Key Points
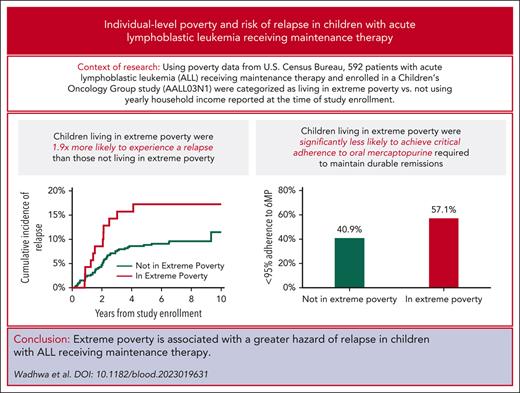
Children living in extreme poverty during maintenance therapy for childhood ALL experience a 1.9-fold greater hazard of relapse.
Lower proportion of children with ALL living in extreme poverty achieve critical adherence (95%) to oral mercaptopurine.
Abstract
The association between individual-level poverty and relapse in children receiving maintenance treatment for acute lymphoblastic leukemia (ALL) remains unclear. In a secondary analysis of COG-AALL03N1, we used data from US Census Bureau to categorize patients living below year-specific federal poverty thresholds, calculated using self-reported annual household income and size of household. Participants with federal poverty thresholds above 120% of their yearly household income were categorized as living in extreme poverty. Hazard of relapse was estimated using multivariable proportional subdistributional hazards regression for patients living in extreme poverty while receiving ALL maintenance therapy after adjusting for relevant predictors. Among 592 patients in this analysis, 12.3% of the patients were living in extreme poverty. After a median follow-up of 7.9 years, the cumulative incidence of relapse at 3 years from study enrollment among those living in extreme poverty was significantly higher (14.3%) than those not living in extreme poverty (7.6%). Multivariable analysis demonstrated that children living in extreme poverty had a 1.95-fold greater hazard of relapse than those not living in extreme poverty; this association was mitigated after the inclusion of race/ethnicity in the model, likely because of collinearity between race/ethnicity and poverty. A greater proportion of children living in extreme poverty were nonadherent to mercaptopurine (57.1% vs 40.9%); however, poor adherence did not completely explain the association between poverty and relapse risk. Future studies need to understand the mechanisms underlying the association between extreme poverty and relapse risk. This trial was registered at www.clinicaltrials.gov as #NCT00268528.
Introduction
Survival after diagnosis of childhood cancer has improved dramatically over the past few decades, with 5-year survival rates now approaching 85%.1 Childhood acute lymphoblastic leukemia (ALL) is one such example, in which 5-year disease-free survival exceeds 90% even among high-risk subgroups.2 Unfortunately, these gains are not observed equally across all patients, and it is increasingly recognized that social determinants of health affect these outcomes. In fact, using Surveillance, Epidemiology, and End Results data, Kehm et al demonstrated that socioeconomic status mediates known racial and ethnic disparities in survival among children with cancer.3 However, their study did not include individual level sociodemographic data and key clinical and behavioral variables. These are especially important when examining outcomes in children with ALL given the strong association of leukemia biology and treatment adherence with risk of relapse.4 Furthermore, previous investigations examining the association between socioeconomic status and risk of relapse in childhood cancer have relied on surrogate markers of poverty, such as neighborhood/community level poverty using area of residence (linked to zip code) or insurance status (public vs private).5,6 About 20% of children in the United States live in households with annual income below federal poverty thresholds.7 It is possible that individual level poverty, measured using annual household income, adversely affects access to health care and ability to adhere to prescribed treatment, ultimately affecting risk of disease relapse.
We addressed these gaps by conducting a secondary analysis of Children’s Oncology Group study COG-AALL03N1 to examine the association between household poverty, measured using yearly federal poverty thresholds provided by the US Census Bureau, and risk of relapse in children with ALL. We hypothesized that children with ALL living in extreme poverty while receiving maintenance therapy would have a greater hazard of relapse than children not living in extreme poverty.
Methods and materials
The eligibility criteria for enrollment on COG-AALL03N1 included age being ≤21 years at diagnosis of ALL in first remission when entering maintenance. All participating sites had approval from local institutional review boards and patients and/or parents/legal guardians provided written informed consent and/or assent before enrollment on the study. The study schema is provided in supplemental Figure 1, available on the Blood website. Briefly, the primary aim of COG-AALL03N1 was to examine adherence to oral 6-mercaptopurine (6MP) during maintenance therapy for ALL. Patients were enrolled after having completed 6 months of maintenance therapy. At the time of study enrollment, patients/parents self-reported race/ethnicity, parental education, household structure, and annual household income. Additional details of COG-AALL03N1 and its primary findings have been previously published.4,8-11 This analysis was restricted to patients living in the United States and those who self-reported annual household income and number of members living in the household at the time of study enrollment to allow for poverty categorization. Furthermore, to allow for homogeneous assessment of dose intensity between poverty groups as a potential explanation of differences in relapse rates, patients with heterozygous or homozygous TPMT-deficiency or mutant NUDT15 genotype (8.9%) were also excluded from this analysis.
Clinical characteristics (age at ALL diagnosis, sex, age at study enrollment, National Cancer Institute [NCI] risk grouping [standard risk: age ≤ 9.99 years and white blood cell count <50 000 cells/μL at ALL diagnosis; high risk if otherwise], ALL subtype, leukemic blast cytogenetics [favorable: t(12;21), hyperdiploidy, trisomy 4 and 10, or trisomy 4, 10, and 17; unfavorable: t(9;22), t(4;11), hypodiploidy, or extreme hypodiploidy; neutral: neither favorable nor unfavorable]) were provided by participating sites. Dose intensity of 6-mercaptopurine (6MPDI) and methotrexate (MTXDI) was calculated by dividing the daily prescribed doses (as reported monthly by participating sites) by planned protocol doses (6MP = 75 mg/m2 per day; MTX = 20 mg/m2 per week). Periodic updates (every 6 months for the first 5 years and then annually for the next 5 years) on clinical outcomes (vital status, relapse or second neoplasm) were collected from participating sites. A subgroup of patients enrolled on COG-AALL03N1 used the electronic medication monitoring device (TrackCap Medication Events Monitoring System [MEMS]; MWV Switzerland Ltd) for measuring 6MP adherence for 6 months; this group was labeled as the MEMS subcohort.8
Statistical analysis
The demographic questionnaire asked parents to report annual household income at study entry using the following categories for response: <$20 000; $20 000 to $49 999; $50 000 to $74 999; $75 000 to $99 999; and ≥$100 000. We used the midpoint of the range as the annual household income for data analysis (eg, $10 000 if a parent reported income between $0 and $20 000; $35 000 if a parent reported income between $20 000 and $49 999; and $150 000 if a parent reported income ≥$100 000). We used data provided by the US Census Bureau to categorize patients as living above or below federal poverty threshold12; annual household income, number of adults and children living in the household, and the year of study participation were used to determine whether the patients lived above or below federal poverty threshold. Among those living in poverty, patients were characterized as living in extreme poverty if the federal poverty threshold was over 120% (median percent difference between income and federal poverty threshold for those in poverty) of their yearly household income; all other patients were categorized as not living in extreme poverty.
We examined demographic and clinical characteristics by poverty status, using standard descriptive statistics. We constructed logistic regression models to examine predictors of extreme poverty. We estimated cumulative incidence of relapse by poverty status, treating second neoplasms and death as competing risks. We examined hazard of relapse from study enrollment using proportional subdistribution hazard regression models. Univariate analyses examined the association between extreme poverty and risk of relapse; other factors examined included demographic and clinical variables (age at study entry, sex, race/ethnicity, NCI risk group, blast cytogenetics, average 6MPDI and MTXDI, and time from start of maintenance to study entry). Multivariable analysis in model 1 examined the hazard of relapse by poverty status after adjusting for all variables with P < .1 in univariate analysis, except race/ethnicity. In model 2, we added race/ethnicity to the model. For patients in the MEMS subcohort, we examined differences in adherence to oral mercaptopurine by poverty group. We then examined the hazard of relapse associated with poverty status with mercaptopurine adherence included in the model.
We used SAS software v9.4 (SAS Institute Inc) for the statistical analyses. Two-sided tests with P < .05 were considered statistically significant.
Results
Of the 742 patients enrolled between 2006 and 2012 on AALL03N1, a total of 592 (79.7%) met criteria for inclusion in this secondary analysis (enrolled at a site in the United States and provided information on annual household income and number of family members in the household). As shown in supplemental Table 1, excluded vs included patients were similar except that excluded patients were older at diagnosis (6 [1-17] vs 5 [1-19] years; P = .003) and at study enrollment (8 [2-20] vs 6 [2-21] years; P = .002) and had lower median 6MPDI (0.73 [0.06-2.05] vs 0.88 [0.03-2.97]; P < .001) and MTXDI (0.78 [0.1-1.41] vs 0.88 [0.2-2.74]; P < .001).
Table 1 highlights the sociodemographic and clinical characteristics of the included patients. The median age at ALL diagnosis was 5 (range, 1-19) years and at study enrollment was 6 (range, 2-21) years. The cohort was followed for a median of 7.9 (range, 0.1-13) years. Most of the patients were male (68.4%). The most commonly reported race/ethnicity was Hispanic (35%), followed by non-Hispanic White (32.4%), African-American or Black (18.2%), and Asian (14.4%). Overall, 34.8% parents reported their education as high school (HS) or lower. The median number of household members was 4 (range, 2-12) and median number of children aged <18 years in the household was 2 (range, 1-10). Most patients had B-lymphoblastic leukemia (88.5%), NCI standard risk disease (58.7%), and neutral cytogenetics (53.7%).
Sociodemographic and disease characteristics of patients with ALL, overall and by poverty status
| Variable . | Entire cohort (n = 592) . | Not in extreme poverty (n = 519) . | In extreme poverty (n = 73) . | P value . |
|---|---|---|---|---|
| Age at diagnosis in years | ||||
| Median (range) | 5 (1-19) | 5 (1-19) | 5 (1-18) | .9 |
| Mean (±standard deviation) | 6.1 (4.4) | 6.1 (4.4) | 6.1 (4.5) | .9 |
| Age at study enrollment in years | ||||
| Median (range) | 6 (2-21) | 6 (6-21) | 6 (2-20) | .8 |
| Mean (±standard deviation) | 7.6 (4.5) | 7.6 (4.5) | 7.8 (4.6) | .7 |
| Length of follow-up in years | ||||
| Median (range) | 7.9 (0.1-13.0) | 7.9 (0.1-13.0) | 7.0 (0.4-10.6) | .05 |
| Sex, n (%) | ||||
| Male | 405 (68.4) | 354 (68.2) | 51 (69.9) | .8 |
| Race/ethnicity, n (%) | ||||
| African-American or Black | 108 (18.2) | 90 (17.3) | 18 (24.7) | <.001 |
| Asian | 85 (14.4) | 77 (14.8) | 8 (11.0) | |
| Hispanic | 207 (35.0) | 163 (31.4) | 44 (60.3) | |
| Non-Hispanic White | 192 (32.4) | 189 (36.4) | 3 (4.1) | |
| Parental education, n (%) | ||||
| ≤HS | 205 (34.6) | 154 (29.6) | 51 (69.8) | <.001 |
| Household structure | ||||
| Number of household members, median (range) | 4 (2-12) | 4 (2-12) | 6 (4-12) | <.001 |
| Total number of children <18 y old, median (range) | 2 (1-10) | 2 (1-10) | 3 (1-6) | <.001 |
| ALL subtype, n (%)∗ | ||||
| B-lymphoblastic leukemia | 521 (88.0) | 454 (87.4) | 67 (91.7) | .2 |
| T-lymphoblastic leukemia | 63 (10.6) | 58 (11.2) | 5 (6.8) | .3 |
| NCI risk group, n (%) | ||||
| Standard risk | 345 (58.2) | 303 (58.3) | 42 (57.5) | .9 |
| Cytogenetics, n (%)† | ||||
| Favorable | 227 (38.3) | 203 (39.1) | 24 (32.8) | .6 |
| Neutral | 298 (50.3) | 260 (50.1) | 38 (52.1) | |
| Unfavorable | 30 (5.1) | 25 (4.8) | 5 (6.8) | |
| 6MP dose intensity | ||||
| Median (range) | 0.88 (0.03-2.97) | 0.88 (0.03-2.97) | 0.89 (0.4-1.50) | .2 |
| MTXDI dose intensity | ||||
| Median (range) | 0.88 (0.2-2.74) | 0.88 (0.2-2.74) | 0.9 (0.26-1.46) | .3 |
| Variable . | Entire cohort (n = 592) . | Not in extreme poverty (n = 519) . | In extreme poverty (n = 73) . | P value . |
|---|---|---|---|---|
| Age at diagnosis in years | ||||
| Median (range) | 5 (1-19) | 5 (1-19) | 5 (1-18) | .9 |
| Mean (±standard deviation) | 6.1 (4.4) | 6.1 (4.4) | 6.1 (4.5) | .9 |
| Age at study enrollment in years | ||||
| Median (range) | 6 (2-21) | 6 (6-21) | 6 (2-20) | .8 |
| Mean (±standard deviation) | 7.6 (4.5) | 7.6 (4.5) | 7.8 (4.6) | .7 |
| Length of follow-up in years | ||||
| Median (range) | 7.9 (0.1-13.0) | 7.9 (0.1-13.0) | 7.0 (0.4-10.6) | .05 |
| Sex, n (%) | ||||
| Male | 405 (68.4) | 354 (68.2) | 51 (69.9) | .8 |
| Race/ethnicity, n (%) | ||||
| African-American or Black | 108 (18.2) | 90 (17.3) | 18 (24.7) | <.001 |
| Asian | 85 (14.4) | 77 (14.8) | 8 (11.0) | |
| Hispanic | 207 (35.0) | 163 (31.4) | 44 (60.3) | |
| Non-Hispanic White | 192 (32.4) | 189 (36.4) | 3 (4.1) | |
| Parental education, n (%) | ||||
| ≤HS | 205 (34.6) | 154 (29.6) | 51 (69.8) | <.001 |
| Household structure | ||||
| Number of household members, median (range) | 4 (2-12) | 4 (2-12) | 6 (4-12) | <.001 |
| Total number of children <18 y old, median (range) | 2 (1-10) | 2 (1-10) | 3 (1-6) | <.001 |
| ALL subtype, n (%)∗ | ||||
| B-lymphoblastic leukemia | 521 (88.0) | 454 (87.4) | 67 (91.7) | .2 |
| T-lymphoblastic leukemia | 63 (10.6) | 58 (11.2) | 5 (6.8) | .3 |
| NCI risk group, n (%) | ||||
| Standard risk | 345 (58.2) | 303 (58.3) | 42 (57.5) | .9 |
| Cytogenetics, n (%)† | ||||
| Favorable | 227 (38.3) | 203 (39.1) | 24 (32.8) | .6 |
| Neutral | 298 (50.3) | 260 (50.1) | 38 (52.1) | |
| Unfavorable | 30 (5.1) | 25 (4.8) | 5 (6.8) | |
| 6MP dose intensity | ||||
| Median (range) | 0.88 (0.03-2.97) | 0.88 (0.03-2.97) | 0.89 (0.4-1.50) | .2 |
| MTXDI dose intensity | ||||
| Median (range) | 0.88 (0.2-2.74) | 0.88 (0.2-2.74) | 0.9 (0.26-1.46) | .3 |
Favorable cytogenetics included t(12;21); hyperdiploidy; trisomy 4 and 10; or trisomy 4, 10, and 17. Unfavorable cytogenetics included t(9;22), t(4;11), hypodiploidy, or extreme hypodiploidy. Neutral cytogenetics implied absence of favorable or unfavorable cytogenetics.
Data were missing for
ALL subtype (n = 8) and
cytogenetics (n = 37).
Seventy-three patients (12.3%) met the criteria for living in extreme poverty. As highlighted in Table 1, there were no differences by poverty group in median age at cancer diagnosis (P = .9) or age at study enrollment (P = .8). Patients not living in extreme poverty had longer median length of follow-up than those living in extreme poverty (7.9 [range, 0.1-13] vs 7.0 [range, 0.4-10.6] years; P = .05). Patients living in extreme poverty were significantly more likely to report Hispanic (60.3% vs 31.4%) or African-American or Black (24.7% vs 17.3%) race/ethnicity (P < .001) than those not living in extreme poverty. Parents of children living in extreme poverty were also significantly more likely to report their education as HS or lower (71.8% vs 29.7%; P < .001). In addition, patients living in extreme poverty had greater number of household members (median: 6 [range, 4-12] vs 4 [range, 2-12]; P < .001) and young children in household (median: 3 [range, 1-6] vs 2 [range, 1-10]; P < .001). There were no differences in disease or treatment intensity by poverty status as shown in Table 1.
As shown in Table 2, non-White race/ethnicity was associated with greater odds of living in extreme poverty (for Asian: odds ratio [OR], 5.8; 95% confidence interval [CI], 1.5-23.5; P = .01; for Black or African-American: OR, 9.8; 95% CI, 2.8-34.6; P = .004; for Hispanic: OR, 7.9; 95% CI, 2.3-27.3; P = .001; reference: non-Hispanic White), as was low parental education (≤HS: OR, 4.1; 95% CI, 2.2-7.5; P ≤ .001; reference: >HS).
Predictors of extreme poverty
| Variable . | OR (95% CI) . | P value . |
|---|---|---|
| Race/ethnicity (reference: non-Hispanic White) | ||
| Asian | 5.8 (1.5-23.5) | .01 |
| Black or African-American | 9.8 (2.8-34.6) | .004 |
| Hispanic | 7.9 (2.3-27.3) | .001 |
| Parental education (reference: >HS) | ||
| ≤HS | 4.1 (2.2-7.5) | <.001 |
| Variable . | OR (95% CI) . | P value . |
|---|---|---|
| Race/ethnicity (reference: non-Hispanic White) | ||
| Asian | 5.8 (1.5-23.5) | .01 |
| Black or African-American | 9.8 (2.8-34.6) | .004 |
| Hispanic | 7.9 (2.3-27.3) | .001 |
| Parental education (reference: >HS) | ||
| ≤HS | 4.1 (2.2-7.5) | <.001 |
Poverty and relapse risk
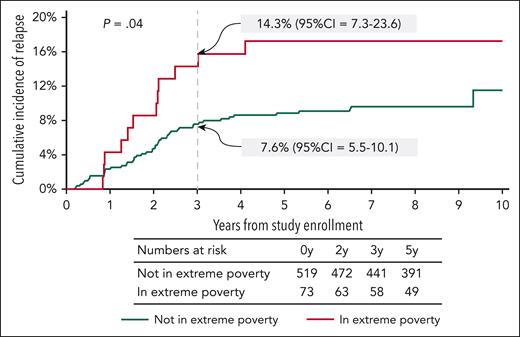
In this secondary analysis of patients enrolled on AALL03N1 and followed for a median of 7.9 (0.1-13.0) years, 61 children (10.3%) experienced relapse of their primary disease. The cumulative incidence of relapse at 3 years from study enrollment was significantly greater among those living in extreme poverty than those not living in extreme poverty (14.3% [95% CI, 7.3-23.6] vs 7.6% [95% CI, 5.5-10.1]; P = .04) (Figure 1). As shown in Table 3, the hazard of relapse among children living in extreme poverty was 1.95-fold (95% CI, 1.03-3.72; P = .04) higher than those not living in extreme poverty, after adjusting for age at study enrollment, NCI risk group, blast cytogenetics, and time from maintenance (model 1). Adjustment for race/ethnicity in model 2 reduced the hazard of relapse among those living in extreme poverty (hazard ratio [HR], 1.68; 95% CI, 0.86-3.28; P = .1), likely owing to collinearity between poverty and race/ethnicity in this cohort. Similarly, addition of parental education to the model incorporating clinical and disease characteristics also attenuated the effect of extreme poverty on relapse risk (HR, 1.69; 95% CI, 0.84-3.41; P = .1, model 3).
Cumulative incidence of relapse from time of enrollment on AALL03N1 by poverty group.
Cumulative incidence of relapse from time of enrollment on AALL03N1 by poverty group.
Hazard of relapse by poverty group
| Variable . | Univariate . | Multivariable model 1 . | Multivariable model 2 . | Multivariable model 3 . | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P value . | |
| Poverty (reference: not in extreme poverty) | ||||||||
| In extreme poverty | 1.89 (1.00-3.55) | .05 | 1.95 (1.03-3.72) | .04 | 1.68 (0.86-3.28) | .1 | 1.69 (0.84-3.41) | .1 |
| Age at study enrollment | ||||||||
| Per year increase | 1.09 (1.03-1.16) | .002 | 1.07 (1.00-1.15) | .05 | 1.07 (1.00-1.15) | .05 | 1.06 (0.99-1.14) | .1 |
| Sex (reference: female) | ||||||||
| Male sex | 0.92 (0.54-1.57) | .7 | - | - | - | - | - | - |
| Parental education (reference: >HS) | ||||||||
| ≤HS | 1.55 (0.93-2.58) | .1 | - | - | - | - | 1.30 (0.75-2.26) | .4 |
| NCI high risk (reference: standard risk) | ||||||||
| High risk | 2.05 (1.22-3.44) | .007 | 1.13 (0.56-2.25) | .7 | 1.15 (0.57-2.32) | .7 | 1.22 (0.60-2.46) | .6 |
| Blast cytogenetics (reference: neutral) | ||||||||
| Favorable | 0.45 (0.24-0.84) | .01 | 0.58 (0.30-1.14) | .1 | 0.58 (0.29-1.13) | .1 | 0.60 (0.30-1.18) | .1 |
| Unfavorable | 0.83 (0.25-2.71) | .8 | 0.77 (0.24-2.52) | .7 | 0.76 (0.23-2.49) | .7 | 0.75 (0.23-2.47) | .6 |
| Average 6MP dose intensity | ||||||||
| Per unit increase | 1.52 (0.57-4.00) | .4 | - | - | - | - | - | - |
| Average MTXDI dose intensity | ||||||||
| Per unit increase | 0.97 (0.33-2.90) | .9 | - | - | - | - | - | - |
| Time from maintenance to study entry | ||||||||
| Per year increase | 0.54 (0.28-1.01) | .06 | 0.5 (0.28-0.86) | .01 | 0.49 (0.28-0.86) | .01 | 0.47 (0.26-0.85) | .01 |
| Race/ethnicity (reference: non-Hispanic White) | ||||||||
| Asian | 1.46 (0.63-3.36) | .4 | - | - | 1.20 (0.46-3.17) | .7 | - | - |
| African-American or Black | 2.50 (1.28-4.88) | .007 | - | - | 1.06 (0.44-2.57) | .9 | - | - |
| Hispanic | 0.98 (0.97-0.99) | .002 | - | - | 2.23 (1.12-4.42) | .03 | - | - |
| Variable . | Univariate . | Multivariable model 1 . | Multivariable model 2 . | Multivariable model 3 . | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P value . | |
| Poverty (reference: not in extreme poverty) | ||||||||
| In extreme poverty | 1.89 (1.00-3.55) | .05 | 1.95 (1.03-3.72) | .04 | 1.68 (0.86-3.28) | .1 | 1.69 (0.84-3.41) | .1 |
| Age at study enrollment | ||||||||
| Per year increase | 1.09 (1.03-1.16) | .002 | 1.07 (1.00-1.15) | .05 | 1.07 (1.00-1.15) | .05 | 1.06 (0.99-1.14) | .1 |
| Sex (reference: female) | ||||||||
| Male sex | 0.92 (0.54-1.57) | .7 | - | - | - | - | - | - |
| Parental education (reference: >HS) | ||||||||
| ≤HS | 1.55 (0.93-2.58) | .1 | - | - | - | - | 1.30 (0.75-2.26) | .4 |
| NCI high risk (reference: standard risk) | ||||||||
| High risk | 2.05 (1.22-3.44) | .007 | 1.13 (0.56-2.25) | .7 | 1.15 (0.57-2.32) | .7 | 1.22 (0.60-2.46) | .6 |
| Blast cytogenetics (reference: neutral) | ||||||||
| Favorable | 0.45 (0.24-0.84) | .01 | 0.58 (0.30-1.14) | .1 | 0.58 (0.29-1.13) | .1 | 0.60 (0.30-1.18) | .1 |
| Unfavorable | 0.83 (0.25-2.71) | .8 | 0.77 (0.24-2.52) | .7 | 0.76 (0.23-2.49) | .7 | 0.75 (0.23-2.47) | .6 |
| Average 6MP dose intensity | ||||||||
| Per unit increase | 1.52 (0.57-4.00) | .4 | - | - | - | - | - | - |
| Average MTXDI dose intensity | ||||||||
| Per unit increase | 0.97 (0.33-2.90) | .9 | - | - | - | - | - | - |
| Time from maintenance to study entry | ||||||||
| Per year increase | 0.54 (0.28-1.01) | .06 | 0.5 (0.28-0.86) | .01 | 0.49 (0.28-0.86) | .01 | 0.47 (0.26-0.85) | .01 |
| Race/ethnicity (reference: non-Hispanic White) | ||||||||
| Asian | 1.46 (0.63-3.36) | .4 | - | - | 1.20 (0.46-3.17) | .7 | - | - |
| African-American or Black | 2.50 (1.28-4.88) | .007 | - | - | 1.06 (0.44-2.57) | .9 | - | - |
| Hispanic | 0.98 (0.97-0.99) | .002 | - | - | 2.23 (1.12-4.42) | .03 | - | - |
Model 1 adjusts for age at study enrollment, NCI risk group, blast cytogenetics, and time from start of maintenance.
Model 2 adds race/ethnicity to model 1.
Model 3 removes race/ethnicity and adds parental education to model 1.
Poverty, adherence to oral mercaptopurine and relapse
We examined differences in adherence to oral mercaptopurine among the MEMS subcohort of AALL03N1 and attempted to understand whether lower adherence explains greater relapse among those living in extreme poverty. Supplemental Table 2 describes the sociodemographic and disease characteristics of 389 children in the MEMS subcohort included in this analysis; of these, 42 (10.8%) were living in extreme poverty. Similar to the entire cohort, there were no significant differences in demographic or clinical characteristics of children by poverty status in the MEMS subcohort, with the exception of higher proportion of Hispanic children (73.8% vs 33.7%; P < .001) living in extreme poverty, and greater proportion of parents reporting their education as HS or lower among those living in extreme poverty (65.9% vs 29.1%; P < .001). Supplemental Table 3 compares children who participated in adherence monitoring with those that did not. The proportion of Black or African-American children was lower among participants in MEMS subcohort than nonparticipants (15.4% vs 23.7%; P = .04)
Using a previously demonstrated critical 6MP adherence level of 95% required to maintain sustained remissions,4,9 we found that children living in extreme poverty had a greater proportion of nonadherers than that of children not living in extreme poverty (57.1% vs 40.9%; P = .04). Unadjusted hazard of relapse among those living in extreme poverty trended toward significance compared with those without extreme poverty (HR, 2.03; 95% CI, 0.88-4.7; P = .09) among patients in MEMS subcohort (Table 4). Adding mercaptopurine adherence to the multivariable regression model (adjusted for age at study enrollment, NCI risk group, blast cytogenetics, and time from start of maintenance to study entry) resulted in minimal attenuation of the association between extreme poverty and relapse risk (adjusted HR = 2.27→HR = 2.11). Finally, addition of race/ethnicity resulted in a reduction of the association between extreme poverty and relapse risk (HR = 2.11→HR = 1.87), likely because of collinearity between race/ethnicity and poverty (Table 4).
Hazard of relapse by poverty group in the MEMS subcohort
| Variable . | Univariate . | Multivariable model 1 . | Multivariable model 2 . | Multivariable model 3 . | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P value . | |
| Poverty (reference: not in extreme poverty) | ||||||||
| In extreme poverty | 2.03 (0.88-4.70) | .09 | 2.27 (0.92-5.59) | .08 | 2.11 (0.85-5.22) | .1 | 1.87 (0.79-4.41) | .2 |
| Age at study enrollment | ||||||||
| Per year increase | 1.11 (1.04-1.19) | .003 | 1.07 (0.98-1.18) | .1 | 1.06 (0.96-1.16) | .2 | 1.06 (0.96-1.17) | .2 |
| Sex (reference: female) | ||||||||
| Male sex | 0.92 (0.54-1.57) | .7 | - | - | - | - | - | - |
| NCI high risk (reference: standard risk) | ||||||||
| High risk | 2.35 (1.22-4.54) | .01 | 1.27 (0.52-3.12) | .6 | 1.31 (0.53-3.22) | .6 | 1.27 (0.50-3.25) | .6 |
| Blast cytogenetics (reference: neutral) | ||||||||
| Favorable | 0.35 (0.15-0.82) | .02 | 0.46 (0.18-1.17) | .1 | 0.47 (0.18-1.22) | .1 | 0.46 (0.18-1.17) | .1 |
| Unfavorable | 0.97 (0.23-4.09) | .9 | 0.86 (0.20-3.61) | .8 | 0.95 (0.22-4.09) | .9 | 0.89 (0.21-3.86) | .9 |
| Average 6MP dose intensity | ||||||||
| Per unit increase | 1.54 (0.62-3.83) | .4 | - | - | - | - | - | - |
| Average MTXDI dose intensity | ||||||||
| Per unit increase | 1.22 (0.35-4.31) | .8 | - | - | - | - | - | - |
| Time from maintenance to study entry | ||||||||
| Per year increase | 0.37 (0.17-0.82) | .02 | 0.37 (0.18-0.74) | .005 | 0.37 (0.17-0.77) | .008 | 0.36 (0.18-0.74) | .005 |
| Race/ethnicity (reference: non-Hispanic White) | ||||||||
| African-American or Black | 1.72 (0.58-5.12) | .3 | - | - | - | - | 1.06 (0.34-3.32) | .9 |
| Asian | 0.92 (0.24-3.55) | .9 | - | - | - | - | 0.67 (0.15-2.97) | .6 |
| Hispanic | 2.66 (1.13-6.26) | .03 | - | - | - | - | 1.97 (0.77-5.04) | .2 |
| Median adherence | ||||||||
| Per unit increase | 0.98 (0.97-0.99) | .002 | - | - | 0.99 (0.98-1.00) | .1 | 0.99 (0.98-1.00) | .2 |
| Variable . | Univariate . | Multivariable model 1 . | Multivariable model 2 . | Multivariable model 3 . | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P value . | |
| Poverty (reference: not in extreme poverty) | ||||||||
| In extreme poverty | 2.03 (0.88-4.70) | .09 | 2.27 (0.92-5.59) | .08 | 2.11 (0.85-5.22) | .1 | 1.87 (0.79-4.41) | .2 |
| Age at study enrollment | ||||||||
| Per year increase | 1.11 (1.04-1.19) | .003 | 1.07 (0.98-1.18) | .1 | 1.06 (0.96-1.16) | .2 | 1.06 (0.96-1.17) | .2 |
| Sex (reference: female) | ||||||||
| Male sex | 0.92 (0.54-1.57) | .7 | - | - | - | - | - | - |
| NCI high risk (reference: standard risk) | ||||||||
| High risk | 2.35 (1.22-4.54) | .01 | 1.27 (0.52-3.12) | .6 | 1.31 (0.53-3.22) | .6 | 1.27 (0.50-3.25) | .6 |
| Blast cytogenetics (reference: neutral) | ||||||||
| Favorable | 0.35 (0.15-0.82) | .02 | 0.46 (0.18-1.17) | .1 | 0.47 (0.18-1.22) | .1 | 0.46 (0.18-1.17) | .1 |
| Unfavorable | 0.97 (0.23-4.09) | .9 | 0.86 (0.20-3.61) | .8 | 0.95 (0.22-4.09) | .9 | 0.89 (0.21-3.86) | .9 |
| Average 6MP dose intensity | ||||||||
| Per unit increase | 1.54 (0.62-3.83) | .4 | - | - | - | - | - | - |
| Average MTXDI dose intensity | ||||||||
| Per unit increase | 1.22 (0.35-4.31) | .8 | - | - | - | - | - | - |
| Time from maintenance to study entry | ||||||||
| Per year increase | 0.37 (0.17-0.82) | .02 | 0.37 (0.18-0.74) | .005 | 0.37 (0.17-0.77) | .008 | 0.36 (0.18-0.74) | .005 |
| Race/ethnicity (reference: non-Hispanic White) | ||||||||
| African-American or Black | 1.72 (0.58-5.12) | .3 | - | - | - | - | 1.06 (0.34-3.32) | .9 |
| Asian | 0.92 (0.24-3.55) | .9 | - | - | - | - | 0.67 (0.15-2.97) | .6 |
| Hispanic | 2.66 (1.13-6.26) | .03 | - | - | - | - | 1.97 (0.77-5.04) | .2 |
| Median adherence | ||||||||
| Per unit increase | 0.98 (0.97-0.99) | .002 | - | - | 0.99 (0.98-1.00) | .1 | 0.99 (0.98-1.00) | .2 |
Model 1 adjusts for age at study enrollment, NCI risk group, blast cytogenetics and time from start of maintenance.
Model 2 adds adherence to model 1.
Model 3 adds race/ethnicity to model 2.
Discussion
In this secondary analysis of data from COG-AALL03N1, we show that children with ALL receiving maintenance therapy and living in extreme poverty experienced an almost 2-fold greater hazard of relapse than those not living in extreme poverty. Moreover, we show that although patients living in extreme poverty are less likely to achieve the critical level of mercaptopurine adherence, lack of adherence does not explain the association between poverty and relapse.
Poverty is associated with inferior health outcomes across several chronic health conditions in children, such as asthma, cystic fibrosis, congenital heart disease, and inflammatory bowel disease, to name a few.13-18 Expectedly, in a disease such as cancer that is associated with significant health care burdens and costs, the association of poverty and relapse risk in children with cancer is not surprising.5,6,19 As a key social determinant of health, poverty creates structural barriers that impede an individual from receiving quality health care. Although an in-depth discussion of the multifaceted framework surrounding poverty and inferior health outcomes is beyond the scope of this paper, we speculate the following mechanisms that may contribute to a greater risk of relapse in children with ALL living in extreme poverty. Individuals living in poverty may have greater difficulty accessing health care, either owing to lack of transportation or difficulty getting time off from work. Maintenance therapy for ALL relies on monthly visits to adjust dosing of oral chemotherapy; lack of transportation to oncology clinic may lead to inadequate surveillance of efficacy of treatment or toxicities during maintenance therapy, thus increasing relapse risk. Moreover, these monthly visits provide the opportunity to emphasize the importance of continued adherence to oral chemotherapy, which may explain the greater proportion of patients living in extreme poverty unable to maintain the critical level adherence required to reduce relapse risk. Nonadherence to oral mercaptopurine could also be associated with nonadherence to other medications during treatment, further affecting relapse risk. Financial distress among those living in poverty may limit their ability to pay out of pocket costs for medications. Poverty may promote food insecurity among families, which may promote prioritization of these basic needs over medication adherence. In addition, poor nutrition among families living in poverty may promote obesity, a well-known risk factor associated with increased risk of ALL relapse.20-23 Last but not least, living in poverty may cause chronic exposure to toxic stress, leading to overall poorer health and subsequently worse cancer-related outcomes.24-26
Several investigators previously have demonstrated the association of poverty with worse outcomes in children with cancer. Gupta et al conducted a meta-analysis demonstrating that low socioeconomic status is associated with worse survival in children with various types of cancers.19 A study by Bona et al in children with high-risk neuroblastoma, which showed inferior event-free and overall survival in children exposed to poverty, particularly underscores the hypothesis that poverty creates chronic stress and affects responses to antineoplastic therapies.5 A separate study by Bona et al in children with ALL examined risk of relapse among children living in poverty using aggregate US census data linked to ZIP codes. Although their study did not show a significantly lower event-free survival among children living in poverty, it did trend toward poorer overall survival while showing that children living in poverty experience relapse earlier (<36 months) than those not living in poverty. However, no study, to our knowledge, has examined individual-level poverty and risk of relapse in children with ALL. These data, along with our results showing greater proportion of patients living in extreme poverty who are unable to maintain critical adherence at a level of 95%, indicate that nonadherence to chemotherapy may partly explain the greater number of early relapses. A trend toward greater hazard of relapse among children living in poverty after adjusting for adherence in our multivariable models further highlights these data. Future studies aimed at improving adherence should consider assessing poverty and designing interventions specifically geared toward these patients.
A key strength of our study is assessing poverty based on individual household income while accounting for the number of household members. Although, to the best of our knowledge, our study is the first to show a greater risk of relapse among children with ALL living in extreme household poverty, the small number of events among each racial and ethnic subgroup as well as the strong correlation of race/ethnicity and poverty limits our statistical power to adjust for both in hazard regression models. As such, we are unable to assess the individual effects of race and ethnicity or poverty on the risk of relapse. However, race is a social construct and provides little to no mechanistic explanation for differences in health outcomes. Rather, differences in social determinant of health between racial groups, such as poverty, access to health care, education, food insecurities, transportation, etc are likely to mediate differences in outcomes and serve as key areas for future interventions. This is evident after inclusion of parental education in our models that reduced the hazard of relapse associated with extreme poverty and thus provides a better mechanistic understanding of the poverty-relapse relationship than simply racial categories.
There are several limitations to recognize in this secondary analysis. AALL03N1 was conducted before measurable residual disease (MRD) assessment was routinely performed as part of clinical care at end of induction (EOI) for patients with ALL. EOI MRD is highly predictive of relapse in ALL.27 Although a subgroup of patients who participated in AALL03N1 were also enrolled on frontline therapeutic trials and likely had EOI MRD assessment, AALL03N1 did not collect data on EOI MRD and subsequent risk stratified modification of therapeutic exposures. Thus, we are unable to adjust for EOI MRD in this analysis. We were also unable to account for the recently recognized high-risk subgroup of Philadelphia-like ALL (Ph-like ALL), more commonly seen in Hispanic children who were overrepresented on AALL03N1. Children of Hispanic ethnicity are disproportionately affected by poverty, have higher rates of Ph-like ALL, and are more likely to be EOI MRD positive than other children; therefore, future studies examining the association between poverty and relapse risk should adjust for all these variables.28,29 We were unable to use exact annual income for poverty assessment and relied on midpoints of income ranges assessed on AALL03N1. The use of 120% (median difference between income and poverty threshold) to categorize patients as living in extreme poverty is arbitrary; future studies are needed to confirm the relapse risk using this definition. In addition, African-American children with ALL were underrepresented in the MEMS subcohort that may have introduced selection bias in these subanalyses. Finally, AALL03N1 assessed income at study enrollment during maintenance. Financial hardships increase with time during early phases of ALL treatment and persist beyond the completion of therapy30,31; therefore, future studies should examine household income at various time points and its associated risk of relapse.
In summary, children with ALL receiving maintenance therapy living in extreme poverty face a greater hazard of relapse than children not living in extreme poverty. Nonadherence to oral chemotherapy is a potential mechanism for which interventions are being actively investigated by COG investigators. Future studies should implement screening measures for poverty and assist families with resources that ameliorate these financial hardships.
Acknowledgments
The authors acknowledge support from NCTN Operations Center grant (U10CA180886), NCTN Statistics & Data Center grant (U10CA180899), St. Baldrick’s Foundation, and NCORP grant (UG1CA189955) to the Children’s Oncology Group, as well as a National Institutes of Health/National Cancer Institute grant (R01 CA096670) to S.B.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: S.B. and W.L. designed AALL03N1; A.W. and S.B. designed this secondary analysis; A.W., Y.C., and S.B. performed statistical analysis; A.W. and S.B. wrote the initial draft of the manuscript; L.H., A.H., A.A., D.S.D., J.P.N., Y.R., A.K.R., A.T., F.L.W., and W.L. reviewed the initial draft of the manuscript and provided critical feedback; and all authors have reviewed and agreed upon this final version of the manuscript.
Conflict-of-interest disclosure: A.A. is a medical director at Servier Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Aman Wadhwa, Institute for Cancer Outcomes and Survivorship, University of Alabama at Birmingham, 1600, 7th Ave South, Lowder 500, Birmingham, AL 35226; e-mail: awadhwa@uabmc.edu.
References
Author notes
Data are available upon reasonable request to the author, Smita Bhatia (smitabhatia@uabmc.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal