Highly prothrombotic, platelet activating PF4-dependent anti-PF4 antibodies can occur without preceding heparin or vaccination.
These antibodies are detectable in some sera obtained before the COVID-19 pandemic and the vaccination program.
Visual Abstract
Platelet-activating anti-platelet factor 4 (PF4)/heparin antibodies and anti-PF4 antibodies cause heparin-induced thrombocytopenia (HIT) and vaccine-induced immune thrombocytopenia and thrombosis (VITT), respectively. Diagnostic and treatment considerations differ somewhat between HIT and VITT. We identified patients with thrombocytopenia and thrombosis without proximate heparin exposure or adenovirus-based vaccination who tested strongly positive by PF4/polyanion enzyme-immunoassays and negative/weakly positive by heparin-induced platelet activation (HIPA) test but strongly positive by PF4-induced platelet activation (PIPA) test (ie, VITT-like profile). We tested these patients by a standard chemiluminescence assay that detects anti-PF4/heparin antibodies found in HIT (HemosIL AcuStar HIT-IgG(PF4-H)) as well as a novel chemiluminescence assay for anti-PF4 antibodies found in VITT. Representative control sera included an exploratory anti-PF4 antibody-positive but HIPA-negative/weak cohort obtained before 2020 (n = 188). We identified 9 patients with a clinical-pathological profile of a VITT-like disorder in the absence of proximate heparin or vaccination, with a high frequency of stroke (arterial, n = 3; cerebral venous sinus thrombosis, n = 4), thrombocytopenia (median platelet count nadir, 49 × 109/L), and hypercoagulability (greatly elevated D-dimer levels). VITT-like serological features included strong reactivity by PIPA (aggregation <10 minutes in 9/9 sera) and positive testing in the novel anti-PF4 chemiluminescence assay (3/9 also tested positive in the anti-PF4/heparin chemiluminescence assay). Our exploratory cohort identified 13 additional patient sera obtained before 2020 with VITT-like anti-PF4 antibodies. Platelet-activating VITT-like anti-PF4 antibodies should be considered in patients with thrombocytopenia, thrombosis, and very high D-dimer levels, even without a proximate exposure to heparin or adenovirus vector vaccines.
Introduction
Antibodies directed against the chemokine platelet factor 4 (PF4) cause some of the most prothrombotic disorders in clinical medicine. The best known is heparin-induced thrombocytopenia (HIT), in which anti-PF4/heparin antibodies bind to epitopes exposed when (cationic) PF4 forms complexes with (anionic) heparin.1 Since 2001, atypical forms of HIT (eg, autoimmune HIT2) have been reported in which there is a high frequency of thrombosis (∼90%) and thrombocytopenia beginning or persisting after heparin discontinuation3 or triggered by very low doses (“flushes”) of heparin. Furthermore, although rare patients with spontaneous HIT4 were identified, they were not exposed to any heparin. The highly pathologic, anti-PF4/heparin antibodies in HIT and atypical HIT strongly activate platelets in the presence of heparin. They are different from the rather frequent (up to 5% of the population) clinically irrelevant antibodies identified in patients with periodontal disease5 or normal blood donors,6 which typically do not activate platelets.
Worldwide interest in anti-PF4 antibody–related prothrombotic disorders was stimulated when such antibodies were identified as the cause of vaccine-induced immune thrombocytopenia and thrombosis (VITT). VITT is characterized by severe thrombotic complications, hypercoagulability (high D-dimer levels), and thrombocytopenia typically occurring between 5 and 20 days after adenovirus vector–based COVID-19 vaccination.7-9 Nearly 90% of patients with VITT develop thrombosis, primarily cerebral venous sinus thrombosis (CVST) and splanchnic vein thrombosis, as well as deep vein thrombosis and pulmonary embolism, and less frequently arterial thrombosis.10,11 Early diagnosis and prompt start of therapeutic-dose anticoagulation, together with high-dose IV immunoglobulin (IVIG), improve patient outcome.12
Although both HIT and VITT antibodies usually test positive by standard microtiter-based anti-PF4/heparin enzyme-immunoassays (EIAs), the anti-PF4 antibodies in patients with VITT differ from the anti-PF4/heparin antibodies implicated in HIT. Antibodies in HIT are polyclonal, whereas VITT antibodies are monoclonal or oligoclonal13 with a very restricted hypervariable light-chain repertoire.14 Furthermore, antibodies in HIT bind to heparin-dependent antigen sites, whereas VITT antibodies bind at the heparin-binding site on PF4.15 A problem for clinical laboratories is that VITT antibodies are not recognized by widespread rapid assays for HIT antibodies16,17; moreover, VITT antibodies often test negative in the classic functional tests for HIT. These test performances have been overcome by a recently developed rapid chemiluminescence assay, which recognizes VITT antibodies but not most HIT antibodies,16 and by modifying the functional test by adding PF4 instead of heparin.7,18
Here, we report a novel clinical-pathological anti-PF4 disorder whereby patients present clinically and serologically similar to VITT (rather than HIT), that is, their clinical picture features such as thrombocytopenia, life-threatening thrombotic complications, and highly elevated D-dimer levels but without any proximate COVID-19 vaccination or heparin exposure. Serologically, the underlying cause is presence of anti-PF4 antibodies detected by anti-PF4/heparin EIAs, with features that are unlike HIT but rather characteristic of VITT (“VITT-like antibodies”), that is, negative testing by a rapid chemiluminescence test for HIT antibodies,19 negative or weakly positive testing by functional assay in the presence of heparin, but strongly positive in the presence of supplemented PF4.
By systematically analyzing sera obtained before 2020 from >300 patients for HIT-like anti-PF4/heparin and VITT-like anti-PF4 antibodies, we also show that VITT-like anti-PF4 antibodies were already present before the SARS-CoV-2 pandemic and the ensuing COVID-19 vaccination campaign. Clinicians should consider testing patients who present with aggressive thrombosis and thrombocytopenia for anti-PF4/heparin and/or anti-PF4 antibodies using a multi-well plate enzyme-linked immunosorbent assay, because this test is sensitive for both HIT and VITT/VITT-like antibodies. Based on novel assays developed to identify VITT antibodies, such patients with a VITT-like anti-PF4 disorder can now be identified.
Material and methods
Patients
VITT-like patients
We aimed to identify patients referred with a laboratory picture consistent with severe HIT or VITT, but without proximate exposure to heparin or (COVID-19) vaccination, and for whom a detailed clinical history was available. “Proximate” exposure was defined as any exposure to heparin or vaccination during the previous 3 months. Patients were included based on the following criteria: thrombocytopenia, thrombosis, and a strong-positive (>1.50 optical density [OD] units) anti-PF4/heparin immunoglobulin G (IgG) EIA, with negative (or weakly positive) heparin-dependent platelet activation assay but strong-positive PF4-dependent platelet activation assay (ie, serological features more suggestive of VITT than HIT). All 9 patients with VITT-like antibodies identified presented with thrombosis, including 7 patients with cerebral vascular occlusions, either arterial stroke (n = 3) or CVST (n = 4). Patients 1,20 2,21 and 822 have been previously reported as having atypical HIT (ie, “spontaneous” HIT). Patient 6 was treated successfully with ibrutinib after this report was submitted.23
Anti-PF4/heparin EIA-negative control groups from before 2020
A total of 155 participants, including 44 healthy blood donors, 79 clinically symptomatic patients with thrombocytopenia and/or thrombosis referred for exclusion of HIT, and 32 patients without thrombosis (controls for prevalence of these antibodies in the healthy population, patients with suspected HIT, and in-hospital patients) were included. All tested negative by anti-PF4/heparin IgG EIA and negative heparin-induced platelet-activation assay (HIPA). Patient sera were referred to the Greifswald laboratory from 2014 to 2019 (inclusive); this time period was chosen so as to avoid overlap with COVID-19 (onset in Europe in early 2020) or vaccination-induced antibodies (since March 2021).
Control group with stroke and thrombocytopenia
To exclude that anti-PF4 antibodies are an epiphenomenon in patients with cerebral vascular occlusions, serum from an additional 59 patients with stroke and thrombocytopenia (platelet count <150 x 103/μL) were identified from 621 patients with mild to moderate incident ischemic stroke from PROSCIS-B24 (study details are given in the supplemental Material [Prospective Cohort with Incident Stroke study (PROSCIS-B)] available on the Blood website).
Patients with antiphospholipid syndrome
We tested 20 patients with confirmed antiphospholipid syndrome, who gave informed consent and are described in detail elsewhere.25 These plasma samples had been collected before the patients received COVID-19 vaccinations; none had a COVID-19 infection before blood sampling.
Patients with HIT from before 2020
Consecutive patients with classic HIT with positive anti-PF4/heparin IgG EIA and HIPA26 and negative reactivity at 0 U/mL heparin (buffer control) (n = 131) were included.
Patients with VITT
Exploratory cohort
Consecutive patients from before 2020 with a laboratory test reactivity pattern potentially indicative of VITT, that is, strong reactivity (defined as >1.5 OD units) in the anti-PF4/heparin IgG EIA but negative HIPA (n = 188). All patients were clinically symptomatic with thrombocytopenia and/or thrombosis and had been referred to exclude HIT, but more detailed clinical history was not available. The aim of this exploratory cohort was to determine whether VITT-like antibodies could be identified in patient samples obtained before the COVID-19 pandemic and the vaccination campaign.
Serum or citrated plasma, prepared from whole blood by centrifugation, was stored at −70°C until use.
Antibody assays
We used 2 rapid assays to detect anti-PF4/heparin and anti-PF4 antibodies, respectively, using chemiluminescence technology (described subsequently): the HemosIL AcuStar HIT-IgG(PF4-H) assay, hereafter called rapid anti-PF4/heparin assay (the assay uses PF4/polyvinyl sulfonate complexes [PVS]); and a novel anti-PF4 antibody assay prototype for the ACL AcuStar, hereafter called new rapid anti-PF4 assay.16 Both assays are 2-step chemiluminescence immunoassays consisting of magnetic particles coated either with PF4 complexed to PVS or with PF4 alone. The new rapid anti-PF4 assay was designed to detect antibodies that only recognize anti-PF4 antibodies (as seen in VITT) with no or minimal cross-reactivity with anti-PF4/heparin antibodies. Serum (1:11) and citrated plasma (1:10) in sample diluent were used for analysis. After incubation, magnetic separation and a wash step, beads were incubated with an isoluminol-labeled anti-human IgG antibody. After a final wash, reagents that trigger the luminescence reaction were added, and the emitted light was measured as relative light units (RLUs). The RLUs are directly proportional to the anti-PF4/heparin or anti-PF4 IgG concentration in the sample. The cutoff for the rapid anti-PF4/heparin assay was calculated using calibrators, as per manufacturer’s instructions. The cutoff of the new rapid anti-PF4 assay was determined by a receiver operating characteristic curve analysis between VITT and control samples. In the commercially available rapid anti-PF4/heparin assay results are usually shown as U/mL. Because no units have yet been established for the new rapid anti-PF4 assay, results for both assays are provided as generic RLUs for better comparability. Stability of sample results over time is shown in supplemental Figure 1.
The microtiter plate-based in-house anti-PF4/heparin IgG EIA was performed as described (assay details are given in the supplemental Material [Anti-PF4/heparin IgG enzyme-immunoassay]),7 with the following criteria: cutoff OD < 0.5; OD, 0.5 to <1.0, weakly positive; OD, 1.0 to <1.5, moderately positive; and OD ≥1.5, strongly positive.
Platelet activation assays
Platelet activation induced by patient antibodies was tested using a washed platelet assay, either in the presence of heparin (0.2 anti-factor Xa U/mL [HIPA]) or PF4 (10 μg/mL [PIPA]; assay details are given in the supplemental Material [PF4-dependent platelet activation assay]).7 A positive result was defined as activation of platelets (lag time ≤30 minutes) of at least 2 of 3 different donors and inhibition at high heparin concentrations (100 IU/mL). Reactivity lag time from 20 to 30 minutes was defined as weak, between 10 and 20 minutes as moderate, and ≤10 minutes as strong reactivity. For sera that tested strongly positive with buffer, we performed dilutions with saline until buffer reactivity became negative and retested them with heparin 0.2 aFXa U/mL and PF4 10 μg/mL. Addition of PF4 enhances reactivity of functional tests for platelet-activating anti-PF4 VITT antibodies, without substantial loss of diagnostic specificity27 (supplemental Material.)
Anti-PF4 antibody purification and mass spectrometric analysis
Proteomic profiling of anti-PF4 antibodies was performed as described previously.14 In brief, anti-PF4 antibodies were purified from patient serum using PF4 protein-coupled MyOne Carboxylic Acid Dynabeads (ThermoFisher). The beads were coated with human PF4 (ChromaTec, Greifswald, Germany) in 15 mM 2-morpholinoethanesulfonic acid (MES) buffer after activation with 100 μL of 1-ethyl-3-(3dimethylaminopropyl) carbodiimide (10 mg/mL). Diluted serum was added to the PF4-coated beads and mixed on a rotator for 2 hours at room temperature. After incubation, unbound sera were removed, and the bound anti-PF4 antibodies were eluted using 100 mM glycine elution buffer (pH 11). Purified IgGs were separated by reduced sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (criterion stain-free TGX gels; Bio-Rad, Hercules, CA). Purified antibodies were retested by anti-PF4-EIA. In-gel tryptic and chymotryptic digests were performed on the 50 kd heavy-chain and 25 kd light-chain gel bands and peptides analyzed using an Orbitrap Exploris 480 mass spectrometer (ThermoFisher Scientific) coupled to an Ultimate 3000 UHPLC (Dionex, USA). Peptide sequences were analyzed by de novo sequencing and International ImMunoGeneTics (IMGT) database matching using Peaks studio XPro software (Bioinformatics Solution Inc, Waterloo, ON, Canada). Parameters for database searches, data refinement and Ig variable region subfamily assignments were described previously14,28,29; in brief, a maximum of 2 missed cleavages, precursor tolerance of <15 parts per million, product ion tolerance of 0.02 Da, precursor charge state of +2 to +4, fixed modification carbamidomethylation, variable modifications oxidation and deamidation, a maximum of 3 modifications allowed, and nonspecific cleavage at 1 end. High-quality de novo peptides were selected based on sequences having an average local confidence score threshold ≥80% and inspected manually to ensure correct assignments. A false discovery rate threshold of 1.0% was applied at the peptide level to each data set. The Ig variable region subfamily is assigned from the presence of a unique peptide corresponding to the subfamily.
Statistical analysis
Data are presented descriptively.
Ethics and data protection rules
The study was approved by the ethics board of the Universitätsmedizin Greifswald (BB 052/21a). Permission to describe patient cases has been obtained. To respect confidentiality, patient ages are given as ranges. For the exploratory cohort, the ethics commission permitted retesting of deidentified repository serum samples from before 2020, selected by the original test result.
Results
VITT-like patients without proximate exposure to heparin or vaccination
Nine patients were identified who met our inclusion criteria of thrombocytopenia, thrombosis, and strongly positive PF4-dependent EIA and for whom clinical information was available. None had proximate heparin exposure or (COVID-19) vaccination. Their platelet activation test profile was more typical for VITT than for HIT (ie, weak/negative HIPA but strong-positive PIPA). The median platelet count nadir was 49 × 109/L (range, 22-81). All 8 patients who had D-dimers measured showed greatly elevated levels (>30-35 mg/L), a characteristic feature of VITT.11 Although most patients who survived exhibited an acute, self-limited prothrombotic disorder, patients 1 and 6 presented with recurrent episodes of thrombocytopenia and thrombosis over 6 and 13 years, respectively. In 5 patients (55.6%), an infection preceded the thrombotic episode (patients 2, 5, and 7-9). Patients 1, 4, and 6 presented with arterial stroke; patients 2, 5, 8, and 9 with CVST. All patients tested negative for antiphospholipid and anti-nuclear antibodies. The clinical characteristics of the patients are shown in Table 1.
Clinical characteristics of the 9 patients with VITT-like prothrombotic disease without proximate exposure to heparin or vaccination
| . | Patient 1∗ . | Patient 2† . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8‡ . | Patient 9 . |
|---|---|---|---|---|---|---|---|---|---|
| Sex, age (y) | Female, 70-80 y | Female, 30-40 y | Female, 70-80 y | Male, 60-70 y | Female, 20-30 y | Female, 30-40 y | Male, 60-70 y | Male, 20-30 y | Male, 5-10 y |
| Symptoms | Variable | Severe Headache | Dyspnea | Neurological symptoms | Common cold, severe headache with hemianopia | N/A | Dyspnea | Severe headache | Severe headache, vomiting, nausea |
| Diagnosis | Recurrent DVT and PE, stroke over 3 y | CVST with secondary bleeding | PE | Stroke with secondary intracranial bleeding | CVST with secondary bleeding, PE, portal vein thrombosis | Recurrent venous and arterial thrombotic events over 13 years, including stroke | Bilateral DVT with PE | CVST with secondary bleeding | CVST |
| Preceding infection (interval to admission) | — | Common cold (7 d) | — | — | Respiratory syncytial virus (unknown, active at admission) | — | Urinary tract infection (11 d) | Common cold (14 d) | Adenovirus (10 d) |
| Other underlying disease | Monoclonal gammopathy | — | — | — | — | — | Crohn‘s disease | — | — |
| Therapy | Multiple anticoagulants and platelet aggregation inhibitors | Argatroban, IVIG | Unfractioned heparin, fondaparinux, apixaban | Plasma exchange, fondaparinux, apixaban | Unfractionated heparin, followed by VKA | Multiple anticoagulants and platelet aggregation inhibitors, over 13 y, IVIG, plasmapheresis | Patient refused further therapy | Unfractioned heparin; followed by fondaparinux; prednisolone | LMWH, rivaroxaban, dexamethasone, acetazolamide |
| Outcome | PLT count stabilized in lower reference range, lately no further thromboembolism | Fatal | Recovered | Recovered with persistent sequelae from stroke | Recovered | Recurrent relapses | Fatal | Recovered with persistent sequelae from CVST | Recovered |
| . | Patient 1∗ . | Patient 2† . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8‡ . | Patient 9 . |
|---|---|---|---|---|---|---|---|---|---|
| Sex, age (y) | Female, 70-80 y | Female, 30-40 y | Female, 70-80 y | Male, 60-70 y | Female, 20-30 y | Female, 30-40 y | Male, 60-70 y | Male, 20-30 y | Male, 5-10 y |
| Symptoms | Variable | Severe Headache | Dyspnea | Neurological symptoms | Common cold, severe headache with hemianopia | N/A | Dyspnea | Severe headache | Severe headache, vomiting, nausea |
| Diagnosis | Recurrent DVT and PE, stroke over 3 y | CVST with secondary bleeding | PE | Stroke with secondary intracranial bleeding | CVST with secondary bleeding, PE, portal vein thrombosis | Recurrent venous and arterial thrombotic events over 13 years, including stroke | Bilateral DVT with PE | CVST with secondary bleeding | CVST |
| Preceding infection (interval to admission) | — | Common cold (7 d) | — | — | Respiratory syncytial virus (unknown, active at admission) | — | Urinary tract infection (11 d) | Common cold (14 d) | Adenovirus (10 d) |
| Other underlying disease | Monoclonal gammopathy | — | — | — | — | — | Crohn‘s disease | — | — |
| Therapy | Multiple anticoagulants and platelet aggregation inhibitors | Argatroban, IVIG | Unfractioned heparin, fondaparinux, apixaban | Plasma exchange, fondaparinux, apixaban | Unfractionated heparin, followed by VKA | Multiple anticoagulants and platelet aggregation inhibitors, over 13 y, IVIG, plasmapheresis | Patient refused further therapy | Unfractioned heparin; followed by fondaparinux; prednisolone | LMWH, rivaroxaban, dexamethasone, acetazolamide |
| Outcome | PLT count stabilized in lower reference range, lately no further thromboembolism | Fatal | Recovered | Recovered with persistent sequelae from stroke | Recovered | Recurrent relapses | Fatal | Recovered with persistent sequelae from CVST | Recovered |
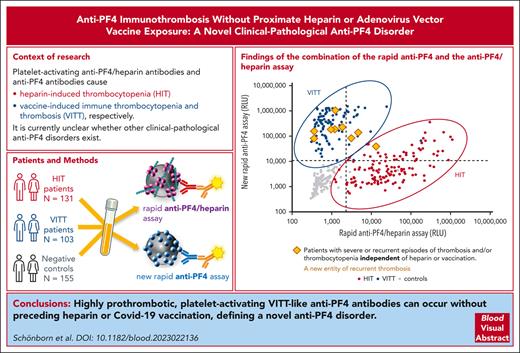
Their laboratory characteristics are given in detail in Table 2. By anti-PF4/heparin IgG EIA, the median OD was 2.55 (range, 1.99-3.10). All showed strong platelet activation (within 5 minutes) in the presence of PF4 (PIPA), with only 3 (patients 4, 7, and 8) also testing (weakly) positive in the HIPA, with low-dose heparin inhibiting the buffer reaction causing a longer lag time (ie, a feature uncharacteristic of HIT). All 9 sera tested positive in the new rapid anti-PF4 assay; 3 of 9 (33%) also tested positive in the rapid anti-PF4/heparin assay (Figure 1). The numbers in Figure 1 refer to the numbers in the Tables 1 and 2. In patients 2, 4, 5, 6, 7, and 8, a monoclonal gammopathy was excluded, and although untested, it was extremely unlikely in patient 9 (age <10 years).
Laboratory characteristics of the 9 patients with VITT-like anti-PF4 antibodies without proximate exposure to heparin or vaccination
| . | Patient 1∗ . | Patient 2† . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8‡ . | Patient 9 . |
|---|---|---|---|---|---|---|---|---|---|
| Platelet count nadir, × 109/L | 81 | 49 | 22 | 41 | 43 | 50 | 55 | 24 | 49 |
| D-dimer level (FEU; < 0.5 mg/L) | 10.4 mg/L | >35 mg/L | >35 mg/L | n.d. | >30 mg/L | >10 mg/L | 124 mg/L | >20mg/L | >35 mg/L |
| Anti-PF4/heparin IgG EIA (OD) | Strongly positive (3.10) | Strongly positive (2.22) | Strongly positive (3.04) | Strongly positive (1.99) | Strongly positive (2.55) | Strongly positive (2.1) | Strongly positive (2.76) | Strongly positive (2.72) | Strongly positive (2.30) |
| HIPA | Negative | Negative | Negative | Weakly positive (inhibited by low-dose heparin§) | Negative | Negative or weakly positive over 13 years (inhibited by low-dose heparin§) | Weakly positive (inhibited by low-dose heparin§) | n.d. (SRA with 0.1 U/mL UFH strongly positive22) | Negative |
| PIPA | Strongly Positive (lag time ≤5 min) | Strongly Positive (lag time ≤5 min) | Strongly positive (lag time ≤5 min) | Strongly positive (lag time ≤5 min) | Strongly positive (lag time ≤5 min) | Strongly positive (lag time ≤5 min) | Strongly positive (lag time ≤5 min) | n.d. (VITT-like profile in the fluid-phase EIA22) | Strongly positive (lag time ≤5 min) |
| HemosIL Acustar HIT IgG (PF4-H) (= rapid anti-PF4/heparin assay) | Negative | Negative | Negative | Positive | Positive | Positive | Negative | Negative | Negative |
| Prototype VITT IgG (PF4) assay (= new rapid anti-PF4 assay) | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
| MS sequencing of the light-chain variable region subfamily | IGKV1-5∗01 | IGKV1-39∗01 | IGLV3-21∗02 | IGLV1-51∗01 | n.d. | IGLV3-21∗02; IGKV3-20∗01 | n.d. | n.d. | n.d. |
| . | Patient 1∗ . | Patient 2† . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8‡ . | Patient 9 . |
|---|---|---|---|---|---|---|---|---|---|
| Platelet count nadir, × 109/L | 81 | 49 | 22 | 41 | 43 | 50 | 55 | 24 | 49 |
| D-dimer level (FEU; < 0.5 mg/L) | 10.4 mg/L | >35 mg/L | >35 mg/L | n.d. | >30 mg/L | >10 mg/L | 124 mg/L | >20mg/L | >35 mg/L |
| Anti-PF4/heparin IgG EIA (OD) | Strongly positive (3.10) | Strongly positive (2.22) | Strongly positive (3.04) | Strongly positive (1.99) | Strongly positive (2.55) | Strongly positive (2.1) | Strongly positive (2.76) | Strongly positive (2.72) | Strongly positive (2.30) |
| HIPA | Negative | Negative | Negative | Weakly positive (inhibited by low-dose heparin§) | Negative | Negative or weakly positive over 13 years (inhibited by low-dose heparin§) | Weakly positive (inhibited by low-dose heparin§) | n.d. (SRA with 0.1 U/mL UFH strongly positive22) | Negative |
| PIPA | Strongly Positive (lag time ≤5 min) | Strongly Positive (lag time ≤5 min) | Strongly positive (lag time ≤5 min) | Strongly positive (lag time ≤5 min) | Strongly positive (lag time ≤5 min) | Strongly positive (lag time ≤5 min) | Strongly positive (lag time ≤5 min) | n.d. (VITT-like profile in the fluid-phase EIA22) | Strongly positive (lag time ≤5 min) |
| HemosIL Acustar HIT IgG (PF4-H) (= rapid anti-PF4/heparin assay) | Negative | Negative | Negative | Positive | Positive | Positive | Negative | Negative | Negative |
| Prototype VITT IgG (PF4) assay (= new rapid anti-PF4 assay) | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
| MS sequencing of the light-chain variable region subfamily | IGKV1-5∗01 | IGKV1-39∗01 | IGLV3-21∗02 | IGLV1-51∗01 | n.d. | IGLV3-21∗02; IGKV3-20∗01 | n.d. | n.d. | n.d. |
FEU, fibrinogen equivalent units; LMWH, low molecular weight heparin; MS, mass spectrometry; n.d., not done; PLT count, platelet count; SRA, serotonin release assay.
Detailed course described in Greinacher et al.20
Detailed course described in Greinacher et al.21
Detailed course described in Warkentin et al.22
Inhibition by low-dose heparin indicates increased lag time (vs buffer). Patient included because >95% serotonin-release was seen at both 0 and 0.1 U/mL heparin, and the fluid-phase EIA confirmed VITT rather than HIT reaction pattern.
Results of the 9 patients with VITT-like features (Tables 1 and 2) with thrombocytopenia and thrombosis without proximate exposure to heparin or vaccination. Results of the rapid anti-PF4/heparin assay are given on the x-axis (cutoff: dashed vertical line). Results of new rapid anti-PF4 assay are given on the y-axis (cutoff: dashed horizontal line). Patient samples shown in green (patients 4, 7, and 8) were positive both in the PF4-dependent functional assay and a heparin-dependent functional assay, whereas patient samples in orange (patients 1, 2, 3, 5, and 6∗) exclusively tested positive in the PF4-dependent functional assay. Detail courses of patients 1,20 2,21 and 822 were previously described. ∗Some samples of patient 6 from previous time points were weakly positive in the heparin-dependent functional assay and strongly positive in the PF4-dependent functional assay. Treatment of this patient after the time covered by this report is summarized in Lindhoff-Last et al.23
Results of the 9 patients with VITT-like features (Tables 1 and 2) with thrombocytopenia and thrombosis without proximate exposure to heparin or vaccination. Results of the rapid anti-PF4/heparin assay are given on the x-axis (cutoff: dashed vertical line). Results of new rapid anti-PF4 assay are given on the y-axis (cutoff: dashed horizontal line). Patient samples shown in green (patients 4, 7, and 8) were positive both in the PF4-dependent functional assay and a heparin-dependent functional assay, whereas patient samples in orange (patients 1, 2, 3, 5, and 6∗) exclusively tested positive in the PF4-dependent functional assay. Detail courses of patients 1,20 2,21 and 822 were previously described. ∗Some samples of patient 6 from previous time points were weakly positive in the heparin-dependent functional assay and strongly positive in the PF4-dependent functional assay. Treatment of this patient after the time covered by this report is summarized in Lindhoff-Last et al.23
Control cohorts
Negative controls
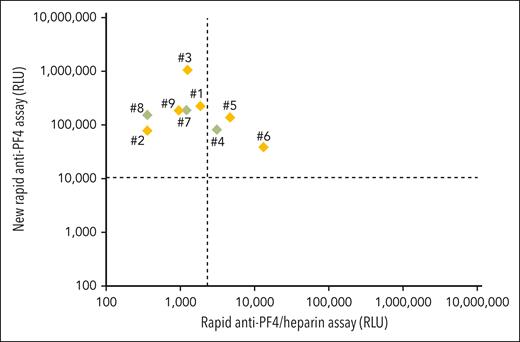
Of 155 negative controls, 152 (98.1%) tested negative in both rapid chemiluminescence assays, and none tested positive in the new rapid anti-PF4 assay (the 3 that tested positive in the rapid anti-PF4/heparin assay belonged to the patient subgroup referred with suspected HIT but who tested negative in the anti-PF4/heparin EIA and the HIPA). All 44 healthy controls and 32 patient controls tested negative in both assays (lower left quadrant Figure 2). Of the 59 patients with stroke and thrombocytopenia, only 1 (1.7%) showed a very weak result in the anti-PF4/heparin IgG EIA (OD, 0.62; results not included into the figures).
Rapid assay results of patient sera with HIT, VITT, and negative controls. Results of the rapid anti-PF4/heparin assay are given on the x-axis (cutoff: dashed vertical line). Results of the new rapid anti-PF4 assay are given on the y-axis (cutoff: dashed horizontal line). Sera of patients with heparin-induced thrombocytopenia are shown in red (n = 131), VITT sera in blue (n = 103), and negative controls in gray symbols (n = 155). None of the 16 VITT sera reacting positive with the rapid anti-PF4/heparin assay (blue dots upper right quadrant) tested positive with 0.2 aFXaU heparin in the heparin-dependent platelet activation assay (12 undiluted; 4 at an informative serum dilution [giving negative buffer reactivity]).
Rapid assay results of patient sera with HIT, VITT, and negative controls. Results of the rapid anti-PF4/heparin assay are given on the x-axis (cutoff: dashed vertical line). Results of the new rapid anti-PF4 assay are given on the y-axis (cutoff: dashed horizontal line). Sera of patients with heparin-induced thrombocytopenia are shown in red (n = 131), VITT sera in blue (n = 103), and negative controls in gray symbols (n = 155). None of the 16 VITT sera reacting positive with the rapid anti-PF4/heparin assay (blue dots upper right quadrant) tested positive with 0.2 aFXaU heparin in the heparin-dependent platelet activation assay (12 undiluted; 4 at an informative serum dilution [giving negative buffer reactivity]).
Of the 20 patients with confirmed antiphospholipid syndrome, none reacted positive in the PF4/heparin microtiter plate EIA, but 6 reacted positive in the new anti-PF4 assay, and 3 yielded borderline positive results (results not included into the figures).
Patients with HIT
In the rapid anti-PF4/heparin assay, 127 sera of 131 (96.9%) tested positive; 40 sera (30.5%) tested positive in the new rapid anti-PF4 assay (upper quadrants Figure 2). In 26 of those 40 sera, enough material was available for retesting in the PF4-dependent platelet activation assay (PIPA); 15 of 26 (57.7%) tested positive, confirming previous observations that addition of PF4 enhances sensitivity for antibodies in HIT.27,30
Patients with VITT
All 103 VITT sera tested positive by PF4-dependent platelet-activation assay, and all but one (99.0%) reacted positive in the new rapid anti-PF4 assay. Sixteen sera (15.5%) also tested positive in the rapid anti-PF4/heparin assay (upper right quadrant Figure 2). However, heparin did not enhance platelet activation in these 16 sera.
Exploratory cohort of patients with strongly positive anti-PF4/heparin EIA but negative HIPA
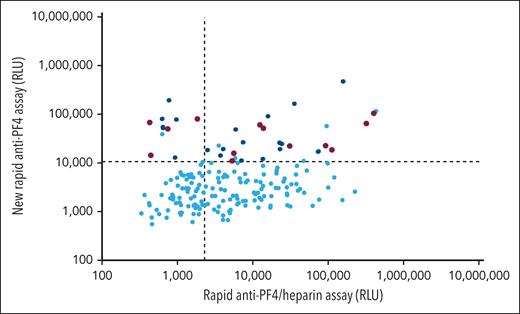
Next, we performed an exploratory analysis to determine whether VITT-like antibodies may have been previously unrecognized among referred sera from patients with thrombocytopenia and/or thrombosis. Because patients with VITT typically show strongly positive results in the anti-PF4/heparin IgG EIA, but often test negative by HIPA, we reanalyzed sera of clinically symptomatic patients (thrombocytopenia and/or thrombosis) referred to our laboratory before 2020 that showed this laboratory profile. Of such 188 unique sera from patients (Figure 3), 40 (21.3%) tested positive by the new rapid anti-PF4 assay (upper left and right quadrant) and 117 (62.2%) tested positive by the anti-PF4/heparin assay (upper and lower right quadrant; it is known that microtiter plate-based anti-PF4/heparin EIAs detect a greater number of clinically irrelevant antibodies compared with the chemiluminescence assay). Of those 40 patients with positive new rapid anti-PF4 assay and sufficient available serum (n = 33), 13 (39.4%) tested positive by PF4-dependent platelet activation assay (PIPA) (4 in the upper left quadrant [ie, strongly VITT-mimicking chemiluminescence assay profile] and 9 in the upper right quadrant). In all 13 patients, the initially negative HIPA result was reproduced (ruling out an initial false-negative result). Because these samples were referred to our laboratory before the COVID-19 pandemic, detection of these VITT-like antibodies cannot be explained either by the COVID-19 pandemic or by adenovirus vector–based vaccines against COVID-19.
Exploratory cohort of patients with strong reactivity in the in-house anti-PF4/heparin IgG EIA but negative heparin-dependent functional assay (n = 188). Results of the rapid anti-PF4/heparin assay are given on the x-axis (cutoff: dashed vertical line). Results of the new rapid anti-PF4 assay are given on the y-axis (cutoff: dashed horizontal line). Of these 188 patients, 40 (21.3%) tested positive by the new rapid anti-PF4 assay (upper left and right quadrant). Of those with sufficient available serum (n = 33, dark blue), 13 (39.4%) tested positive by PF4-dependent platelet activation assay (dark blue with red outline; 4 in the upper left quadrant and 9 in the upper right quadrant). ASA, acetylsalicyl acid; DVT, deep vein thrombosis; LMWH, low molecular weight heparin; PE, pulmonary embolism; VKA, vitamin K antagonists.
Exploratory cohort of patients with strong reactivity in the in-house anti-PF4/heparin IgG EIA but negative heparin-dependent functional assay (n = 188). Results of the rapid anti-PF4/heparin assay are given on the x-axis (cutoff: dashed vertical line). Results of the new rapid anti-PF4 assay are given on the y-axis (cutoff: dashed horizontal line). Of these 188 patients, 40 (21.3%) tested positive by the new rapid anti-PF4 assay (upper left and right quadrant). Of those with sufficient available serum (n = 33, dark blue), 13 (39.4%) tested positive by PF4-dependent platelet activation assay (dark blue with red outline; 4 in the upper left quadrant and 9 in the upper right quadrant). ASA, acetylsalicyl acid; DVT, deep vein thrombosis; LMWH, low molecular weight heparin; PE, pulmonary embolism; VKA, vitamin K antagonists.
Discussion
We report a novel clinical-pathological anti-PF4 disorder characterized by thrombosis, thrombocytopenia, and presence of VITT-like, rather than HIT/HIT-like, antibodies, despite the absence of proximate heparin treatment or vaccination. Affected patients were of all age groups and presented with venous and arterial thromboses, some at unusual sites, as seen in VITT11 (Table 1). Typically, D-dimer levels were highly elevated, and platelet counts ranged from 22 × 109 to 81 × 109 per liter (nadir values). Besides thrombosis at unusual sites, a history of infection often preceded the thrombotic episodes by 5 to 14 days, as seen in patients 2, 5, 7, 8, and 9 (5 of 9 [56%]), an observation that parallels VITT, in which a similar time interval was seen after vaccination with an adenovirus vector–based vaccine. Of interest, in patient 9, adenovirus was identified as the pathogen responsible for the preceding infection. (Information on preceding viral infections was not obtained systematically.)
Three of our patients with VITT-like antibodies had been recognized in the past and had been classified as spontaneous HIT.20-22 However, in the functional test for HIT antibodies, heparin inhibited platelet activation of these sera (buffer reactivity stronger than heparin reactivity; Table 2), which is unusual for spontaneous HIT.4 The other patients we describe do not resemble spontaneous HIT, because they tested negative in the presence of heparin in the functional HIPA assay. In addition, it was recently shown using fluid-phase EIA that serum antibodies identified from patients with spontaneous HIT after orthopedic surgery generally do not show VITT-like serological features (rather, these sera contain heparin-dependent antibodies, as seen in HIT22). Potentially, similar patients with VITT-like antibodies as described here have not been recognized in the past, because referring samples for HIT antibody testing is not common in the absence of heparin exposure. Patient 9 was only referred because concurrence of CVST and thrombocytopenia in the setting of an adenovirus infection triggered suspicion of a VITT-like disorder by the clinician.
Of clinical relevance, the thrombotic episodes in these patients with VITT-like features are similarly life-threatening as in VITT. In VITT, early recognition of patients and treatment with therapeutic-dose anticoagulation and high-dose IVIG12,31 reduced mortality by up to 90%.32 As in the entity known as pre-VITT,33 4 of our patients initially presented with severe headache in the emergency room (with initially negative imaging studies) and only later developed overt radiologically-evident CVST (patient 2, 5, 8, and 9), progression of which might have been prevented by early treatment with IVIG.33
The triad of thrombosis, thrombocytopenia, and high D-dimer levels should prompt clinical suspicion for the presence of VITT-like platelet-activating anti-PF4 antibodies. Confirmation of the diagnosis is now possible using our novel rapid immunoassay specific for anti-PF4 antibodies that target the heparin-binding site on PF4, in combination with an established rapid antigen assay for anti-PF4/heparin antibodies, that is, antibodies recognizing PF4 when heparin is bound (Figure 1). This combination of 2 rapid chemiluminescence immunoassays that target 2 distinct antigen sites on PF4 now allows for rapid testing for the presence of these 2 distinct anti-PF4 antibody specificities featuring heparin-dependent and heparin-independent platelet-activating properties, respectively. Patients can also be recognized by commercially available standard anti-PF4/heparin EIA and a functional assay for PF4-dependent platelet-activating antibodies. Without availability of a functional assay, a strongly positive standard anti-PF4/heparin EIA and a negative rapid anti-PF4/heparin assay in patients presenting with the triad of thrombosis, thrombocytopenia, and high D-dimer levels are consistent with VITT-like antibodies and can support clinical decision making. These antibodies can also be differentiated by a recently reported fluid-phase EIA,22 as well as by their different reactivity profiles when performing platelet activation assays in the presence of heparin or PF4. However, these assays are neither widely available nor suitable for screening large patient cohorts.
As reported previously,34,35 we found anti-PF4 antibodies in a subset of patients with confirmed antiphospholipid syndrome. Further systematic studies are needed to better understand the potential clinical relevance.
To further characterize the VITT-like anti-PF4 antibodies, we affinity-purified these antibodies in 5 patients and determined the sequence of their light-chain variable region subfamily by mass spectrometry (Table 2). Similar to VITT, these antibodies were monoclonal or oligoclonal.13 Although in VITT IGLV3-21∗02 seems to be the predominant subtype,14 patients with VITT-like antibodies showed a more heterogenous pattern.
The exploratory analysis of 188 patient sera from before 2020 (Figure 3) shows that these VITT-like antibodies are not a new phenomenon caused by the SARS-CoV-2 pandemic or vaccination with adenovirus vector–based vaccines. Although they tested negative in the HIPA, 13 of these patients showed very strong platelet activation within 5 minutes when we added PF4. Unfortunately, because these sera were obtained from before 2020, this time frame and ethics restrictions made it unfeasible to obtain detailed clinical information of these patients. This does not, however, change the important finding that these antibodies preceded the COVID-19 vaccination campaign. This observation is of relevance for further development of adenovirus vector–based vaccines, which are affordable for many parts of the world and interpretation of anti-PF4 antibody responses in the context of new vaccination programs.
We also identified an additional VITT-like reactivity profile in 30% of the 131 patients with classic HIT. Potential heparin contamination of patient serum does not influence the chemiluminescence assays because of the marked dilution of patient serum. Such additional anti-PF4 antibodies in heparin-treated patients might very well be the reason why addition of PF4 enhances the sensitivity of functional tests for HIT.27,30 An important future question is whether these patients have a more severe clinical presentation and whether they may benefit from treatment with high-dose IVIG in addition to alternative anticoagulation.2,36
Finally, our data indicate 3 groups of platelet-activating anti-PF4 antibodies: (1) antibodies activating platelets in a heparin-dependent fashion (classic and atypical HIT; lower right quadrant in Figure 2); (2) antibodies activating platelets in a PF4-dependent fashion (VITT and VITT-like antibodies; upper left quadrant in Figure 2); and (3) a combination of both (upper right quadrant in Figure 2). It is an important question for future prospective studies to assess the prevalence and the potential impact of these different antibodies on clinical outcome and implications for treatment in various patient cohorts.
The lessons learned from HIT were instrumental to rapidly recognize the mechanisms of VITT. Understanding VITT was now the key to identify VITT-like PF4-dependent platelet-activating antibodies as a new cause for severe thrombosis and thrombocytopenia. These patients present with acute venous and/or arterial thrombosis, thrombocytopenia, and high D-dimer levels even without proximate exposure to heparin or vaccination. This presentation should prompt consideration of thrombosis at unusual sites; for example, CVST, especially if severe headache is also present, and by analogy with VITT, treatment with high-dose IVIG together with anticoagulation.
Acknowledgments
The authors thank the staff of the transfusion medicine platelet laboratory for their excellent technical support: Ulrike Strobel, Carmen Freyer, Ricarda Raschke, Ines Warnig, Katrin Stein, and Nicole Lembke. The authors thank all the patients and treating physicians who sent blood samples for follow-up and provided us with clinical information. Medical University of Innsbruck: Gerhard Klingenschmid, Alois Schiefecker, Bettina Pfausler (cared for patients). The authors thank Tim Chataway and Alex Colella (Flinders Proteomics Facility) and Bridie Armour (SA Pathology) for technical support with antibody proteomics.
The study has been funded by the Deutsche Forschungsgemeinschaft (DFG): 374031971-TRR240, GR 2232/9-1, SCHO 2052/1-1, TH 2320/3-1. This work was supported by the American Society of Hematology (ASH) with the ASH Global Research Award and within the Gerhard Domagk Research Program by University Medicine Greifswald (L.S.). Some parts of the study have been funded by European Medicines Agency (EMA) service contract number EMA/2021/17/TDA. This project has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under Marie Skłodowska-Curie grant agreement no. 801342 (Tecniospring INDUSTRY) and the Government of Catalonia's Agency for Business Competitiveness (ACCIÓ). M.E. reports funding from the DFG under Germany´s Excellence Strategy (EXC-2049–390688087), Collaborative Research Center ReTune TRR 295-424778381, Bundesministerium für Bildung und Forschung, Deutsches Zentrum für Neurodegenerative Erkrankungen, Deutsches Zentrum für Kardiovaskuläre Erkrankungen, European Union, Corona Foundation, and Fondation Leducq. T.L. reports funding from the DFG (project number 491524754).
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its committees or working parties.
Authorship
Contribution: L.S. performed the study and took care for patients; A.G. and L.S. developed the concept, analyzed data, and took care for patients and laboratory studies; J.W. and M.B. performed the biostatistical analyses, created the figures, and helped writing the manuscript; E.L.-L., T.H., L.A., F.L., C.B., E.B., L.G., and T.E.W. took care for patients; O.E., P.D., M.B., S.R.P., R.T., J.S., R.L., and M.P. developed the new assay; J.F. tested patients; J.J.W. and T.P.G. performed the proteomic studies. M.E. and T.L. performed the patients with stroke studies; A.G., L.S., M.P., T.T., and T.E.W. wrote the manuscript; all authors have critically revised and approved the final version of the manuscript; and L.S., J.W., M.B., and A.G. have accessed and verified the underlying data.
Conflict-of-interest disclosure: A.G. reports grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme (MSD), Bristol Myers Squibb (BMS), Paringenix, Bayer HealthCare, Gore Inc, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, MSD, Macopharma, BMS, Chromatec, and Werfen; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, GTH e.V. outside the submitted work. O.E., P.D., M.B., S.R.P., R.T., J.S., R.L., and M.P. are employees of Werfen. O.E., P.D., M.B., R.L., and A.G. have filed a patent application (PCT/EP2023/062615) covering the design and use of the new rapid anti-PF4 assay. E.L.-L. has received lecture honoraria and advisory fees from Bayer AG, Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, Daiichi Sankyo, Portola, CSL Behring, Viatris, Werfen, Norgine, and Aspen; and institutional research support from Bayer AG, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, and CSL Behring for a different research project. F.L. has received personal fees for lectures or consultancy from Alexion, AstraZeneca, Bayer, BioMarin, BioNTech, Bristol Myers Squibb, Chugai, CSL Behring, Daiichi Sankyo, Grifols, Janssen-Cilag, LEO Pharma, Mitsubishi Tanabe Pharma, Novo Nordisk, Pfizer, Roche, Swedish Orphan Biovitrum, Takeda, Viatris, and Werfen; and institutional research support from Bayer, Chugai, CSL Behring, Intersero, Novo Nordisk, Pfizer, and Swedish Orphan Biovitrum. L.A. reports institutional research support from Bayer, CSL Behring, Novartis, Novo Nordisk, Octapharma, Roche, Sobi, Takeda/Shire, and Werfen; and fees for lectures or consultancy from Bayer, Biotest, Boehringer-Ingelheim, CSL Behring, Daiichi Sankyo, Novo Nordisk, OrPha Swiss, Roche, Sanofi-Aventis, Sanofi-Genzyme, Siemens, Sobi, Takeda/Shire, Viatris, and Werfen. M.E. reports grants from Bayer; and fees paid to the Charité from Abbot, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Sanofi, Novartis, and Pfizer, all are outside the submitted work. T.T. reports personal fees and/or other from Bristol Myers Squibb, Pfizer, Bayer, Chugai Pharma, Novo Nordisk, Novartis, Daichii Sankyo, and LFB Pharma, all of which are outside the submitted work. L.S. receives a young investigator grant of the medical faculty of the Universitätsmedizin Greifswald and a Global Research Award of the American Society of Hematology. T.H. has received lecture honoraria and advisory fees from CSL Behring, Werfen, and Takeda. C.B. has received lecture honoraria from AbbVie; and travel grants from Pfizer and Sanofi outside the submitted work. T.E.W. has received lecture honoraria from Werfen (Instrumentation Laboratory), and royalties from Informa (Taylor & Francis); consulting service fees from Ergomed, Paradigm Pharmaceuticals, Octapharma, and Veralox Therapeutics; research funding from Werfen (Instrumentation Laboratory); and has provided expert witness testimony relating to heparin-induced thrombocytopenia (HIT) and non-HIT thrombocytopenic and coagulopathic disorders. The remaining authors declare no competing financial interests.
Correspondence: Andreas Greinacher, Institut für Transfusionsmedizin, Universitätsmedizin Greifswald, Sauerbruchstr, D-17489 Greifswald, Germany; email: andreas.greinacher@med.uni-greifswald.de.
References
Author notes
∗L.S. and O.E. contributed equally.
The data underlying this publication will be made available upon written request to the corresponding author, Andreas Greinacher (andreas.greinacher@med.uni-greifswald.de). The details of individual patients beyond the information given in this publication will not be made available according to data protection regulations.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Rapid assay results of patient sera with HIT, VITT, and negative controls. Results of the rapid anti-PF4/heparin assay are given on the x-axis (cutoff: dashed vertical line). Results of the new rapid anti-PF4 assay are given on the y-axis (cutoff: dashed horizontal line). Sera of patients with heparin-induced thrombocytopenia are shown in red (n = 131), VITT sera in blue (n = 103), and negative controls in gray symbols (n = 155). None of the 16 VITT sera reacting positive with the rapid anti-PF4/heparin assay (blue dots upper right quadrant) tested positive with 0.2 aFXaU heparin in the heparin-dependent platelet activation assay (12 undiluted; 4 at an informative serum dilution [giving negative buffer reactivity]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/26/10.1182_blood.2023022136/2/m_blood_bld-2023-022136-gr2.jpeg?Expires=1769080204&Signature=mNcKfAkkL8ExZdhIsa54IOjM0Xs4wLZ2BRtCA~x-YonO3C2IlIpHVAMEGz0gNIWEa0SlRqlx3pfxNni6vaoCyCFA-Hmo4orLUJ5LAPEs5aWFQWwQJ74sfcQLQehMhK9uHZMgkxSnO5S3U6ibNeipDxe2mBzLIMF-l46Wumi7XTyOpBaz4aZisM3IzEygulH~Ya~UltRMFUg364OGTzB1TQROSTkBBhAF1P~uIYM-sDTkkmh1sD3Mh3IZn59EmxN7omB0PRxm8saNkbF9Ua4DYgNHDZj0IdU0TJKZcHT0qZxJGQEfDLi~mMulwzMprmPBgOlSctK0X5J5ZAnz~ATBkw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal