Abstract
Neutrophilia and neutropenia commonly lead to inpatient hematology consultation. Quantitative neutrophil abnormalities have a broad differential and include diagnoses that are important to recognize because they may be associated with increased mortality. Neutrophilia can reflect etiologies such as infection, medications, inflammation, splenectomy, and congenital disorders. Neutropenia can arise from infection, medications, autoimmune destruction, sequestration, nutritional deficiency, malignancy, and congenital neutropenia syndromes. In the evaluation of all abnormalities of neutrophil number, the timing of the change, and the patient’s historical neutrophil count are crucial.
Abnormalities of neutrophil number often lead to inpatient hematology consultation. In the context of 4 illustrative cases, we discuss the differential diagnosis of elevated white blood cell (WBC) count (neutrophilia) and decreased WBC count (neutropenia) with an emphasis on etiologies that occur in the hospital setting.
Neutrophil kinetics
Neutrophils are fully differentiated cells that progress from hematopoietic stem cells to myeloblasts to promyelocytes to myelocytes to metamyelocytes to bands to segmented neutrophils. This is mediated by cytokines, including granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF). The earlier neutrophil precursors (myeloblasts, promyelocytes, and myelocytes) can undergo cellular division and are referred to as the neutrophil proliferative pool. In contrast, metamyelocytes, bands, and segmented neutrophils cannot divide and make up the neutrophil storage pool,1 also termed the bone marrow reserve. Neutrophils are maintained in the bone marrow reserve by chemokine receptors.2 Maturation of neutrophils in the bone marrow takes 7 to 10 days, circulation in the peripheral blood lasts 3 to 24 hours, and duration in the tissues averages 2 to 3 days. Average bone marrow counts in healthy patients range from 1% to 2% myeloblasts, 1% to 3% promyelocytes, 3% to 14% myelocytes, 4% to 16% metamyelocytes, 10% to 15% bands, and 7% to 29% segmented neutrophils.3 Peripheral blood counts can vary by laboratory, but healthy individuals normally have neutrophil and band percentages of 40% to 60% and 0% to 3%, respectively, of the total WBC count.
The major mechanisms of neutrophilia include demargination (release of cells adherent to the endothelial lining of peripheral blood vessels and movement into the peripheral circulation), release from the bone marrow storage pool, and increased neutrophil production. To exit the bone marrow, the storage neutrophils leave the hematopoietic cords and cross the sinusoidal endothelium, which separates the blood and the bone marrow.2 Approximately 50% of neutrophils are marginated on the endothelium.4 In contrast, neutropenia is most often caused by decreased cell production in the bone marrow but can also be caused by increased sequestration and peripheral destruction.
Evaluation of a patient with an elevated neutrophil count (neutrophilia)
The normal range of WBCs is approximately 4400 to 11 000 cells per μL, depending on the laboratory; a differential should always be obtained when an elevated white count is found. The normal range for absolute neutrophil count (ANC) can vary widely among laboratories but is typically between 1500 and 7000 cells per μL.5 When evaluating neutrophilia, one should assess for the presence or absence of other cell types, degree of neutrophil elevation, timing, and clinical scenario.
If neutrophilia is found, one should assess if there are other immature cells present, including bands, metamyelocytes, myelocytes, and blasts; when these are present, the differential is thought to be “left-shifted.” A left-shifted smear is very often associated with infection and can also occur when the bone marrow is stressed for any reason, owing to early release of cells by the bone marrow to the periphery before the cells are mature. In terms of the degree of elevation, certain infections, such as Streptococcus pneumoniae and Clostridium difficile, can present with very high WBC counts, which can be as high as 30 000 cells per μL. A WBC count of >50 000 cells per μL should raise concern for a leukemoid reaction or leukemia. A leukemoid reaction is a significant leukocytosis >50 000 cells per μL that is not due to a malignant cause. The most common cause is infection, especially mycobacterial and fungal infections. Although leukemoid reactions can sometimes be mistaken for leukemia, 1 differentiating aspect is the clinical picture, with infection and inflammatory syndromes leading to leukemoid reactions that improve with fixing the underlying cause.6 Factors that make one concerned for leukemia include having circulating blasts present without other left-shifted elements. Concomitant anemia and thrombocytopenia may suggest malignant involvement of the bone marrow.
The timing of neutrophilia can also be very helpful, if patients have had recent laboratory testing. If neutrophilia is found in a patient in the outpatient setting, in the absence of any signs of infection and previously documented neutrophilia, a repeat complete blood count and differential should be obtained after 6 weeks to assess for potential transient causes. An acute rise in neutrophil count in hospitalized patients should prompt evaluation for infection, bone marrow stress, or malignancy.
Most importantly, one should also always consider the clinical scenario when determining the cause of a neutrophilia (Figure 1). This includes considering the patient’s past medical history, social history, and reason for presentation to the hospital. Links between neutrophilia and obesity have been documented, however this association is complex. One should consider the pathophysiology of inflammation from adipose tissue and insulin resistance to understand this link and refrain from associating neutrophilia with general terms like obesity and elevated body mass index. Neutrophilia occurs because of chronic inflammation from adipose tissue. Adipose tissue produces inflammatory mediators, which leads to increased production and degranulation of neutrophils, and produces leptin, which activates neutrophils.7 Neutrophilia is thus also linked to insulin resistance because chronic inflammation leads to neutrophil infiltration into adipose tissue, and then these neutrophils release chemokines and proteases that lead to insulin resistance.7 To avoid inappropriate bias, those with larger healthy bodies, regardless of an elevated body mass index, without increased adipose tissue or insulin resistance, should not be linked to neutrophilia. Smoking also has been found to be associated with neutrophilia and increased neutrophil activation because of increased production of inflammatory cytokines.8 An extensive review of systems should be performed to assess for infectious causes. If a patient has new bruising, bleeding, fevers, chills, night sweats, and weight loss (“B symptoms”), a malignant etiology involving the bone marrow should be considered.
Differential of neutrophilia with inpatient consultative questions highlighted in pink.
Differential of neutrophilia with inpatient consultative questions highlighted in pink.
Patient 1
A 43-year-old woman presented to the hospital owing to development of watery diarrhea over the past 3 days. She was a healthy woman who took no medications or supplements and did not have any pertinent family history. She recently had a urinary tract infection and received antibiotics for treatment. She had baseline labs obtained in the emergency department and hematology was consulted owing to an elevated WBC count of 29 000 cells per μL with an 80% neutrophil predominance and normal hemoglobin and platelet levels. Her kidney function showed mild acute kidney injury, due to dehydration. She had further stool testing, which found she was positive for C. difficile. She was started on vancomycin orally and over the next couple of days, her WBC count decreased to 13 000 cells per μL. The primary team saw the differential associated with this WBC count and noticed that there were metamyelocytes, myelocytes, and blasts listed thus they became concerned and consulted hematology owing to continued elevation of WBC count and left shift.
Follow-up of patient 1
The initial clinical picture reflected the patient’s acute infection, supported by the observation that her WBC count improved appropriately with antibiotics. It can take days to a week for WBC counts to normalize after treatment with appropriate therapy because there is still ongoing inflammation. Therefore, the left-shifted nature of this patient’s differential is not concerning and is consistent with a stressed marrow due to infection. On review of her smear, the neutrophils were very granulated and there were numerous bands present, all of which fit with the clinical picture.
Many other diagnoses can cause the neutrophil count to be slightly elevated. For example, any stress can lead to demargination of WBCs. Other chronic etiologies should be explored; history and physical exam showed no other infectious etiologies, inflammatory conditions, smoking history, nor increased adipose tissue. It was confirmed that she was not currently pregnant. She had also not exercised recently, which can increase the WBC count. Her prior laboratory values are important to assess to determine if this is a more chronic process. Prior laboratory studies from 5 years prior showed a WBC count of 6000 cells per μL. Medications were reviewed, and she was not taking nor had she recently been taking medications, including corticosteroids, beta-agonists, or lithium. Her erythrocyte sedimentation rate and C-reactive protein were within normal limits. She had never had splenectomy or other surgery.
If her WBC count had still been elevated on discharge, she should have had follow-up in 6 weeks with her primary care doctor to assess. If neutrophilia persisted past treatment and resolution of her symptoms, other causes should be considered. Although acute hematologic malignancies can present with symptoms such as fevers, chills, night sweats, weight loss, bruising or bleeding, and fatigue, it is common for chronic myeloid leukemia to be found as an incidental laboratory finding, as it is a chronic disease usually presenting without symptoms.9 In the absence of any of the other patient-specific factors previously mentioned, further laboratory studies should be sent, including BCR-ABL p210. The BCR-ABL fusion protein has 2 isoforms, p210 and p190. P210 is found in chronic myelogenous leukemia and p190 is found in most Philadelphia chromosome–positive acute lymphoblastic leukemia.10
The patient was discharged home and referred to outpatient primary care follow-up, where labs were repeated 6 weeks later and showed normalization of her WBC and neutrophil counts. No further hematology follow-up specific to this issue was indicated.
Patient 2
A 75-year-old man with mitral valve prolapse and mitral regurgitation presented to the hospital for mitral valve repair and had symptoms and signs of heart failure and jaundice. After further evaluation, he was diagnosed with a cholangiocarcinoma. His laboratory values showed a WBC count of 54 × 103/μL, hemoglobin of 12.8 g/dL, and platelets of 148 × 103/μL. The differential showed ANC of 30.5 × 103/μL, absolute eosinophil count of 10.9 × 103/μL, absolute lymphocyte count of 270/μL, absolute monocyte count of 1.29 × 103/μL, and no precursors or blasts. The peripheral blood smear confirmed mature cells with no evidence of blasts. Molecular studies, including FIP1L1-PDGFRA, PDGFRB, and BCR-ABL1, showed no rearrangements. Of note, laboratory values 5 months before presentation showed a normal WBC count of 6.77 × 103/μL and normal hemoglobin and platelet quantities; no differential was obtained at that time. Hematology was consulted to determine the etiology of neutrophilia.
Follow-up of patient 2
Elevated neutrophil counts of >50 000 cells per μL should raise concern for a leukemoid reaction or hematologic malignancy (such as chronic myeloid leukemia or chronic neutrophilic leukemia). In solid tumor patients, neutrophilia may be a paraneoplastic phenomenon associated with tumor-specific secretion of GM-CSF or G-CSF. G-CSF enhances proliferation and induces maturation of myeloid progenitors leading to overproduction of neutrophils and may also be associated with increased basophils. In contrast, GM-CSF has broader activity, enhancing proliferation and induction of common myeloid progenitors, and is typically associated with increased production of neutrophils, monocytes, and eosinophils.11
Although a rare occurrence, many different tumor types have been reported to secrete G-CSF, including bladder cancer, oropharyngeal squamous cell carcinoma, thyroid cancer, and especially lung cancer.12,13 An array of solid cancers have also been reported to secrete GM-CSF, including lung cancer,14 colorectal cancer, gastric cancer, invasive bladder cancer, breast cancer, and glioma.14-16
Pertinent to patient 2, intrahepatic cholangiocarcinomas have been associated with secretion of GM-CSF.17 Elevation in myeloid cells is associated with a poorer prognosis in intrahepatic cholangiocarcinoma, as high concentrations of GM-CSF may contribute to cancer cell survival and proliferation.17 One study found that patients with cancer and an associated WBC count of >100 000 cells per μL were twice as likely to die than those with WBC counts between 11 000 and 40 000 cells per μL.18
The patient was found to have metastatic cholangiocarcinoma with liver, lung, and bone involvement. The onset of his neutrophilia parallels the diagnosis of his metastatic cholangiocarcinoma. He received gemcitabine, cisplatin, and durvalumab.19 After 2 months of treatment, he had significant response to cancer-directed treatments and his WBC decreased to 5.62 × 103/μL, with hemoglobin of 11.2 g/dL, a normal platelet count, ANC of 3.66 × 103/μL, and absolute eosinophil count of 420/μL. The hypothesis that his neutrophilia reflected tumor secretion of GM-CSF is further supported by the fact that his WBC count (and neutrophilia and eosinophilia) decreased significantly with the response to treatment of his cancer.
Summary of evaluation of neutrophilia
Evaluate the time course of neutrophilia and compare to prior laboratory values.
Use the degree of neutrophilia to narrow your differential diagnosis; elevated neutrophil counts of >50 × 103/μL are suggestive of specific infections, leukemoid reaction, hematologic malignancy, or rare etiologies, such as tumor cytokine production.
Determine etiology of neutrophilia by associating clinical signs and symptoms with pathophysiologic mechanisms; if neutrophilia is persistent after clinical improvement, consider alternative causes.
If neutrophilia does not resolve by discharge, the patient should have a follow-up complete blood count 6 weeks later to determine if further evaluation is needed.
If neutrophilia is persistent, a test for BCR-ABL should be performed.
When there is no cause determined for persistent neutrophilia, a bone marrow biopsy should be performed.
Evaluation of the patient with a low neutrophil count
Neutrophils are produced in the bone marrow and neutropenia can occur because of decreased production, demargination, or immune destruction. Neutropenia is defined by the ANC (which includes neutrophils and bands) being <1500 cells per μL, with mild neutropenia being defined as an ANC between 1000 and 1500 cells per μL, moderate as an ANC from 500 to 1000 cells per μL, and severe as an ANC of <500 cells per μL. Agranulocytosis is defined as an ANC of <200 cells per μL. These “normal” neutrophil counts are defined in the United States based historically on white individuals. ANC reference ranges in Africa, for example, recognize that an ANC of <1500 cells per μL is common and usually normal. Certain populations, such as those with sub-Saharan African ancestry, Sephardic Jewish populations, and some Arab populations that live in regions where malaria is endemic, often have the Duffy-null phenotype, in which they lack erythrocyte expression of the Duffy antigen. The Duffy antigen is the receptor for Falciparum ovale, and therefore being Duffy-null is protective against malaria.20 This genetic variation has previously been termed “benign ethnic neutropenia.” However, it is recommended to adopt the term “Duffy-null associated neutrophil count,” to better reflect the underlying biology of lower neutrophil counts and underscore that this is a normal variant and does not represent pathology.
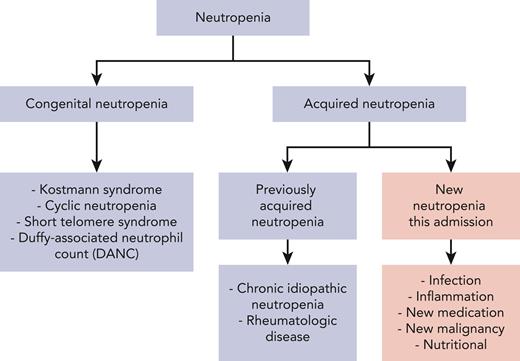
The differential diagnosis of acquired neutropenia includes medications, toxic exposures, infections, and nutritional deficiencies21 (Figure 2). It is critical to consider a low neutrophil count in the context of the patient’s prior history to distinguish whether the neutropenia is acute or chronic (Table 1). Often previous laboratory values may not be available, in which case, historical details regarding signs and symptoms may be critical. Detailed personal history and family history can assist in making the diagnosis. Patients with chronic neutropenia can give a history of symptoms such as mouth sores and recurrent bacterial infections, including sinusitis, gingivitis, and pneumonia. If the neutropenia is of new onset, or indeterminant, evaluation depends on the degree of neutropenia. Profound neutropenia or agranulocytosis associated with fever is a medical emergency, and workup should not delay immediate initiation of antibiotic therapy.
Differential of neutropenia with inpatient consultative questions highlighted in pink.
Differential of neutropenia with inpatient consultative questions highlighted in pink.
Causes, laboratory findings, and treatments of conditions associated with neutropenia
| Cause . | Mutations or laboratory recommendations . | Treatment . |
|---|---|---|
| Severe congenital neutropenia | ELANE, HAX1, other less-frequent mutations (G6PC3, CSF3R, GFI1, etc) | Lifelong G-CSF Surveillance for MDS/AML |
| Immunodeficiencies (Shwachman-Diamond syndrome, Chediak-Higashi syndrome, G6PD deficiency, cartilage-hair hypoplasia, GATA2 deficiency, primary humoral deficiencies) | SBDS CHS1 (LYST) G6PD RMRP GATA2 Many mutations | G-CSF if needed Surveillance for myeloid malignancy |
| Cyclic neutropenia | ELANE gene mutation | G-CSF if symptoms |
| Duffy-null associated neutrophil count (DANC) | Duffy antigen blood typing | No treatment required |
| Short telomere syndrome | Obtain telomere length | Surveillance for aplastic anemia, myeloid malignancy Counseling regarding pregnancy |
| Rheumatologic/autoimmune conditions (primary or secondary autoimmune, Felty syndrome [rheumatoid arthritis], Sjogren syndrome, inflammatory bowel disease, granulomatosis with polyangiitis) Autoimmune lymphoproliferative syndrome | ESR, CRP, antibodies as indicated by symptoms FAS, FASLG, CASP10 | Treat underlying cause |
| Medications/toxins (antibiotics such as sulfa drugs, phenothiazine, chemotherapy, rituximab, tyrosine kinase inhibitors, benzene, insecticides, azathioprine, mycophenolate mofetil) | Medication review, can check some drug levels; toxicology blood and urine testing | Drug discontinuation if severe |
| Infection | Hepatitis A, B, and C; HIV, EBV, bacteria, parasites, Rickettsia, tuberculosis | Treat underlying cause G-CSF for fever and neutropenia |
| Malignancy (aplastic anemia, leukemia, MDS, large granular lymphocytic leukemia, hairy cell; can present as pancytopenia) | Flow cytometry; bone marrow | Diagnosis-directed therapy G-CSF for fever and neutropenia |
| Nutritional (ask about diet [vegan] and alcohol consumption; can present as pancytopenia) | Vitamin B12, MMA, homocysteine, folate, copper | Replete nutritional deficiency |
| Chronic idiopathic neutropenia | Diagnosis of exclusion | No treatment required if no symptoms G-CSF for treatment of aphthous ulcers, frequent infections |
| Cause . | Mutations or laboratory recommendations . | Treatment . |
|---|---|---|
| Severe congenital neutropenia | ELANE, HAX1, other less-frequent mutations (G6PC3, CSF3R, GFI1, etc) | Lifelong G-CSF Surveillance for MDS/AML |
| Immunodeficiencies (Shwachman-Diamond syndrome, Chediak-Higashi syndrome, G6PD deficiency, cartilage-hair hypoplasia, GATA2 deficiency, primary humoral deficiencies) | SBDS CHS1 (LYST) G6PD RMRP GATA2 Many mutations | G-CSF if needed Surveillance for myeloid malignancy |
| Cyclic neutropenia | ELANE gene mutation | G-CSF if symptoms |
| Duffy-null associated neutrophil count (DANC) | Duffy antigen blood typing | No treatment required |
| Short telomere syndrome | Obtain telomere length | Surveillance for aplastic anemia, myeloid malignancy Counseling regarding pregnancy |
| Rheumatologic/autoimmune conditions (primary or secondary autoimmune, Felty syndrome [rheumatoid arthritis], Sjogren syndrome, inflammatory bowel disease, granulomatosis with polyangiitis) Autoimmune lymphoproliferative syndrome | ESR, CRP, antibodies as indicated by symptoms FAS, FASLG, CASP10 | Treat underlying cause |
| Medications/toxins (antibiotics such as sulfa drugs, phenothiazine, chemotherapy, rituximab, tyrosine kinase inhibitors, benzene, insecticides, azathioprine, mycophenolate mofetil) | Medication review, can check some drug levels; toxicology blood and urine testing | Drug discontinuation if severe |
| Infection | Hepatitis A, B, and C; HIV, EBV, bacteria, parasites, Rickettsia, tuberculosis | Treat underlying cause G-CSF for fever and neutropenia |
| Malignancy (aplastic anemia, leukemia, MDS, large granular lymphocytic leukemia, hairy cell; can present as pancytopenia) | Flow cytometry; bone marrow | Diagnosis-directed therapy G-CSF for fever and neutropenia |
| Nutritional (ask about diet [vegan] and alcohol consumption; can present as pancytopenia) | Vitamin B12, MMA, homocysteine, folate, copper | Replete nutritional deficiency |
| Chronic idiopathic neutropenia | Diagnosis of exclusion | No treatment required if no symptoms G-CSF for treatment of aphthous ulcers, frequent infections |
AML, acute myeloid leukemia; CRP, C-reactive protein; EBV, Epstein-Barr virus; ESR, erythrocyte sedimentation rate; MDS, myelodysplastic syndrome; MMA, methylmalonic acid.
Acute, isolated severe neutropenia is almost uniformly attributable to a drug or a toxin. To ensure other dangerous diagnoses are not missed, workup should include assessment of the peripheral blood smear to assess for abnormal cells or concurrent anemia and/or thrombocytopenia, which may suggest a malignancy. If there is concern for malignant cells on peripheral smear, flow cytometry should be done to further assess. Although drug- or toxin-induced neutropenia does not usually require bone marrow examination, in the absence of an identifiable cause, a bone marrow examination should be performed to rule out a primary hematologic malignancy.
Patient 3
A 90-year-old woman was admitted to the hospital from her nursing home with a new cough, fever, and intermittent confusion. She was hemodynamically stable but had an oxygen saturation of 87% on admission, which improved to 97% with 3 L of supplemental oxygen. On admission, her WBC count was 15.6 × 103/μL; hemoglobin was 13.6 g/dL and platelets were 300 × 103/μL. She had a radiograph, which showed a right lower lobe opacity, concerning for pneumonia. She was admitted and treated with ceftriaxone and azithromycin. Her morning laboratory values 2 days after being on these medications showed a WBC count of 1.65 × 103/μL, hemoglobin of 13.4 g/dL, and platelets of 286 × 103/μL. Her ANC was 1.2 × 103/μL. Her oxygenation improved and she was afebrile, thus her antibiotics were changed to amoxicillin-clavulanate and azithromycin.
Commentary on patient 3
Newly acquired acute neutropenia is almost always attributable to medications or toxins. In a patient who has acute onset of newly acquired neutropenia while in the hospital without anemia or thrombocytopenia, medication-induced etiologies are most likely. Many medications have been found to be associated with mild neutropenia, usually dose-related, including antibiotic drugs such as macrolides, antifungals such as amphotericin, antiepileptics such as carbamazepine and valproate, and diuretics such as thiazides and acetazolamide.21 Mild neutropenia must be distinguished from agranulocytosis because those patients with agranulocytosis often present with acute febrile illness or sepsis. Both mild neutropenia and agranulocytosis have been seen with antibiotics such as penicillins, cephalosporins, and vancomycin. In addition, drugs like clozapine, sulfasalazine, procainamide, methimazole, sulfa drugs, chloramphenicol, and dapsone have been associated with high rates of idiosyncratic reactions leading to agranulocytosis.21 The cause of antibiotic-induced neutropenia is still controversial. Dose-related mild neutropenia is likely to reflect a toxic effect; however, idiosyncratic agranulocytosis is likely immune-mediated. In bone marrow biopsies of individuals with drug-induced agranulocytosis, patients usually have myeloid maturation arrest without other abnormalities,22 favoring an antibody-mediated process. Repeat exposure can rapidly precipitate recurrent neutropenia, again suggesting an immune-mediated mechanism23 with an anamnestic response.
Another frequent cause of neutropenia is infection, especially when complicated by sepsis, and is usually already present when the patient is admitted with symptoms. Neutropenia in the setting of sepsis is commonly seen in patients with reduced bone marrow reserve, such as in infants and in the older population, and in patients with concomitant marrow injury. Because neutropenia can put one at risk for infections and infections can also cause neutropenia, establishing whether the patient had a previously normal neutrophil count can help distinguish whether neutropenia was already present and contributed to the development of infection or whether the neutropenia is caused by sepsis. Neutropenia from infection usually improves rapidly with treatment; it can occur because of marrow suppression, peripheral destruction, and localization of neutrophils to the area of infection. In addition to bacterial infections, viral infections, such as with HIV, Epstein-Barr virus, cytomegalovirus, and hepatitis A virus, can cause neutropenia.
When a drug is suspected to be the cause of mild dose-related neutropenia, the decision of whether to stop the drug should be based on a balance of risk and benefit. If there is an alternative medication that works similarly, the alternative medication can be tried. However, some drugs, like antipsychotic or antiepileptic medications, work well for symptom control in a patient and thus mild neutropenia can be tolerated with close monitoring. However, if a patient presents with agranulocytosis, the indicated medication should never be given to the patient again.
Testing for neutrophil drug-dependent antibodies is rarely performed because access to such testing is limited and has poor sensitivity.24 The lower yield of the tests may be owing to the fact that the assay only tests the parent drug compounds and does not account for metabolites of the drug, which could also cause immune-mediated neutropenia.25 If needed, neutrophil drug-dependent antibodies can be sent to certain specialized laboratories, but a negative result does not rule out drug-induced neutropenia.
In patient 3, the most likely medication etiology was ceftriaxone, a cephalosporin. This appears especially likely because it occurred after drug administration and improved after drug discontinuation. The rapid fall in her neutrophil count is suggestive of rapidly developing agranulocytosis, and ceftriaxone as well as any other potential contributory drugs should be stopped and replaced by alternative agents. In fact, on closer questioning, the family thought she had had a similar problem with ceftriaxone in the past, explaining the very rapid onset of neutropenia. Given this, we would not recommend that she be given ceftriaxone in the future. She completed her total course of antibiotics and on day of discharge, her WBC count was back to normal at 8 × 103/μL with a normal differential 1 week after initial presentation.
Patient 4
A 60-year-old male presented to the emergency department with acute intoxication and fall. His fall was witnessed and occurred when he accidentally slid out of a low chair; he did not hit his head. Laboratory values showed a WBC count of 1 × 103/μL, hemoglobin of 13.8 g/dL, and platelets of 256 × 103/μL. The differential showed ANC of 0/μL, with the rest of the differential being normal with no precursors or blasts. His kidney function was normal, and his liver enzymes were normal. Alcohol level was 0. Toxicology screen was positive for cocaine but negative for all other evaluated drugs. When his vital signs were checked, he was found to have a fever at 102°F, with a heart rate of 110 beats per minute and a blood pressure of 140/75 mmHg. On physical exam, he was diaphoretic and had swelling on his arm from a recent injury. Given his fever and signs concerning for cellulitis, he was treated with antibiotics, fluids, and G-CSF. He had blood cultures drawn before the administration of antibiotics, which were ultimately negative. Over the next week, his fever abated and his neutrophil count slowly improved.
Commentary on patient 4
When a patient comes into the hospital with acute severe neutropenia and has not been on prescribed medications, one should ask about over-the-counter supplements and medications, illicit drugs, or other toxins. Patient 4 tested positive for cocaine on toxicology screen.
Levamisole-adulterated illicit drugs, such as cocaine and heroin, have been found to be associated with agranulocytosis. Levamisole was previously used in the treatment of rheumatoid arthritis and certain cancers, before being removed from the market in the United States owing to reports of agranulocytosis; it is currently still used in veterinary medicine.26 In recent years, levamisole has been found in cocaine in increasing concentrations, increasing from 1% of cocaine in 2001 to 10% of cocaine in 2009. Furthermore, in 2009, 69% of cocaine entering the United States was found to include levamisole.26,27 Bone marrow biopsy analysis from 2 patients with agranulocytosis after levamisole exposure showed decreased granulocytic proliferation and maturation, suggesting a toxin-mediated effect; the marrows were otherwise normal.28 The mechanism of levamisole-induced agranulocytosis is not well understood, although it is thought to be immune related, and early studies suggested that it was more common in patients positive for HLAB27.29 However, there are suggestions of both toxin-mediated and immune-mediated contributions to levamisole-induced neutropenia and agranulocytosis. Ultimately, it was felt that patient 4 had received cocaine laced with levamisole; after discharge, the patient returned 2 weeks later for follow-up and had a negative drug screen with normal WBC and neutrophil counts. Overall, medications, supplements, and illicit substances can cause neutropenia and agranulocytosis and a close history and medication reconciliation must always be performed.
Treatment of neutropenia in hospitalized patients
Treatment of in-hospital neutropenia depends on the severity of the neutropenia and the associated clinical scenario. If driven by infection, appropriate antimicrobials and supportive care should be given. In cases of drug- or toxin-induced neutropenia, G-CSF or filgrastim (trade names: Neupogen, Granix, and Zarxio) may be recommended. If a patient has mild to moderate neutropenia with no infectious symptoms or mild neutropenia with mild infectious symptoms, they likely do not need G-CSF. Patients with moderate to severe neutropenia or agranulocytosis with infectious symptoms should receive G-CSF. G-CSF can also be considered in a patient with an infection, if the neutrophil count is quickly trending downward and will soon be moderate to severe. The recommended dosing is G-CSF 5 μg/kg per day, given daily until the neutrophil count is >1000 cells per μL for 3 days. The mortality of drug-induced agranulocytosis is 7% across all age-groups and 11% in those aged >64 years, thus it should be acted upon quickly.30 G-CSF can shorten the recovery phase for patients with neutropenia and agranulocytosis.31 Because it takes 7 to 10 days for neutrophils to mature in the bone marrow, response to G-CSF should be expected to be delayed by up to a week or potentially longer. Older age and comorbidities that decrease bone marrow reserve should prompt providers to have a lower threshold for starting G-CSF. Although we do not give G-CSF reflexively for a specific ANC, most patients with agranulocytosis present with fever and should receive G-CSF. Although the long-acting G-CSF pegfilgrastim (Neulasta) is the agent of choice for reducing chemotherapy-related neutropenia, long-acting G-CSF agents are not recommended for the treatment of acute neutropenia.
Summary of evaluation of neutropenia
Isolated acute neutropenia is almost uniformly related to a drug or toxin.
Consider prior history, signs, and symptoms to determine the chronicity and etiology.
With severe neutropenia, consider the clinical context and give G-CSF if moderate to severe neutropenia and evidence of infection.
Drug-induced agranulocytosis has a high rate of morbidity and mortality, supporting the use of G-CSF to shorten the period of profound neutropenia.
When there is no cause determined for persistent neutropenia, a bone marrow biopsy should be performed.
Authorship
Contribution: R.L.Z. wrote the initial draft; and N.B. edited and refined the manuscript.
Conflict-of-interest disclosure: R.L.Z. is a consultant and stockholder for Amagma Therapeutics. N.B. declares no competing financial interests.
Correspondence: Rebecca L. Zon, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: becky_zon20@dfci.harvard.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal