21.67 at the individual end of treatment and to 20.00 at follow-up in the 12 patients of the per-protocol set, with all 3 values available. There was no relevant change in pain, as measured using the NRS, during the study. Nevertheless, on average, pain was reported as <2 of 10 points on an NRS and thus was rather irrelevant. Pruritus, however, decreased, on average, from 3.00 points (of 10 points) at screening to 2.17 points at end of treatment and to 2.36 points at follow-up in the 12 patients, with all 3 values available. Therefore, the study medication could relieve pruritus in several patients with CTCL, which is often one of the most vexing symptoms.9
Safety
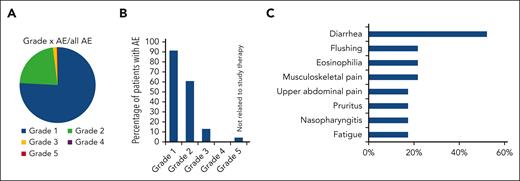
Regarding safety, all patients of the per-protocol cohort had at least 1 AE; 75.9% of the AEs were classified as mild, 1.7% of all AEs were classified as severe (CTCAE grade ≥3), and 4 of 23 patients (17.4%) experienced an AE of grade ≥3, of whom 1 patient died because of an AE that was not related to the study treatment or the disease treated in the study (Figure 3A-B; Table 2). Of the 5 AEs of grade ≥3 that occurred in 4 patients, only 1 was classified as related to DMF study therapy. Of the AEs, 70.1% could be resolved under the study, and 88.8% of the AEs did not even necessitate a dose adjustment within the study. Three patients experienced an AE that required the drug to be withdrawn. The main side effects observed under the study were diarrhea (52.2% of the patients experienced diarrhea at least once), eosinophilia (21.7%), pain in extremity (21.7%), flushing (21.7%), upper abdominal pain (17.4%), fatigue (17.4%), pruritus (17.4%), and nasopharyngitis (17.4%) (Figure 3C). Detailed AEs per affected organ systems are listed in Table 2.
AEs with DMF study therapy. (A) AEs sorted based on CTCAE grades. Depicted is the percentage of AEs of each grade based on the total number of AEs. (B) Percentage of patients who experienced an AE of each CTCAE grade. (C) Most frequent AE with DMF study therapy sorted based their frequency and depicted by the percentage of patients in the per-protocol cohort (n = 23) who experienced them. All AEs that occurred in >10% of patients are listed.
AEs with DMF study therapy. (A) AEs sorted based on CTCAE grades. Depicted is the percentage of AEs of each grade based on the total number of AEs. (B) Percentage of patients who experienced an AE of each CTCAE grade. (C) Most frequent AE with DMF study therapy sorted based their frequency and depicted by the percentage of patients in the per-protocol cohort (n = 23) who experienced them. All AEs that occurred in >10% of patients are listed.
AEs based on system organ class in the per-protocol cohort (n = 23)
| . | AEs . | AEs (CTCAE grade ≥3) . |
|---|---|---|
| Total | 241/23 (100.0%) | 5/4 (17.4%) |
| Gastrointestinal disorders | 87/16 (69.6%) | 1/1 (4.3%) |
| Reduction in general state of health, fatigue | 19/11 (47.8%) | |
| Infections | 13/9 (39.1%) | 3/2 (8.7%) |
| Skin and subcutaneous tissue disorders | 27/10 (43.5%) | |
| Nervous system disorders | 11/6 (26.1%) | |
| Blood and lymphatic system disorders | 11/7 (30.4%) | |
| Musculoskeletal and connective tissue disorders | 12/6 (26.1%) | |
| Vascular disorders | 23/6 (26.1%) | 1/1 (4.3%) |
| Abnormalities of laboratory values of liver enzymes, CRP, or blood count | 7/5 (21.7%) | |
| Injury or orthostatic problems | 7/4 (17.4%) | |
| Psychiatric disorders | 4/4 (17.4%) | |
| Respiratory, thoracic, and mediastinal disorders | 4/4 (17.4%) | |
| Metabolism and nutrition disorders | 4/3 (13.0%) | |
| Cardiac disorders | 3/2 (8.7%) | |
| Ear and labyrinth disorders | 2/2 (8.7%) | |
| Eye disorders | 2/2 (8.7%) | |
| Neoplasms: benign, malignant, and unspecified (including cysts and polyps) | 2/2 (8.7%) | |
| Renal and urinary disorders | 2/2 (8.7%) | |
| Reproductive system and breast disorders | 1/1 (4.3%) |
| . | AEs . | AEs (CTCAE grade ≥3) . |
|---|---|---|
| Total | 241/23 (100.0%) | 5/4 (17.4%) |
| Gastrointestinal disorders | 87/16 (69.6%) | 1/1 (4.3%) |
| Reduction in general state of health, fatigue | 19/11 (47.8%) | |
| Infections | 13/9 (39.1%) | 3/2 (8.7%) |
| Skin and subcutaneous tissue disorders | 27/10 (43.5%) | |
| Nervous system disorders | 11/6 (26.1%) | |
| Blood and lymphatic system disorders | 11/7 (30.4%) | |
| Musculoskeletal and connective tissue disorders | 12/6 (26.1%) | |
| Vascular disorders | 23/6 (26.1%) | 1/1 (4.3%) |
| Abnormalities of laboratory values of liver enzymes, CRP, or blood count | 7/5 (21.7%) | |
| Injury or orthostatic problems | 7/4 (17.4%) | |
| Psychiatric disorders | 4/4 (17.4%) | |
| Respiratory, thoracic, and mediastinal disorders | 4/4 (17.4%) | |
| Metabolism and nutrition disorders | 4/3 (13.0%) | |
| Cardiac disorders | 3/2 (8.7%) | |
| Ear and labyrinth disorders | 2/2 (8.7%) | |
| Eye disorders | 2/2 (8.7%) | |
| Neoplasms: benign, malignant, and unspecified (including cysts and polyps) | 2/2 (8.7%) | |
| Renal and urinary disorders | 2/2 (8.7%) | |
| Reproductive system and breast disorders | 1/1 (4.3%) |
The values are given as x/y (z.z%): x, number of events; y, number of patients with events; and z.z, percentage of patients with events.
CRP, C-reactive protein.
Influence of DMF on NF-κB activity and cell death in CTCL cells of the skin and blood
In 3 patients with SS who completed study procedures we had the opportunity to assess the T-cell death of the malignant T-cell population in the peripheral blood and the NF-κB activity upon DMF therapy, because we had shown in 2016 that DMF induces cell death mainly via inhibition of NF-κB in CTCL cells.19 These patients included patient 2 who responded in the blood with a complete remission (B2 to B0) and patients 3 and 4 who showed a good PR in the skin, as observed in patient 2 but with no blood involvement (Figure 1; supplemental Figure 2). In parallel, we also investigated these effects in skin samples of 3 study patients each who responded and did not respond to treatment, respectively.
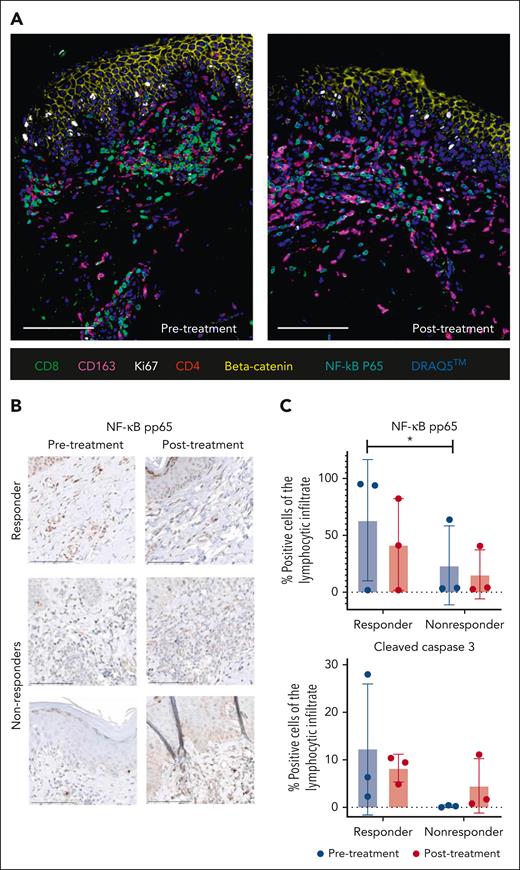
Firstly, we stained skin samples for different cell proliferation, cell death, and cell type–defining markers using IHC and CODEX highly multiplexed tissue imaging. In a responder after therapy, we observed lower levels of p65 as a readout for NF-κB activity and decreased numbers of CD4+ and CD8+ T cells, whereas CD163+ macrophages increased in the dermal infiltrate (Figure 4A). IHC staining revealed that pretreatment pp65 levels were significantly higher in responders vs nonresponders, and that these levels decreased under therapy, suggesting that NF-κB activity correlates with response to DMF therapy in this small cohort (Figure 4B-C). Apoptotic cell numbers, reflected by cleaved caspase 3 staining, were also higher in pretreatment responders vs nonresponders but the differences did not reach statistical significance (Figure 4C).
Skin response to DMF correlates with NF-κB, changes in microenvironment and T-cell death. (A) CODEX 7-color overlay images from skin samples of a representative responders before and after DMF study treatment. The paraffin samples were stained for CD8, CD163, CD4, β-catenin, and NF-κB p65, and the DNA dye DRAQ5 was used for nuclear staining. (B) Representative IHC pictures of skin samples from 1 responder and 2 nonresponders stained for NF-κB pp65 before and after study DMF treatment. (C) Statistical analysis of pp65 and cleaved caspase-3 staining in 3 representative responders and 3 nonresponders before and after DMF study therapy. Depicted is the percentage of positive lymphocytes from the complete lymphocytic infiltrate. ∗P < .05.
Skin response to DMF correlates with NF-κB, changes in microenvironment and T-cell death. (A) CODEX 7-color overlay images from skin samples of a representative responders before and after DMF study treatment. The paraffin samples were stained for CD8, CD163, CD4, β-catenin, and NF-κB p65, and the DNA dye DRAQ5 was used for nuclear staining. (B) Representative IHC pictures of skin samples from 1 responder and 2 nonresponders stained for NF-κB pp65 before and after study DMF treatment. (C) Statistical analysis of pp65 and cleaved caspase-3 staining in 3 representative responders and 3 nonresponders before and after DMF study therapy. Depicted is the percentage of positive lymphocytes from the complete lymphocytic infiltrate. ∗P < .05.
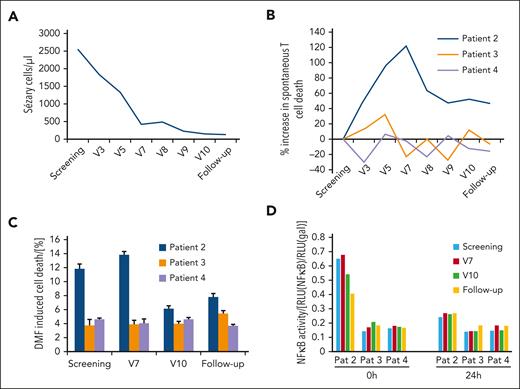
We also assessed NF-κB activity and cell death in patients’ blood Sézary cells (SCs). In Figure 5A, the blood response in patient 2 is depicted. Initially, there was a dramatic decrease of SCs in the blood from >2500/μL at screening to <500/μL at seventh follow-up visit, which then continued at a slower rate to <130/μL at subsequent follow-up. We isolated blood CD4+ T cells of the 3 patients at screening, visit 3, and every 4 weeks thereafter until the follow-up, and measured spontaneous cell death of the CD26− population (Figure 5B). We found that throughout all visits the relative T-cell death rate compared with that of the screening baseline was at least twofold higher in patient 2 compared with that of patients 3 and 4, which hints toward a higher cell death sensitivity toward DMF in this patient. Although cell death rates did not change in patients who did not respond, relative cell death rates in patient 2 increased until visit 7 and then dropped again and remained at a constant level. The spontaneous cell death correlates very well with the blood response to DMF therapy, for which, at visit 7, saturation was almost reached.
Blood response upon DMF treatment depends on SC cell death sensitivity and NF-κB activity. (A) Time curve of the Sézary cells (SC) count of the only patient that responded on DMF therapy with a CR. Depicted are the SC values throughout the study (visit number reflects sampling every 4 weeks) in absolute number per μL. (B) Change in spontaneous CD4+ T-cell death rate in patient 2 (responder) and patients 3 and 4 (nonresponders) throughout the study. Spontaneous cell death was measured directly in CD4+ T cells freshly isolated from patients. (C) DMF sensitivity reflected by DMF-induced cell death in the CD4+ T cells of patients 2, 3, and 4 throughout the study. DMF-induced cell death was measured after stimulation of isolated CD4+ T cells of the respective patients with 30 μM DMF for 24 hours. (D) Specific luminescence in the NF-κB luciferase assay in isolated CD4+ T cells from patients 2, 3, and 4 spontaneously at 0 hours and 24 hours after transfection with treatment with 50 μM DMF for 24 hours. The specific NF-κB activity is normalized to a control plasmid and depicted as the ratio of both luminescence values.
Blood response upon DMF treatment depends on SC cell death sensitivity and NF-κB activity. (A) Time curve of the Sézary cells (SC) count of the only patient that responded on DMF therapy with a CR. Depicted are the SC values throughout the study (visit number reflects sampling every 4 weeks) in absolute number per μL. (B) Change in spontaneous CD4+ T-cell death rate in patient 2 (responder) and patients 3 and 4 (nonresponders) throughout the study. Spontaneous cell death was measured directly in CD4+ T cells freshly isolated from patients. (C) DMF sensitivity reflected by DMF-induced cell death in the CD4+ T cells of patients 2, 3, and 4 throughout the study. DMF-induced cell death was measured after stimulation of isolated CD4+ T cells of the respective patients with 30 μM DMF for 24 hours. (D) Specific luminescence in the NF-κB luciferase assay in isolated CD4+ T cells from patients 2, 3, and 4 spontaneously at 0 hours and 24 hours after transfection with treatment with 50 μM DMF for 24 hours. The specific NF-κB activity is normalized to a control plasmid and depicted as the ratio of both luminescence values.
To test DMF sensitivity, we then treated the isolated T cells ex vivo with 30 μM DMF for 24 hours and measured cell death again upon additional DMF stimulation. Again, we found that the T cells of patient 2 showed a high additional cell death sensitivity toward DMF treatment up to visit 7, which then decreased but still surpassed the cell death sensitivity of the T cells of patients 3 and 4, which were considerably more resistant to DMF therapy (Figure 5C). We then checked NF-κB activity in the patient’s isolated T cells via a luminescence reporter gene assay. Confirming our previous in vitro data, we found that NF-κB activity in the T cells of patient 2 was >3 times higher than that in the T cells of the other 2 patients, an effect that decreased over time during the study but was still measurable at follow-up (Figure 5D). Twenty-four hours of stimulation with DMF ex vivo decreased NF-κB activity in all patient T-cell samples, proving that the inhibition of constitutively activated NF-κB is the causative mechanism for DMF effect on T-cell death.
Discussion
This report describes the results of a single-arm, open-label multicenter phase 2 trial of oral DMF monotherapy in patients with relapsed or refractory MF or SS.
Overall, DMF treatment presented promising clinical activity in this study with an overall response rate of 30.4% (7/23; 95% CI, 13.2-52.9) in the skin of the patients. Thus, the clinical efficacy is comparable with other targeted therapeutics already approved for this MF/SS population including mogamulizumab12 and brentuximab vedotin11 as well as recently published phase 1 and 2 trials on novel promising drug candidates such as tenalisib or pembrolizumab in CTCL.32-34
In our study, we did not detect significant differences in response between patients with MF (27%) and SS (33%); in both diseases we found deep and durable responses to DMF treatment. The compartment with the deepest responses was the skin, reflected by a decrease in mSWAT score. In the blood, only 1 patient (16.7%; 1 of 6) showed a decrease in malignant T-cell population by >50% and showed a CR. Both of these findings are intriguing, because the constitutive NF-κB activity, the primary therapeutic target of DMF treatment, is reportedly higher in SS than in MF and vaguely correlates with a higher stage,15,16,18,19 although some studies also describe a constant activation of NF-κB throughout all stages.35 The missing difference between SS and MF might derive from the fact that all patients in the study were pretreated and thus showed different tumor burdens at study entry, disregarding their CTCL subtype and original stage. Based on CTCL consensus criteria, patients cannot be downstaged during the course of disease, leading to a possible bias in the study cohort presented herein.36,37 Therefore, to better reflect tumor burden at baseline, we selected a subpopulation of our patients with SS with >1000 malignant cells per μL blood (B2) and severe skin involvement (mSWAT score > 100) at study start, and found a response rate of 80% (4 of 5 patients) in the skin of this subpopulation. Thus, our results suggest that DMF is most effective in high-stage CTCL, as expected, as well as that NF-κB inhibition is the primary mechanism of DMF action in CTCL. We gained further evidence in support of this mechanism of action by additional translational experiments. We had the opportunity to investigate malignant skin and blood samples of responders and nonresponders throughout the complete course of the study. Additional samples of more patients could not be collected because of limitations by the restricted patient collective as well as the sensitive functional assays that did not allow for the transport of blood samples between the centers. Nevertheless, in these experiments we found a correlation between response to DMF treatment and a high NF-κB activity in the skin and blood, as well as specific cell death sensitivity in CTCL cells, further supporting NF-κB activation as the primary target of DMF. Beyond CTCL, this has implications for other tumor entities, because our clinical data as well as our previous preclinical work on the DMF mechanisms promote DMF as a promising NF-κB–targeting agent also for other NF-κB–dependent tumor entities such as glioblastoma,38 colorectal cancer,39 or B-cell leukemia,40 because NF-κB represents a prominent general factor promoting and maintaining malignancy in many cancers.41 In many CTCL therapies, like mogamulizumab, resistance toward treatment often develops during medication by different mechanisms and thus limits the duration of response.42 Because of its mechanism of action that targets cell death resistance far downstream, resistance toward DMF therapy in CTCL is not expected.19 Beyond NF-κB inhibition, DMF has several pleiotropic immunomodulatory effects on T cells and cells from the skin microenvironment that have been studied in other diseases (supplemental Table 5). These will be investigated in CTCL in more detail within ongoing projects.
We did not observe changes in quality of life induced by DMF in our study. Although these would at least have been expected in the responders, there might be several reasons for the missing improvement: firstly, many patients develop gastrointestinal side effects by DMF treatment; in our study >50% of the patients were affected. Although only mild in most cases, quality of life can be impaired by these side effects, which might mask possible improvements by CTCL response. Secondly, there was no CTCL-specific assessment tool, so we used the DLQI that has known weaknesses in assessing quality of life in CTCL.8 Thirdly, the timespan of 24 weeks was rather short for assessing quality of life. Nevertheless, pruritus is often the most vexing symptom in CTCL and, thus, on average, impaired quality of life decreased in our study, showing that DMF can exert a pruritus-relieving effect in several patients with CTCL.
One of the greatest strengths of DMF compared with other CTCL drugs is its excellent tolerability. In our study, only 1.7% of the AEs were severe, 89% of all AEs did not even necessitate a change of study medication. The number of the patients with AEs of grade ≥3 was, at 17.4%, much lower compared with those reported for mogamulizumab and brentuximab-vedotin phase 3 trials (41% each).11,12 These findings are congruent with the long clinical experience with DMF treatment in psoriasis and MS.22,43 Also, the side effect spectrum and its distribution are very similar to psoriasis and MS, additional toxicity in malignant disease did not occur. Several of the AEs, such as pruritus or fatigue, are also typical symptoms of CTCL, so whether they derive from study therapy or the disease itself cannot be precisely distinguished. DMF-induced lymphopenia that might favor the development of progressive multifocal leukoencephalopathy as a fatal AE was not relevant in our study.44,45 This excellent tolerability makes DMF especially suitable for patients with comorbidities or polymedication, because interactions with other medication are also very rare.
Because of its very favorable side effect profile and its good efficacy in the skin, DMF is not only suitable for monotherapy in CTCL but also presents as an ideal partner for combination therapies, especially together with therapy mainly effective in the blood such as mogamulizumab, venetoclax or ECP.12,20 Further clinical studies will be performed to identify other combination partners for DMF in CTCL and to confirm our findings in a larger collective of patients with CTCL.
In conclusion, this clinical phase 2 study presents DMF as a promising therapeutic option in CTCL also in a clinical setting. DMF is characterized by a good efficacy, especially in late-stage CTCL with high NF-κB activity but can also be effective in earlier stages. In addition, it shows an excellent tolerability with a favorable side effect profile compared with other CTCL drugs. Therefore, DMF not only represents a promising monotherapy option in CTCL but also an ideal combination partner for a wide variety of targeted and nontargeted established CTCL therapies such as mogamulizumab or ECP. Preclinical and clinical work on DMF combinations, for example with Bcl-2 inhibitors or ECP, is already ongoing to optimize efficacy and tolerability of CTCL therapy further. In addition, to better stratify patients suitable for DMF therapy, further translational projects to investigate DMF mechanism of action and influence on cellular signaling in CTCL have started.
Acknowledgments
The authors thank Sabine Gack, Holger Wilden, Marion Nonn-Anastasiadis, and Johannes Hüsing from the KKS Heidelberg for excellent support of the study. In addition, the authors thank Christine Beschorner and Karen Greif (Department of Pathology, University Hospital Tübingen, Germany) for excellent technical assistance. The authors also thank the pharmacy of the University Hospital Heidelberg for collaboration concerning the study drug.
This work was supported by the Deutsche Forschungsgemeinschaft (NI1407/1-2) and the Helmholtz-Alliance for Immunotherapy of Cancer. The study did not receive any industrial or commercial funding but was completely funded by independent sponsoring. The sponsor of the study was the University of Heidelberg, Heidelberg, Germany.
Authorship
Contribution: J.P.N. and P.H.K. designed the study and developed the protocol; J.P.N. was the principal investigator; J.D.A., C.A., E.D., R.S., J.S.U., U.W., and M.W. were clinical investigators at the respective centers; J.P.N., S.M., J.Z., S.S., C.M.S., and K.G. performed translational experiments; J.P.N., S.M., I.B., C.M.S., K.G., S.G., and J.S.U. evaluated clinical and experimental data; J.P.N. and I.B. prepared and wrote the manuscript; and all authors evaluated and discussed the manuscript.
Conflict-of-interest disclosure: J.P.N. received travel and congress participation funding from Teva and Novartis, as well as consulting fees from Teva, Almirall, Biogen, Novartis, Kyowa Kirin, Innate Pharma, Takeda and Actelion, UCB Pharma, and Recordati. S.M. received honoraria from Kyowa Kirin. C.A. has performed consultancies for Takeda, Kyowa, Helsinn, Recordati, and 4SC. U.W. has performed consultancies for Takeda, Therakos, Kyowa Kirin, Recordati Rare Diseases, Stemline, Mundipharma, Helsinn, and Galderma; and has lectured at educational events sponsored by MSD, Takeda, Galderma, Kyowa Kirin, Stemline, and Recordati Rare Diseases. M.W. received funding for congress participation and consulting fees from Kyowa Kirin, Takeda, Recordati Rare Diseases, and Stemline Therapeutics. K.G. received consulting fees from Biogen and supports BioMed X as an academic mentor. C.M.S. is a scientific adviser to AstraZeneca plc, and is on the scientific advisory board of, has stock options in, and has received research funding from, Enable Medicine, Inc, all outside the current work. J.S.U. is on the advisory board of, has received honoraria from, and travel support from, Amgen, Bristol Myers Squibb, GSK, Immunocore, LEO Pharma, Merck Sharp and Dohme, Novartis, Pierre Fabre, Roche, and Sanofi outside the submitted work. P.H.K received consulting fees from Biogen. The remaining authors declare no competing financial interests.
Correspondence: Jan P. Nicolay, University Medical Center Mannheim, Department of Dermatology, Building 27, Theodor-Kutzer-Ufer 1-3, 68167 Mannheim, Germany; e-mail: jan.nicolay@umm.de.
References
Author notes
Complete study protocol, analytic code, statistical plans, or information on translational research are available on request from the corresponding author, Jan P. Nicolay (jan.nicolay@umm.de).
Individual participant data will not be shared because of restrictions by the government and institution.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal