Abstract
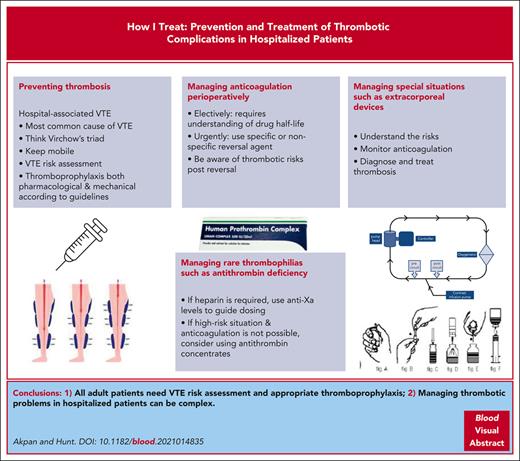
This article uses case-based discussion to review prevention and management of thrombotic problems in hospitalized patients that involve a clinical hematologist. There is variation in the clinical hematologist’s role in thrombosis practice throughout the world, and we discuss this where indicated. Hospital-associated venous thromboembolism (VTE), or hospital-associated thrombosis (HAT), is the term to cover VTE occurring during admission and for 90 days postdischarge and is a common patient safety problem. HATs are the most common cause of VTE accounting for 55% to 60% of all VTE, with an estimated 10 million occurring globally. VTE risk assessment alongside evidence-based thromboprophylaxis reduces this risk significantly. Many hospitalized patients, especially older patients, use direct oral anticoagulants (DOACs), mainly to prevent stroke in atrial fibrillation. DOACs require perioperative management and may need urgent reversal. Other complex interventions such as extracorporeal membrane oxygenation which require anticoagulation are also discussed. Lastly, those with uncommon high-risk thrombophilias, especially those with antithrombin deficiency, produce unique challenges when hospitalized.
Introduction: the role of the hematologist in the prevention and treatment of VTE in hospitalized patients
Venous thromboembolism (VTE) risk factors were famously described by Virchow in 18461,2; he described changes in blood flow and the vessel wall and/or prothrombotic changes in blood constituents. It is now recognized that hospital admission is the most common cause of VTE, accounting for 55% to 60% of all VTE. Hospital-associated VTE and hospital-associated thrombosis (HAT) are the terms used to describe VTE in hospital and for the at-risk period of 90 days postdischarge.2 The World Health Organization (WHO) estimated there are over 10 million cases of HAT annually.3 There is an urgent need to ensure that any adult admitted to hospital has VTE risk assessment on admission, followed by evidence-based thromboprophylaxis as an approach that is safe and effective in reducing morbidity and mortality from HAT.2 The Patient Safety division of the WHO is working with the International Society for Thrombosis and Haemostasis (ISTH) to improve global HAT prevention. In both medical and surgical settings, most HATs occur after discharge; thus hospital staff may be unaware of the magnitude of the problem. Ideally each hospital should institute mandated VTE risk assessment and provide local thromboprophylaxis protocols based on evidence-based guidelines. In some countries such as the United Kingdom, hematologists are usually heavily involved in designing, implementing, updating, and monitoring such guidelines. In contrast, a global survey has shown that many hospitals have yet to attain effective thromboprophylaxis for at-risk-hospitalized patients.4 Moreover, evidence-based guidelines for thromboprophylaxis are changing with increasing recognition of the poor evidence supporting antiembolic stockings when compared to the efficacy of pneumatic compression devices.5 For those with a diagnosed VTE, management usually requires anticoagulation to prevent further propagation of the thrombus; the duration of anticoagulation depends on whether the VTE occurred in the presence or absence of provoking factors. In some settings anticoagulation is contraindicated due to the patient’s high bleeding risk and in such cases the use of an inferior vena cava filter needs consideration.
Many hospitalized patients are already receiving anticoagulation; the most common indication being for stroke prevention in atrial fibrillation. Indeed, the numbers are likely to increase, for an estimated 12.1 million individuals are expected to have atrial fibrillation in the United States by 2030.6 Management of anticoagulated patients perioperatively or during urgent anticoagulation reversal can be complex. Additionally, some patients with high-risk thrombophilias need individualized thromboprophylaxis, especially those with antithrombin deficiency, who require skilled management of heparin thromboprophylaxis.
This article discusses cases that highlight the management of difficult thrombotic problems in hospitalized patients.
HAT and reversal of anticoagulants
Case 1
A 75-year-old woman with a body mass index of 34 kg/m2presented with abdominal pain and was diagnosed with diverticulitis. She was admitted for intravenous fluids and antibiotics. VTE risk assessment was performed and thromboprophylaxis with weight-adjusted dose of low-molecular-weight heparin (LMWH) was started as recommended by the hospital thromboprophylaxis guideline. She had a protracted stay and after seven days complained of left calf pain. Duplex ultrasound revealed a left popliteal vein thrombosis, extending into the common femoral vein and so the patient’s anticoagulation was increased from prophylactic to therapeutic dose. As she was now eating and drinking, subcutaneous injections of enoxaparin 1 mg/kg every 12 hours was stopped and rivaroxaban 15 mg every 12 hours, given with food, was started. The following day, midmorning, she complained of sudden onset of dizziness and severe abdominal pain. On physical examination, she had a blood pressure of 80/60 mmHg, pulse rate of 126 beats per minute, and an acute abdomen. A complete blood count (CBC) showed that her hemoglobin had declined to 90 g/L from 130 g/L. An abdomen and pelvis computed tomography (CT) scan showed a perforated bowel which had bled and caused extensive intra-abdominal bleeding. A coagulation screen showed activated partial thromboplastin time (APTT) 53 seconds (normal 34-39), PT 28 seconds (normal 15-18 seconds), and fibrinogen 0.9 g/L (normal 1.9-3.5). She was resuscitated with intravenous fluids, packed red blood cells, 2 pools of cryoprecipitate, and approximately 20 mL/kg of fresh frozen plasma and was prepared for surgery. An urgent call was made to the hematologist on call to assist with reversal of her anticoagulation.
Hospital-associated thromboprophylaxis failure
Despite thromboprophylaxis, VTE sometimes occurs because LMWH reduces thrombotic risk but does not prevent all HATs. It is a reminder that there is a need to find better anticoagulants with a more effective antithrombotic profile without increased bleeding risk. This woman received a weight-adjusted dose of thromboprophylaxis, in keeping with local policy, but not with international guidelines, for weight-adjusted thromboprophylaxis has not been studied formally in clinical trials.7 The original trials of thromboprophylaxis vs placebo were conducted more than 20 years ago and all used empirical doses of LMWH. Since that time there has been an obesity pandemic.8 The Centers for Disease Control and Prevention (CDC) cites an obesity prevalence in the United States of 41.9%.9 We feel strongly that the use of weight-adjusted thromboprophylaxis should be a priority in thrombosis research, to assess whether higher anticoagulation doses for obese patients reduce the risk of VTE without increasing bleeding.
Reversal of DOACs
Direct oral anticoagulants (DOACs) include a direct thrombin inhibitor (dabigatran) as well as factor Xa inhibitors (FXa) (rivaroxaban, apixaban, edoxaban), and are preferred over vitamin K antagonists (VKAs) for the management of VTE for multiple reasons, which include their lower risk of major bleeding.10-13 DOACs have fewer drug-drug interactions and have predictable pharmacokinetics, so routine monitoring of anticoagulation levels is not required.14,15 However, there are occasions when it is helpful to know the activity level of a DOAC. In case 1, the patient had taken rivaroxaban with her breakfast (rivaroxaban is better absorbed with food) and thus could be predicted to have high rivaroxaban concentration levels midmorning. Therefore, measuring DOAC activity levels would be helpful to guide reversal management.
ISTH and American College of Cardiology (ACC) guidelines suggest direct reversal of DOACs if the patient has had a major bleed, which include a bleed at a critical site or a hemoglobin drop ≥2 g/dL.16,17 Fast-turnaround testing for DOAC anti-FXa levels, as well as dilute thrombin time (dTT) and ecarin-based assays for measuring dabigatran effects, is extremely helpful if a significant drop in blood levels is suspected.17 A normal thrombin time excludes clinically significant plasma concentrations of dabigatran but a normal APTT cannot exclude the presence of a DOAC with anti-FXa activity.18,19 Most anti-FXa inhibitors have a nonlinear relationship with routine tests such as APTT and prothrombin time (PT), and thus they are not reliable for anti-FXa DOAC measurements. Only rivaroxaban’s anti-FXa levels have a linear relationship with the PT.20,21 However, rivaroxaban’s anti-FXa level probably does not have a direct relationship with PT prolongation in this patient, for due to intra-abdominal bleeding she has a low fibrinogen level and decrease in hemoglobin level; and thus much of the PT prolongation may be due to coagulation consumption. Specific chromogenic anti-FXa assays provide drug levels but the correlation is less reliable at low drug levels (rivaroxaban concentration level <30 ng/ml, apixaban <15 ng/mL, edoxaban <10 ng/mL) and at concentration levels >500 ng/mL.21-23 The ISTH expert consensus states that DOAC reversal may be necessary if a concentration level is >50 ng/mL in a bleeding patient or >30 ng/mL in a perioperative patient.24
Management of hemorrhage with factor Xa inhibitors
Andexanet alfa is an FDA-approved direct reversing agent for apixaban and rivaroxaban25 (see Table 1). Andexanet also decreases edoxaban levels in healthy volunteers and in patients with acute bleeding.31,39 Therefore, andexanet alfa is recommended for off-label reversal of edoxaban by the Anticoagulation Forum.32 Four-factor prothrombin complex concentrates (PCCs) are also widely used to counteract the effects of factor Xa inhibitors. Oral activated charcoal is a consideration for anticoagulation reversal if the anticoagulants have been ingested within 2 to 4 hours.17,40
Reversal agents of direct oral anticoagulants
| . | Idarucizumab17,26-28 . | Andexanet alfa17,25,27,29 . | 4-Factor PCC17,27,30 . |
|---|---|---|---|
| References | 17,30-33 | 17,25,31,34,35 | 17,31,36 |
| Type of structure | Humanized monoclonal antibody fragment | Recombinant modified factor Xa protein | Coagulation factors II, VII, IX, and X |
| Cost per dose | $350037 | $24 200–$48 40038 | $4050–$810030 |
| FDA approved | 2015 | 2018 | Off-label use |
| Approval based on | RE-VERSE AD study | ANNEXA-4 trial | Multiple observational studies |
| Drug reversed | Dabigatran | Apixaban, rivaroxaban, edoxaban∗ | Dabigatran, apixaban, rivaroxaban, edoxaban, warfarin |
| Mechanism of action | Binds dabigatran and its metabolites with an affinity for dabigatran that is ∼350× greater that of thrombin | Binds and sequesters the factor Xa inhibitors | Contain vitamin K dependent coagulation factors that promote hemostasis (factors II, IX, X, and VII) Some 4-factor PCC contain heparin† Some countries only have access to 3-factor PCC (missing Factor VII) |
| Onset | Within minutes | Rapid | Rapid |
| Dose | 5 g IV (given as 2 separate 2.5 g doses ≤15 minutes apart) | IV bolus (400 or 800 mg), then a continuous infusion for up to 120 min (4 or 8 mg/min) based on the last dose of rivaroxaban (≤10 or >10 mg/unknown) or apixaban (≤5 or >5 mg/unknown), and time of the last dose of rivaroxaban or apixaban (<8 h/unknown or ≥8 h) | PCC for FXa inhibitor reversal, IV 25-50 U/kg or fixed dose of 2000; for dabigatran reversal, IV 50 U/kg |
| Adverse effects | Thromboembolism | Thromboembolism, cardiac arrest, stroke | Thrombosis, stroke, hypotension |
| Thromboembolic events after 30 days | 3.8%-5% | 10%-14% | 4%-8% |
| . | Idarucizumab17,26-28 . | Andexanet alfa17,25,27,29 . | 4-Factor PCC17,27,30 . |
|---|---|---|---|
| References | 17,30-33 | 17,25,31,34,35 | 17,31,36 |
| Type of structure | Humanized monoclonal antibody fragment | Recombinant modified factor Xa protein | Coagulation factors II, VII, IX, and X |
| Cost per dose | $350037 | $24 200–$48 40038 | $4050–$810030 |
| FDA approved | 2015 | 2018 | Off-label use |
| Approval based on | RE-VERSE AD study | ANNEXA-4 trial | Multiple observational studies |
| Drug reversed | Dabigatran | Apixaban, rivaroxaban, edoxaban∗ | Dabigatran, apixaban, rivaroxaban, edoxaban, warfarin |
| Mechanism of action | Binds dabigatran and its metabolites with an affinity for dabigatran that is ∼350× greater that of thrombin | Binds and sequesters the factor Xa inhibitors | Contain vitamin K dependent coagulation factors that promote hemostasis (factors II, IX, X, and VII) Some 4-factor PCC contain heparin† Some countries only have access to 3-factor PCC (missing Factor VII) |
| Onset | Within minutes | Rapid | Rapid |
| Dose | 5 g IV (given as 2 separate 2.5 g doses ≤15 minutes apart) | IV bolus (400 or 800 mg), then a continuous infusion for up to 120 min (4 or 8 mg/min) based on the last dose of rivaroxaban (≤10 or >10 mg/unknown) or apixaban (≤5 or >5 mg/unknown), and time of the last dose of rivaroxaban or apixaban (<8 h/unknown or ≥8 h) | PCC for FXa inhibitor reversal, IV 25-50 U/kg or fixed dose of 2000; for dabigatran reversal, IV 50 U/kg |
| Adverse effects | Thromboembolism | Thromboembolism, cardiac arrest, stroke | Thrombosis, stroke, hypotension |
| Thromboembolic events after 30 days | 3.8%-5% | 10%-14% | 4%-8% |
DOAC, direct oral anticoagulant; PCC, prothrombin complex concentrates.
Activated charcoal can be used for reversal for known ingestion of DOAC within 2 to 4 hours.
Not FDA approved for edoxaban, off-label use recommended by 2020 American College of Cardiology Expert Consensus.
Some PCC contains heparin, so avoid use of heparin-containing PCC in patients with a history of heparin induced thrombocytopenia.
Idarucizumab is a monoclonal antibody that is the specific reversal agent of dabigatran.26 If idarucizumab is not available, then activated PCC 50 U/kg IV is recommended.32 Renal replacement therapy (RRT) is also effective at reducing the dabigatran activity and can be used to manage patients with renal impairment on dabigatran who need to reduce their plasma levels of the drug rapidly.40 Ciraparantag is another reversal agent that binds noncovalently to heparin, LMWH, and DOACs,41 but is still in clinical trials.42
A meta-analysis of 21 studies that compared andexanet alfa with PCC showed similar effectiveness at 12 hours (82% vs 88%); 24hrs (71% vs 76%); average 30-day symptomatic VTE rate (5% vs 1.9%), average total VTE rate (10.7% vs 3.1%), and mean in hospital mortality (23.3% vs 15.8%).43 A further meta-analysis which included 36 studies showed no difference in the proportion of anticoagulation reversal, thromboembolic rates or mortality rates between andexanet alfa, idarucizumab, and PCC.27 Overall, DOAC-specific reversal agents have not been directly compared with PCCs in randomized controlled trials, and we await these data with interest.27,43
Thrombotic risks with reversal agents
Unfortunately, discontinuing a DOAC and using reversal agents carry a thrombotic risk. The Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors (ANNEXA-4) study showed a thrombotic event in 10% of patients within 30 days.25 A recent meta-analysis that investigated the use of idarucizumab, andexanet alfa, and PCC for reversal of severe DOAC-associated bleeding showed a thromboembolism rate of 4.6% (95% confidence interval: 3.3% to 6.0%), with a very high rate with andexanet (10.7%; 95% confidence interval: 6.5% to 15.7%), and lower with the other agents: PCC (4.3%) and idarucizumab (3.8%).28
Case 1 (continued)
Our patient received 50 U/kg of 4-factor PCC at anesthetic induction. Serial coagulation testing and platelet count were performed during the procedure and 2 liters of blood were removed from her abdomen. Her bowel perforation was surgically managed. If andexanet alfa were available, she would have received high-dose andexanet alfa (initial 800 mg IV bolus at a target rate of 30 mg/min followed by continuous infusion at 8 mg/min) prior to emergency surgery given that she had taken rivaroxaban 15 mg less than 8 hours prior. Postoperatively she was transferred to intensive care and ventilated. She did not receive a thromboprophylactic dose of LMWH 6 hours postoperatively because there was blood oozing from her drains. She could not be fitted with intermittent pneumatic compression devices either, due to an acute deep vein thrombosis (DVT). A decision was made to insert a temporary inferior vena cava filter until she was fit for anticoagulation.
Inferior vena cava filters
For patients who are unable to tolerate anticoagulation (perhaps due to acute bleeding and/or recent surgery) and/or have hemodynamic instability, an inferior vena cava (IVC) filter placement may be indicated temporarily to prevent a pulmonary embolism (PE). There is an international trend to reduce the use of IVC filters due to their limited efficacy and complication rate. Modern advice is to use an IVC filter temporarily and only when VTE risk is very high but the risk of bleeding with anticoagulation is unacceptably high.44 The PREPIC and PREPIC 2 studies randomized patients with an acute PE associated with a lower extremity DVT treated with anticoagulation to receive or not receive an IVC filter. The studies showed that the placement of an IVC filter did not significantly reduce recurrent PE or mortality but may increase the risk of DVT in some patients.45,46
Currently, retrievable IVC filters are preferable so that these filters can be removed as soon as no longer needed. Our patient had a recent DVT but due to bleeding postoperatively an IVC filter was inserted with a plan to remove the IVC filter before discharge and after starting anticoagulation. Multiple guidelines, including those of the American College of Chest Physicians47 and the British Society for Haematology48 and the recent and comprehensive Society of Interventional Radiology guideline,49 all recommend against IVC filter placements in patients who can tolerate anticoagulation.
IVC filter retrieval
Unfortunately, many filters are forgotten and not retrieved. Having hospital registries for IVC filters may improve retrieval rates. In patients with a fresh thrombus, the filter should be retrieved after the patient has resumed anticoagulation.50
IVC filter–associated complications
There is no survival benefit if an IVC filter is left in place permanently and complications such as caval thrombosis and VTE occur.51 Less frequent complications include filter migration to the right atrium, caval perforation, strut fracture, and filter leg endothelialization causing difficulty or failure to remove the filter.51 Other complications of IVC filters include development of hematoma, pneumothorax during insertion, and recurrent DVT (5%-35%) and IVC/filter thrombosis (2%-30%) in the late setting.51 Lastly, postthrombotic syndrome is more common in patients with long-term filters.
When resuming therapeutic anticoagulation prior to filter retrieval, consider initiating anticoagulation at a prophylactic dose with either unfractionated heparin (UFH) or LMWH, and if tolerated then gently up titrate to therapeutic dose anticoagulation. It may be helpful to obtain an ultrasound to assess residual thrombus to gauge how quickly anticoagulation should be increased. Ultimately switch from UFH or LMWH to a DOAC once it is clear that the patient no longer requires additional procedures.
Perioperative management of anticoagulation in hospitalized patients
Case 2
A 65-year-old man with a history of diabetes, hyperlipidemia, compensated heart failure, inherited protein S deficiency, and recurrent unprovoked VTE, who is on long term rivaroxaban 20 mg by mouth daily, with a creatinine clearance of 48 mL/min is admitted with cholecystitis and requires a cholecystectomy. Although the surgical teams assessed the patient and recommended that the laparoscopic cholecystectomy be performed later as an outpatient case, the patient says he lives alone and is several hours from a major hospital, therefore would prefer to undergo the surgery prior to discharge. The hematology team is consulted for advice on perioperative anticoagulation management.
Management of DOACs perioperatively for elective procedures and surgeries
The ISTH has provided guidelines on how to manage DOACs perioperatively.52 A multicenter landmark study that assessed patients with atrial fibrillation on a DOAC and undergoing an elective procedure or surgery showed that bridging is not required in most elective procedures.53 Extrapolating from the PAUSE (Perioperative Anticoagulant Use for Surgery Evaluation) study, the European consensus statement on perioperative management of DOACs, and the Anticoagulation Forum, the measurement of DOAC concentration levels or reversal of DOAC activity is not necessary for elective procedures.32,53,54 Instead a DOAC should be stopped a day or two prior to surgery based on the bleeding risk of the surgery and the renal function of the patient32,53-55 (Table 2).
Perioperative DOAC management55
| Bleeding risk procedure . | Factor Xa inhibitors dabigatran (CrCl ≥ 50 ml/min) . | Dabigatran (CrCl < 50 ml/min) . |
|---|---|---|
| Low | Hold 1 day before procedure and resume 1 day after | Hold 2 days before procedure and resume 1 day after |
| High | Hold 2 days before procedure and resume 2-3 days after | Hold 4 days before procedure and resume 2-3 days after |
| Bleeding risk procedure . | Factor Xa inhibitors dabigatran (CrCl ≥ 50 ml/min) . | Dabigatran (CrCl < 50 ml/min) . |
|---|---|---|
| Low | Hold 1 day before procedure and resume 1 day after | Hold 2 days before procedure and resume 1 day after |
| High | Hold 2 days before procedure and resume 2-3 days after | Hold 4 days before procedure and resume 2-3 days after |
DOAC, direct oral anticoagulant.
High-bleeding-risk procedures have a 2-day risk of major bleed of ≥2% and include gastrointestinal surgery, neurosurgical procedures, transurethral resection of the prostrate, epidural corticosteroid injections, bowel resection, and nephrectomy/renal biopsy. Low-bleeding-risk procedures include laparoscopic cholecystectomy, coronary angiography, and arthroscopy. Procedures with minimal bleeding risks include cataract and minor dermatologic procedures. It is also recommended that patients are stratified into either high-, intermediate-, or low-perioperative thromboembolic categories.56 High-thrombotic-risk patients include patients with recent VTE (<3 months), protein C deficiency, antithrombin deficiency, protein S deficiency, combined thrombophilia, and triple positive antiphospholipid antibodies. Intermediate-risk patients include those with VTE within 3 to 12 months, recurrent VTE, and active cancer while low-risk patients are those with a VTE >12 months previously.56 See recent CHEST guidelines for detailed risk stratification for periprocedural bleeding and thrombotic risks.56 While DOACs should be held for procedures with high- and low-bleeding risks, procedures associated with minimal bleeding do not require interruption of anticoagulation, though in some cases, it may be prudent to hold it on the day of the procedure.56 In the patients undergoing elective procedures/surgeries, bridging from a DOAC with LMWH is not usually necessary unless they have high-thrombotic risk and need to be off the DOAC longer than planned, for example, if the procedure/surgery is delayed.56,57 Also, recent studies have demonstrated that it is not necessary to adjust the PAUSE management protocol to further minimize bleeding risks.58
Case 2 (continued)
Our 65-year-old man with recurrent, unprovoked VTE on indefinite anticoagulation has a high-thrombotic risk and undergoing a low-bleeding-risk procedure. Rivaroxaban was held for 24 hours (2-3 half-lives) prior to the surgery and restarted 24 hours postoperatively, once adequate hemostasis was achieved (seeTable 2).
Managing thrombosis during and after ECMO
Case 3
A previously healthy 32-year-old woman was transferred to the intensive therapy unit for management of her severe respiratory distress syndrome secondary to postinfluenza cavitating Staphylococcus aureus pneumonia. Despite mechanical ventilation and treatment for septic shock her condition deteriorated. She was severely hypoxic and required veno-venous extracorporeal membrane oxygenation (VV-ECMO) with internal jugular and left femoral cannulas inserted. She received intravenous heparin following the Extracorporeal Life Support Organization (ELSO) guideline59for anticoagulation aiming to achieve an activated clotting time (ACT) of 180 to 220 seconds. After a period of 10 days, during which there were periods when increasing amounts of heparin infusion were required to maintain an adequate ACT (which responded to the use of antithrombin concentrate) and 2 bleeding episodes, her condition improved and ECMO was withdrawn. Bilateral lower extremity edema was noted on physical examination, and ultrasound of both legs demonstrated an acute left DVT from the point of insertion of the femoral cannula extending down to the popliteal vein.
ECMO and hemostasis
High rates of both thrombosis and bleeding are seen with ECMO. The rates are variable between different units, affected by flow rates and anticoagulation protocols, but in general, bleeding is a more frequent complication than thrombosis. Most units use UFH as the anticoagulant of choice, but there is a trend towards use of direct thrombin inhibitors such as bivalirudin and argatroban, especially with severe COVID-19 infections and in cases where heparin-induced thrombocytopenia and thrombosis occurred. International guidelines describe the different modes of monitoring UFH, which range from ACT to APTT, anti-FXa activity levels, and viscoelastometry, but do not give recommendations for 1 method, for all have drawbacks, and there have been no comparative studies.59 When UFH is used in a sick patient, sometimes there is a need to increase the rate of infusion to maintain the same ACT. This is indicative of acquired antithrombin deficiency, due to consumption of antithrombin as an anticoagulant, and/or decreased antithrombin production due to liver dysfunction.60 Over a third of ECMO centers monitor antithrombin activity levels and supplement with antithrombin concentrate or fresh frozen plasma.61 Pragmatically, measurement of antithrombin activity levels and use of antithrombin concentrate to attain antithrombin levels of 80% to 120%, or infusion of fresh frozen plasma seems reasonable until we have more data.62
It is not uncommon to see a DVT after removal of femoral catheters. For example, patients with COVID variants in 2020 and 2021 were found to have post–ECMO DVT rates as high as 50%. These should be treated with standard therapeutic anticoagulation.63
Case 3 (continued)
Our patient, who had normal renal function, and a platelet count of 154 × 109/L was started on therapeutic LMWH. Because she was eating a normal diet 36 hours after being decannulated from ECMO, she was switched to rivaroxaban 15 mg twice daily with food, and with an initial plan to continue therapeutic anticoagulation for three months only, as this was a provoked VTE.
Bridging patients with inherited antithrombin deficiency
Case 4
A 32-year-old woman was admitted for elective delivery of a 38-week fetus, her second pregnancy. In her first pregnancy she developed an iliofemoral DVT at 30 weeks and was found to have low levels of antithrombin activity (47 IU/dL [normal range 76-124 IU/dL]) and antithrombin antigen (52 IU/dL [normal range 80-120 IU/dL]). Genetic assessment showed a mutation in the SERPINC1 gene consistent with type 1 antithrombin deficiency. At the time of a positive pregnancy test, the patient weighed 75 kg and was immediately started on therapeutic-dose LMWH. Monthly pre- and 4 hours post-anti-FXa activity levels were tested. As expected, her requirements for enoxaparin increased rapidly, and at the time of admission she was taking 120 mg twice daily to attain adequate anti-FXa levels.
It was agreed that the last dose of enoxaparin needed to be 24 hours before cesarean section, and she was listed first on the elective cesarean section list so that 50 U/kg of a plasma-derived antithrombin concentrate could be given intravenously 12 hours preoperatively. The cesarean section was undertaken under regional anesthesia and was uneventful with a blood loss of <500 ml, and she restarted on enoxaparin 40 mg 6 hours postoperatively. She wanted to breastfeed for six months and it was agreed that she would switch to warfarin under cover of enoxaparin.
Heparins act as anticoagulants by increasing the anticoagulant effect of antithrombin 10 000-fold, thus antithrombin deficiency results in an inability to attain adequate anticoagulation with standard doses of unfractionated and low-molecular-weight heparin.64 This can be remedied by either increasing concentration levels of heparin supratherapeutically and/or providing supplementary antithrombin in the form of antithrombin concentrate or fresh frozen plasma, as with acquired antithrombin deficiency in case 3. Pregnancy in those with antithrombin deficiency is associated with high rates of VTE unless meticulous monitoring of anticoagulation is used. Women with a type II heparin binding site (HBS) defect, have a slightly lower risk of VTE, but higher rates of miscarriage, especially those with homozygous HBS defects.64 The most recent American Society of Hematology guidelines on managing VTE in pregnancy recommend against giving antenatal thromboprophylaxis to those with antithrombin deficiency without a family history. They do, however, recommend postpartum antithrombotic prophylaxis (strong recommendation, moderate certainty in evidence about effects), but do suggest both antenatal and postnatal thromboprophylaxis in those with a family history based on a very low certainty of evidence.65
In order to ensure adequate anticoagulation, monitoring of anticoagulation with an anti-FXa assay that does not contain supplementary antithrombin is required in order to avoid spuriously elevated anti-FXa concentration levels. There are no international guidelines recommending target trough (predose) and peak (4-6 hours postdose of LMWH) anti-FXa concentration levels. Pragmatically, based on clinical experience we aim for trough anti-FXa levels of >0.11 U/ml in those with previous thromboses and peaks between 0.5 to 1.0 U/ml, with emphasis on the troughs being adequate.66 Cost efficacy dictates that increasing heparin levels is preferred to giving antithrombin concentrate regularly; indeed we estimate the cost of taking a woman with antithrombin deficiency through pregnancy using only antithrombin concentrate would be $300 000 for the cost of the concentrate alone. However, there are times where heparin is contraindicated, such as a cesarean section, in which case antithrombin concentrates can be used to attain normal concentrations of antithrombin. The half-life of antithrombin concentrates varies between different products. The 2 forms of antithrombin concentrate, plasma derived and recombinant are both approved by the US Food and Drug Administration (FDA) and the European Medicines Agency. Recombinant antithrombin, has a shorter half-life of 11 to 17 hours and is produced in genetically engineered goats, which secrete antithrombin in their milk. Although immunogenicity is a theoretic issue it has never been observed.34 Plasma-derived antithrombin has a half-life of 2 to 3 days and is usually given as bolus injections. During the period after administering antithrombin concentrate, normal doses of LMWH should be used as thromboprophylaxis, but the LMWH dose should be increased as the activity levels of antithrombin wane from the time of antithrombin concentrate infusion.
Timing of dosing of LMWH is critical to allow access to obstetric regional anesthesia. The anesthetist needs to understand that restoring normal activity levels of antithrombin with antithrombin concentrate in the absence of heparin has no excess anticoagulant effects. Anesthetic guidelines usually stipulate that the last dose of therapeutic LMWH needs to be given 24 hours preoperatively and thromboprophylactic doses 12 hours preoperatively, and that coagulation testing is normal and the platelet count is >70 × 109/L.33,36
Lastly preimplantation genetics is being used increasingly in mothers with a strong family history of thrombosis to produce a child without antithrombin deficiency. In this case the mother did not want preimplantation genetics and so gene analysis of the neonate is necessary to see if the neonate has the same defect.35 Performing genetic analysis is preferable to looking at plasma concentration levels in a neonate because it does not require a blood sample but a buccal smear and plasma antithrombin concentration levels are lower than in adults and may be difficult to interpret.
Conclusion
In conclusion, although caring for a thrombophilic patient in the hospital can be challenging, a multidisciplinary team involving a hematologist with a clear understanding of bleeding and thrombotic risks is instrumental in ensuring the safety of the patient. Also, the use of institutional policies based on evidence-based national or international guidelines are extremely helpful in delivering care; they require regular updates to ensure the patient is receiving the optimal level of care.
Authorship
Contribution: I.J.A. and B.J.H. contributed equally to this article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Beverley J. Hunt, Guy’s & St Thomas' NHS Foundation Trust, Westminster Bridge Rd, London SE1 7EH, United Kingdom; e-mail: beverley.hunt@gstt.nhs.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal