In this issue of Blood, Tremblay et al demonstrate that STAT5B activation drives proliferation, self-renewal, and chemoresistance of leukemia stem cells (LSCs) in an early T-cell precursor acute lymphoblastic leukemia mouse model and explore STAT5B as a direct therapeutic target (see figure).1
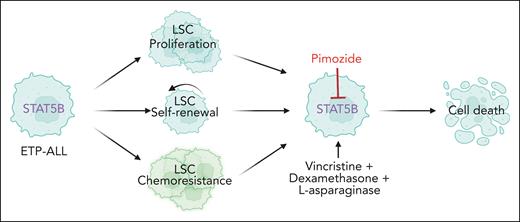
Activation of STAT5B confers proliferation, self-renewal, and chemoresistance of LSCs in early T-cell precursor acute lymphoblastic leukemia. Pimozide is a STAT5 inhibitor that sensitizes the LSCs to chemotherapy.
Activation of STAT5B confers proliferation, self-renewal, and chemoresistance of LSCs in early T-cell precursor acute lymphoblastic leukemia. Pimozide is a STAT5 inhibitor that sensitizes the LSCs to chemotherapy.
JAK/STAT signaling is a central pathway controlling differentiation, proliferation, and survival in the hematopoietic system and is constitutively activated in a variety of hematological malignancies.2 This activation can occur through mutations in receptors, the JAK kinases, the STAT transcription factors, and/or the negative regulators of this pathway. From the different STAT proteins, STAT5 has received most attention in hematological malignancies as it is widely implicated in oncogenic signaling.2,3 For example, STAT5 is known to be a key downstream effector of JAK2 in cases with JAK2 fusion genes, JAK2 mutations, or receptor overexpression/mutation (such as cytokine receptor like factor 2 overexpression or erythropoietin receptor mutations). Moreover, STAT5 is involved in other pathways beyond the classic JAK/STAT pathway and is also critical downstream of many other oncogenic kinases, such as BCR::ABL1 and mutant FLT3.2-4
STAT5 is, however, a misleading name because there are 2 STAT5 genes in the human genome, STAT5A and STAT5B, which are located next to each other on chromosome 17, in the same region as STAT3. The STAT5A and STAT5B proteins are similar, and they were initially considered to have redundant functions. An elegant and detailed study from Kollmann et al recently clarified important differences between these 2 STAT proteins in the hematopoietic system.4 They identified STAT5B as a critical protein for the self-renewal properties of normal hematopoietic stem cells, whereas STAT5A inactivation had minimal consequences.4 Moreover, knockout of Stat5b in BCR::ABL1 LSCs prevented leukemia development in immune-deficient mice, whereas Stat5a knockout did not impact leukemia development. LSCs derived from BCR::ABL1, FLT3-ITD, or JAK(V617F) leukemia models only showed strong STAT5B and not STAT5A phosphorylation in the nucleus, further indicating that STAT5B is the most important STAT5 downstream of oncogenic kinases in hematological malignancies.4
In T-cell acute lymphoblastic leukemia (T-ALL), STAT5B also seems to be the most important of the 2 STAT5 proteins. Several subtypes of T-ALL show a high frequency of mutations in the interleukin-7 receptor (IL-7R) signaling pathway, including mutations in the IL-7R itself, in JAK1 or JAK3 kinases, often in STAT5B, but never in STAT5A.5 Data from the Catalogue of Somatic Mutations in Cancer (COSMIC)6 reveal that the STAT5B N642H mutation is a clear hotspot mutation that is detected in various T-cell and myeloid malignancies, whereas there are no highly recurrent STAT5A mutations. Strikingly, retroviral expression of the STAT5B N642H mutant in mouse hematopoietic stem and progenitor cells is sufficient to drive their expansion ex vivo and to cause leukemia development in vivo, indicating that activation of the STAT5B target genes is critical for normal hematopoietic stem cells (HSCs) and LSCs.7
In the current study, Tremblay et al investigate the role of STAT5 in early T-cell precursor acute lymphoblastic leukemia (ETP-ALL). ETP-ALL cases are characterized by a high frequency of IL-7R/JAK/STAT pathway mutations and are known to be sensitive to JAK kinase inhibitors, such as ruxolitinib (which inhibits JAK2 and JAK1), even in the absence of IL-7R/JAK/STAT mutations.8 Using an Lmo2-driven T-ALL mouse model in which the Il7r gene was inactivated (Il7r–/–), Tremblay et al demonstrate that expression of activated STAT5B is sufficient to rescue the self-renewal capacity of the Il7r–/– LSCs. Moreover, using a novel mouse model with inducible expression of a constitutively activated STAT5B (H298R + S716F) mutant (also known as STAT5B1∗6), they show that activated STAT5B enhances T-ALL development in the Lmo2 leukemia model, and confers a cell-intrinsic advantage as well as chemoresistance to the LSCs.
On the basis of the central role of STAT5B in ETP-ALL, the authors questioned if direct inhibition of STAT5 would be a viable therapeutic avenue. Pimozide is one of the older drugs known to inhibit STAT5A and STAT5B, and on the basis of recent modeling, it was suggested that this drug binds STAT5B at the Asn642 amino acid, thereby preventing its phosphorylation.9 Despite the fact that high concentrations of pimozide are required to reduce STAT5 phosphorylation and to block cell proliferation mediated by STAT5, these effects seem specific.1 Indeed, cells transformed by IL-7R mutants or FLT3 mutants were sensitive to pimozide, whereas NRAS-transformed cells were not.1 Treatment of mice transplanted with Lmo2-driven LSCs demonstrated synergy between chemotherapy and pimozide treatment, as shown by effects on leukemia burden and survival. Although pimozide alone had only mild effects on leukemia burden in patient-derived xenograft models of ETP-ALL, pimozide enhanced the response to chemotherapy.1
This study, together with previous work on STAT5B, provides strong arguments for targeting STAT5B in T-ALL as well as other hematological malignancies.1,4,10 Pimozide is an interesting drug candidate, because it is already US Food and Drug Administration approved, but many questions remain. Pimozide has inhibitory effects on STAT5 but also impacts several other proteins, including receptors and ion channels. Currently, it is used as an antipsychotic drug at doses that do not affect STAT5 phosphorylation. It will be interesting to determine if pimozide or other agents that target STAT5 can be converted to more selective and more potent STAT5 targeting agents, perhaps by converting these to proteolysis-targeting chimeras. Studies to develop novel pimozide derivatives are underway,10,11 but the fact that STAT5 is also important in HSCs and T-cell development and that the drug would also target various ion channels might be problematic.4 That should not stop us from developing and testing more potent and selective STAT5B inhibitors, as kinase inhibitors were initially also received with skepticism.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal