In this issue of Blood, Kaczmarek et al1 elucidate distinct pathways of factor VIII (FVIII) uptake, trafficking, antigen presentation and CD4+ T-cell stimulation following IV administration to hemophilia A (HA) mice. FVIII avoided potential tolerizing interactions in the splenic red pulp, while innate immune and inflammatory pathways were shown to contribute to the anti-FVIII immune response.
Therapeutic options available to HA patients, whose bleeding disorder results from a lack of functional FVIII protein, have expanded considerably in the past 10 years.2 In addition to the development of engineered recombinant FVIII proteins with extended half-lives (EHL), which provide the significant benefit of requiring fewer infusions, the FVIII-mimicking bispecific antibody emicizumab now enables prophylactic treatment via even less frequent subcutaneous injections. Other novel therapies targeting anticoagulant pathways are under investigation but not currently approved by regulatory agencies, and FVIII replacement via gene therapy is also showing promise, but widespread availability, particularly for children, remains a future goal. The impact of emicizumab has been particularly striking as an effective therapy now available to the approximately 30% of patients with HA (among those with access to hemophilia care) who develop a neutralizing antibody (“inhibitor”) response to FVIII. However, despite these impressive achievements, breakthrough bleeds still occurred in some patients who were enrolled in emicizumab clinical trials, indicating FVIII supplementation will still be required or preferred periodically.
As real-world data become available, the efficacy and safety of both EHL-FVIII and non-FVIII replacement therapies will come into better focus. Even at this early stage of gaining experience with these novel therapies, however, FVIII replacement therapy remains the standard of care to treat breakthrough bleeds and in settings of trauma and to support surgeries.3 The major impediment to effective FVIII administration remains the development of inhibitors. Therefore, further progress in understanding the molecular and cellular mechanisms contributing to FVIII immunogenicity is needed to develop targeted therapies that can prevent or reverse inhibitor development.
Therapeutic FVIII is administered IV, and therefore its initial presentation to the FVIII-naïve immune system of a patient with severe HA occurs as it is filtered by the spleen. Fifteen years ago, van Schooten et al demonstrated, by fluorescent and immunohistochemistry imaging of tissues from von Willebrand factor (VWF)-deficient mice, that IV-administered FVIII and VWF were both internalized by splenic and liver macrophages.4 Subsequent studies have characterized splenic trafficking and cellular interactions of FVIII in mouse HA (FVIII-knockout) models,5-7 as well as splenic transcriptomes,8 following IV FVIII infusions. Both splenectomy and marginal zone B-cell depletion attenuated the anti-FVIII antibody responses of FVIII-knockout mice.5,6
The study in this issue by Kaczmarek et al sheds further light on the trafficking and immune sequelae of IV-administered FVIII. First, to follow the journey of FVIII after splenic uptake, the same dose of fluorescently labeled FVIII or ovalbumin (OVA) was administered to HA mice. Flow cytometry analysis of their splenocytes shortly afterwards showed both proteins were internalized by dendritic cells, marginal zone B cells and macrophages, and follicular and transitional B cells. Interestingly, only OVA was found in red pulp macrophages, indicating a distinct trafficking pattern of FVIII in the spleen.
In 1 set of parallel experiments, a B-domain–deleted FVIII construct with an OVA epitope, OVA323-339, engineered into the residual B-domain linker region (“FVIII-OVA”), or chicken OVA protein, or FVIII + OVA, or human factor IX (as an irrelevant antigen) was administered to FVIII-knockout mice. Labeled CD4+ T cells collected from OT-II transgenic mice, which are highly specific for the OVA323-339 epitope, were then injected into the mice, their spleens collected 5 days later, and the in vivo CD4+ T-cell proliferation analyzed by flow cytometry. FVIII-OVA was markedly more immunogenic than OVA protein administered at the same dose. Although T-cell proliferation was seen after OVA was injected at a 10 times higher dose, proliferation was enhanced when OVA was administered together with B-domain–deleted FVIII, suggesting FVIII had an adjuvant effect on the anti-OVA immune response. These results were consistent with an earlier study that compared FVIII and OVA immunogenicity.9 Further experiments showed that (nonhemophilic) mice with inflammatory pathway signaling deficiencies were less likely to develop inhibitors than wild-type mice injected with the same doses of human FVIII. Together, these results suggested that FVIII has intrinsic immunostimulatory/inflammatory properties. This conclusion is consistent with results of a recent transcriptomics study demonstrating up-regulation of both inflammatory and innate immune markers in FVIII-stimulated CD4+ T cells obtained from human subjects with HA and a current inhibitor.10
Specific types of antigen-presenting cells were then selectively depleted by chemical treatment or use of appropriate transgenic mice, and decreased proliferation of their splenocytes following injection of FVIII-OVA confirmed their roles in priming the immune response to FVIII. Additional experiments showed FVIII immunogenicity was enhanced when administered with a Toll-like receptor-9 agonist, and MyD88-deficient mice were less likely to develop anti-FVIII antibodies than wild-type mice. These results further indicated a role for inflammation in the risk of developing an inhibitor response to FVIII.
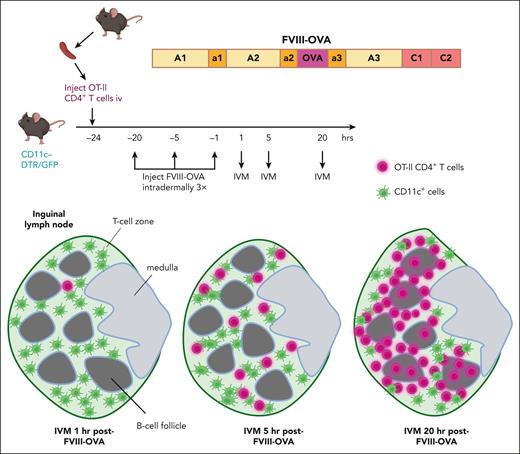
Finally, multiphoton intravital microscopy (IVM) experiments allowed visualization of specific CD4+ T cell trafficking through a lymph node shortly after administration of FVIII-OVA. (The inguinal lymph node is more amenable than the spleen to IVM, and it is a relevant model for the splenic white pulp area.) First, cell trace violet dye–labeled CD4+ cells from OT-II mice were injected into transgenic mice with green fluorescent protein-expressing CD11c+ cells. FVIII-OVA was injected intradermally several hours later, with IVM performed on the draining lymph node. The T cells formed clusters with the antigen-presenting cells throughout the T-cell zone 5 hours after FVIII-OVA administration, whereas at the 20-hour time point, the T cells were clustered at the T-B cell border, suggesting they had differentiated into T-follicular helper cells (see figure).
Tracking of specific CD4+ T cells following FVIII administration. CD4+ T cells from OT-II mice, which recognize an immunodominant OVA peptide that was engineered into a FVIII-OVA hybrid protein, were labeled with cell trace violet. These cells were then adoptively transferred into transgenic C57BL/6-CD11c-DTR/GFP mice, which were subsequently injected intradermally with 5 μg of FVIII-OVA 1, 5, or 20 hours before performing IVM of the draining lymph node. The violet T cells initially entered the green (CD11c+GFP+) T-cell zone, and by 20 hours after FVIII-OVA injection, most of these cells were clustered at the T-B cell zone at the border of B-cell follicles. This trafficking pattern suggested they had differentiated into T-follicular cells by 20 hours after exposure to FVIII-OVA. DTR, diphtheria toxin receptor; GFP, green fluorescent protein.
Tracking of specific CD4+ T cells following FVIII administration. CD4+ T cells from OT-II mice, which recognize an immunodominant OVA peptide that was engineered into a FVIII-OVA hybrid protein, were labeled with cell trace violet. These cells were then adoptively transferred into transgenic C57BL/6-CD11c-DTR/GFP mice, which were subsequently injected intradermally with 5 μg of FVIII-OVA 1, 5, or 20 hours before performing IVM of the draining lymph node. The violet T cells initially entered the green (CD11c+GFP+) T-cell zone, and by 20 hours after FVIII-OVA injection, most of these cells were clustered at the T-B cell zone at the border of B-cell follicles. This trafficking pattern suggested they had differentiated into T-follicular cells by 20 hours after exposure to FVIII-OVA. DTR, diphtheria toxin receptor; GFP, green fluorescent protein.
Overall, this elegant study provides both independent and confirmatory evidence indicating that intrinsic immunostimulatory properties of the FVIII protein contribute to its immunogenicity. As the authors point out, efficient trafficking of FVIII to splenic dendritic cells may well contribute to its immunogenicity by evading processes in the red pulp that promote tolerance to blood-borne self-antigens. Future studies could build on the present work by further characterizing the most relevant antigen-presenting cell subsets, as well as immunodominant epitopes, posttranslational modifications, and receptor-binding interactions of FVIII that contribute to its immunogenicity. Some engineered EHL-FVIII products may show distinct splenic interactions affecting immunogenicity, and the potential influence of VWF on FVIII trafficking11 could also be further explored.
The opinions or assertions contained herein are the private ones of the author and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal