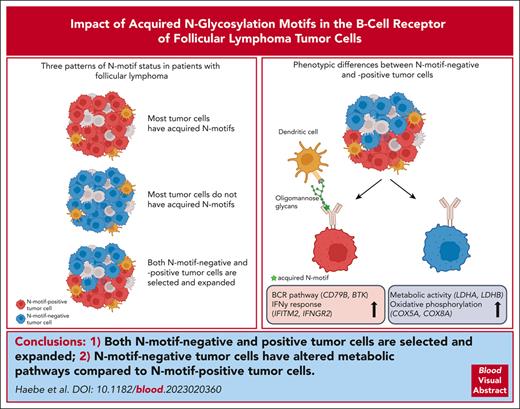
Both N-motif–negative and –positive tumor cells can be selected and expanded during tumor evolution.
N-motif–negative tumor cells upregulate metabolic pathways, which may underlie their ability to thrive despite presumably less BCR signaling.
Visual Abstract
An early event in the genesis of follicular lymphoma (FL) is the acquisition of new glycosylation motifs in the B-cell receptor (BCR) due to gene rearrangement and/or somatic hypermutation. These N-linked glycosylation motifs (N-motifs) contain mannose-terminated glycans and can interact with lectins in the tumor microenvironment, activating the tumor BCR pathway. N-motifs are stable during FL evolution, suggesting that FL tumor cells are dependent on them for their survival. Here, we investigated the dynamics and potential impact of N-motif prevalence in FL at the single-cell level across distinct tumor sites and over time in 17 patients. Although most patients had acquired at least 1 N-motif as an early event, we also found (1) cases without N-motifs in the heavy or light chains at any tumor site or time point and (2) cases with discordant N-motif patterns across different tumor sites. Inferring phylogenetic trees of the patients with discordant patterns, we observed that both N-motif–positive and N-motif–negative tumor subclones could be selected and expanded during tumor evolution. Comparing N-motif–positive with N-motif–negative tumor cells within a patient revealed higher expression of genes involved in the BCR pathway and inflammatory response, whereas tumor cells without N-motifs had higher activity of pathways involved in energy metabolism. In conclusion, although acquired N-motifs likely support FL pathogenesis through antigen-independent BCR signaling in most patients with FL, N-motif–negative tumor cells can also be selected and expanded and may depend more heavily on altered metabolism for competitive survival.
Introduction
Follicular lymphoma (FL), a malignancy of germinal center B cells, is the second most common form of lymphoma worldwide.1 Despite the availability of efficacious treatments, FL remains an incurable disease. Two key features of FL pathogenesis are its functional retention of surface immunoglobulin (Ig; the B-cell receptor [BCR]) despite the loss of 1 allele by the hallmark t(14;18) translocation2 and ongoing somatic hypermutation (SHM) of the rearranged Ig variable (IGV) regions of the heavy and light chains that compose the BCR. These mutations are more often located in the IGV region of the heavy chain than in that of the light chain3 and result in substantial intraclonal heterogeneity of the IGV gene sequence within each FL tumor clone. Additionally, recombination and SHM can introduce amino acid motifs, termed N-linked glycosylation motifs (N-motifs),4-7 consisting of N-X-S/T, in which X denotes any amino acid except proline, and these acquired N-motifs have been found in 75% to 90% of FL cases.4,8 In contrast to N-linked glycosylation at conserved sites in the Ig constant regions, which includes complex sugars, the oligosaccharides attached at acquired N-motifs within the IGV region are of the high-mannose type.9-11 These mannose-terminated glycans interact with lectins, in particular DC-SIGN, found on dendritic cells and tumor-associated macrophages, triggering BCR signaling in an antigen-independent manner,12-14 promoting FL B-cell survival and proliferation.
The acquisition of N-motifs appears to be an early and clonal event in FL, supporting its important role in tumor pathogenesis.5,15,16 Tracking acquired N-motifs during the clinical course of 6 patients, a recent study demonstrated that acquired N-motifs are conserved during tumor evolution and progression in almost all tumor subclones.16 Given the observed scarcity of N-motif–negative tumor subclones in this study, the authors suggested that N-motif–positive tumor clones are selected and expanded preferentially in FL and that rare N-motif–negative FL tumor cells are lost or negligible for tumor evolution. Although N-motif–negative tumor subclones were rare in this study, there was evidence that they could proliferate, as they underwent further rounds of SHM. Another recent report revealed the synchronous presence of N-motif–positive and –negative tumor cells in 1 patient sample.17
To better understand the role and impact of Ig N-linked glycosylation in FL tumor evolution over time, we have reexamined the question by applying and integrating single-cell BCR sequencing (scBCR-seq) and single-cell RNA sequencing (scRNA-seq) of 90 tumor specimens from 17 patients with FL collected from different tumor sites over time.
Methods
Detailed and additional methods are available in the supplemental Methods, available on the Blood website.
Patient cohort
Our cohort consisted of 17 patients with FL participating in clinical trials (ClinicalTrials.gov identifiers NCT02927964 and NCT03410901). All patients had grade 1 to 3A FL based on morphological and immunohistochemical characteristics, confirmed by expert hematopathologists. Details of the patient cohort are provided in supplemental Table 1. Fine needle aspiration (FNA) specimens from the 17 patients with FL were synchronously obtained from 2 tumor sites (tumor sites A and B) before (T0) and after 8 days (T1) and 6 weeks (T2) on treatment. In some patient cases, FNA samples were collected from a third tumor site (tumor site C) from a later, variable, time point. Single-cell suspensions from these specimens were then subjected, typically within an hour after acquisition, to scBCR-seq, scRNA-seq, and flow cytometry. Written informed consent was obtained from all participants, and all protocols were approved by the Institutional Review Board at Stanford University and conducted in accordance with good clinical practice as defined in International Conference on Harmonization guidelines and the US Code of Federal Regulations.
Preparation of single-cell libraries and initial data processing
Single-cell libraries were prepared using the Chromium Single-Cell 5′ Library and Gel Bead Kit and the Chromium Single-Cell V(D)J Enrichment Kit (10x Genomics) following the manufacturer’s protocol, as previously described.18 Amplified complementary DNA was used for BCR enrichment, followed by scBCR-seq library construction and for gene expression library construction. All scRNA-seq libraries from 1 patient were sequenced together on Illumina NovaSeq, whereas sequencing of the scBCR libraries was performed on Illumina NextSeq and/or NovaSeq (supplemental Table 2). After sequencing, scBCR-seq and scRNA-seq data were processed using Cell Ranger VDJ and Cell Ranger (10x Genomics), respectively.
scRNA-seq data processing and cell annotation
After filtering, based on quality control metrics, all tumor samples from all patients were merged. Standard scRNA-seq analyses (normalization, scaling, and clustering) were performed using the R package Seurat (version 4.3.019; see supplemental Methods for details). The 22 most informative principal components and a resolution of 1.4 were then used for shared nearest neighbor–based clustering and uniform manifold approximation and projection dimension reduction. Cell-cycle stage was estimated using methods implemented in Seurat. Cells were then annotated based on the top differentially expressed gene in each cluster as well as known canonical cell markers, as previously described.18 In addition, cluster markers were cross-referenced with previously characterized gene expression profiles of sorted immune cell subsets.20,21 Clusters of patient-specific FL B cells were distinguished from nonmalignant B cells based on homogeneity of BCR light chain v-gene expression and the expression of FL hallmark genes.18,22
Calculation of the adjusted Rand index23 for overlap between batch identity (sample date, 10x chromium chip, or sequencing run) and cell-type identity, based on unsupervised clustering of all nonmalignant cells from all tumor samples revealed that cluster assignments were near-random with respect to batch identity (adjusted Rand index, 0.002-0.007), providing evidence for a lack of significant batch effects.
scBCR-seq analysis
For each patient, high-quality, productive single-cell IGV heavy and light chain sequences (combining tumor cells from all samples for each patient) were aligned to reference gene segments encoding variable (diversity) joining (V(D)J) with the package Change-O.24 The IMGT/HighV-QUEST database was used for germ line sequence reconstruction. After identifying paired heavy and light chain sequences belonging to the dominant V(D)J tumor clone for each patient using the length-normalized Hamming distance, the tools IMGT/HighV-QUEST and IgBLAST were used to analyze the sequences for acquired N-linked glycosylation amino acid motifs, consisting of N-X-S/T, in which X denotes any amino acid except proline. Phylogenetic trees of evolving tumors were constructed based on the SHM profiles of patient’s single-cell IGH VDJ sequences using the R package alakazam (version 1.2.0).25 For visualization, the R package igraph (version 1.4.0)26 was applied. See supplemental Methods for details.
Differential gene expression analysis
Differential expression analyses were performed using the R package Seurat (version 4.3.0).19 Genes with >0.2 log fold-change, at least 20% expression in tested groups, and an adjusted P value < .05 (based on Bonferroni correction) were regarded as significant.
Statistical analysis
Analysis and visualization of the scBCR-seq and scRNA-seq data were performed with the R software (version 4.1.2). Detailed descriptions are provided in the specific method sections. For differential gene expression analysis, we used the Wilcoxon rank sum test with Bonferroni correction implemented in the R package Seurat (version 4.3.0).19
Results
Identifying of tumor cells via paired heavy and light chain analysis
We assessed the impact of N-motifs in FL tumor cells by performing scBCR-seq and scRNA-seq on 90 FNA specimens from 17 patients with FL, including tumor samples from different time points and tumor sites for each patient. Details of the patients and their samples are provided in supplemental Tables 1 and 2. We first identified the FL tumor cells in the scRNA and scBCR data sets. For the scRNA data set, we applied graph-based clustering (supplemental Figure 1A) and annotated the cells based on canonical marker genes (supplemental Figure 1B). Tumor B cells were further defined by clonal light chain v-gene expression and distinct patient-specific clustering, as previously described.18 For the scBCR data set, which contained full-length BCR V(D)J data, we first identified the dominant, rearranged heavy and light chain clonal tumor sequences for each patient and included only cells expressing the patient-specific dominant rearranged tumor clone for both the heavy and light chain V(D)J genes for analysis (supplemental Table 3; supplemental Figure 2). Collectively, we identified on average ∼3300 tumor cells with paired heavy and light chain sequences per sample (supplemental Table 2).
N-motifs are stable during FL evolution
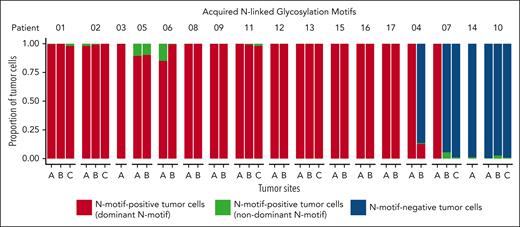
We next evaluated the paired IGH and IGK/L V(D)J sequences for acquired N-motifs. In line with previous results, we detected at least 1 acquired N-motif that was conserved across tumor sites over time in 13 of 17 patients (77%; Figure 1; supplemental Table 4) despite ongoing SHM (mean IGH VDJ SHM frequency, 12% [range, 4%-21%], mean IGK/L VJ SHM frequency, 6% [range, 3%-11%]). Notably, only 1.5% (range, 0%-15%) of tumor cells harbored N-motifs different from the amino acid sequence and/or localization of the dominant tumor clone. Acquired N-motifs were detected predominantly in the IGH VDJ, whereas 7 patients also acquired N-motifs in the IGK/L VJ. We detected up to 4 acquired N-motifs in the IGH VDJ and up to 3 in the IGK/L VJ within 1 patient’s sample (supplemental Table 4). Interestingly, most tumor cells did not gain additional N-motifs across the sampled time (ranging from 4 months to 1.7 years) in our patient cohort.
Prevalence of acquired N-linked glycosylation motifs during FL evolution. Stacked bars showing proportions of N-motif–positive tumor cells with the N-motif of the dominant tumor clone (dominant N-motif, red), N-motif–positive tumor cells with a different acquired N-motif (with a different amino acid sequence and/or location) compared with that of the dominant tumor clone (nondominant N-motif, green), and N-motif–negative tumor cells (blue) across multiple tumor sites and time points for 17 patients with FL. Bars reflect data from all available time points for a given site (see supplemental Tables 1 and 2 for detailed information). N-motif–positive cells can harbor N-motifs in Ig heavy and/or light chain V(D)J.
Prevalence of acquired N-linked glycosylation motifs during FL evolution. Stacked bars showing proportions of N-motif–positive tumor cells with the N-motif of the dominant tumor clone (dominant N-motif, red), N-motif–positive tumor cells with a different acquired N-motif (with a different amino acid sequence and/or location) compared with that of the dominant tumor clone (nondominant N-motif, green), and N-motif–negative tumor cells (blue) across multiple tumor sites and time points for 17 patients with FL. Bars reflect data from all available time points for a given site (see supplemental Tables 1 and 2 for detailed information). N-motif–positive cells can harbor N-motifs in Ig heavy and/or light chain V(D)J.
Patients without acquired N-motifs
In 2 patients, patients 10 and 14, the majority of tumor cells (>97%) did not acquire any glycosylation motifs in either the heavy or light chain IGV genes at any tumor site or time point (Figure 1; supplemental Table 4) despite high levels of ongoing SHM (mean IGH VDJ, 21% [patient 10] and 10% [patient 14]; mean IGK/L VJ, 8% [patient 10] and 5% [patient 14]). Investigating other features that may lead to tonic BCR signaling, we observed that patient 14 was 1 of 3 patients whose rearranged heavy chain used the IGHV4-34 gene (supplemental Table 3). The IGHV4-34 gene encodes intrinsically self-reactive antibodies, and the usage of this gene has been linked to enhanced autoantigen reactivity in lymphoma.27-30 However, 2 other patients with IGHV4-34 usage in our cohort still acquired N-motifs in their heavy and/or light chain V(D)J genes, suggesting that IGHV4-34 usage alone might not be sufficient for BCR stimulation in FL.
N-motif–negative subclones can outcompete N-motif–positive subclones
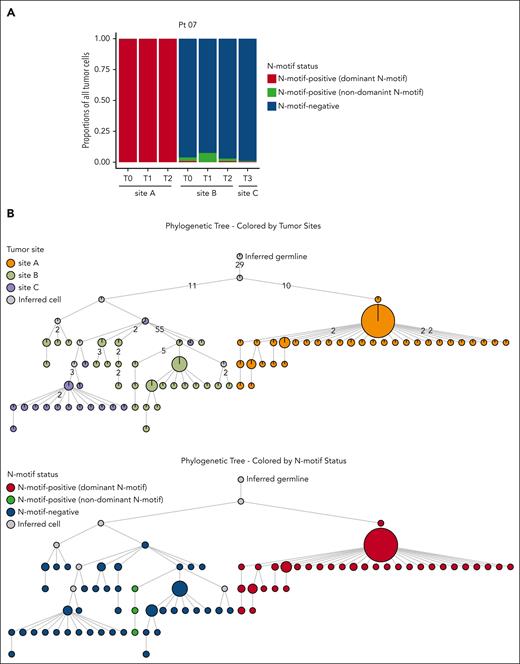
Although the N-motif status of tumor cells in most patients was consistent across tumor sites and time points, 2 patients harbored both N-motif–positive and N-motif–negative tumor cells. In patient 07, we detected a clear discordant N-motif pattern across tumor sites (Figure 1). Tumor cells from site A harbored a novel N-motif (NGT) in the IGH CDR3 at all time points, whereas at site B, the majority of tumor cells did not harbor any N-motifs at any time point (Figure 2A; supplemental Table 4). Only a small tumor subclone at site B gained a novel N-motif, and this was different in its amino acid sequence and location compared with the acquired motif in site A (Figure 2A; supplemental Figure 3A). Importantly, all tumor cells were derived from the same clonal precursor cell, as evidenced by their shared rearranged IGH and IGK/L V(D)J genes (supplemental Table 3). To better understand tumor evolution and N-motif acquisition timing in this patient, we inferred the clonal architecture among the distinct tumor sites over time using the patient’s single-cell IGH VDJ sequences (Figure 2B). When we overlaid the site of origin (Figure 2B, top; supplemental Figure 3B, top), we observed that major tumor subclones were often site-restricted, indicating that the 2 lymphoma areas arose from a single precursor tumor cell but then evolved independently. Moreover, overlaying the N-motif status, the least mutated tumor subclones from site A harbored the dominant acquired N-motif, whereas the ones from site B did not, suggesting that this motif was acquired after dissemination of early tumor cells to distinct tumor sites (Figure 2B, bottom; supplemental Figures 3B, bottom, and 4). Interestingly, clinical tumor progression (tumor site C) in this patient arose from the selection and expansion of N-motif–negative subclones despite the putative survival advantage conferred by acquired N-motifs.
Patient 07 shows discordant N-motif pattern across multiple tumor sites. (A) Stacked bar plot showing proportions of N-motif–positive tumor cells with the N-motif of the dominant tumor clone (dominant N-motif, red), N-motif–positive tumor cells with a different acquired N-motif (with a different amino acid sequence and/or location) compared with that of the dominant tumor clone (nondominant N-motif, green), and N-motif–negative tumor cells (blue) across multiple tumor sites and time points for patient 07. N-motif–positive cells can harbor N-motifs in the heavy and/or light chains. (B) Phylogenetic trees inferred for patient 07 by using the patient’s single-cell heavy chain BCR sequences. Only tumor subclones with at least 18 cells are included. Each circle displays a specific tumor subclone, with the size of circle representing the number of tumor cells within each subclone. Subclones are colored according to the tumor site (top) and N-motif status (bottom). N-motif–positive subclones are distinguished into subclones with N-motifs of the dominant tumor clone (dominant N-motif, red) and cells with a different acquired N-motif (nondominant N-motif, green) compared with that of the dominant clone. N-motif–positive cells harbor N-motifs in the heavy and/or light chains. Numbers on branches indicate that >1 mutation separates 1 subclone from the other. Gray circles represent inferred tumor subclones.
Patient 07 shows discordant N-motif pattern across multiple tumor sites. (A) Stacked bar plot showing proportions of N-motif–positive tumor cells with the N-motif of the dominant tumor clone (dominant N-motif, red), N-motif–positive tumor cells with a different acquired N-motif (with a different amino acid sequence and/or location) compared with that of the dominant tumor clone (nondominant N-motif, green), and N-motif–negative tumor cells (blue) across multiple tumor sites and time points for patient 07. N-motif–positive cells can harbor N-motifs in the heavy and/or light chains. (B) Phylogenetic trees inferred for patient 07 by using the patient’s single-cell heavy chain BCR sequences. Only tumor subclones with at least 18 cells are included. Each circle displays a specific tumor subclone, with the size of circle representing the number of tumor cells within each subclone. Subclones are colored according to the tumor site (top) and N-motif status (bottom). N-motif–positive subclones are distinguished into subclones with N-motifs of the dominant tumor clone (dominant N-motif, red) and cells with a different acquired N-motif (nondominant N-motif, green) compared with that of the dominant clone. N-motif–positive cells harbor N-motifs in the heavy and/or light chains. Numbers on branches indicate that >1 mutation separates 1 subclone from the other. Gray circles represent inferred tumor subclones.
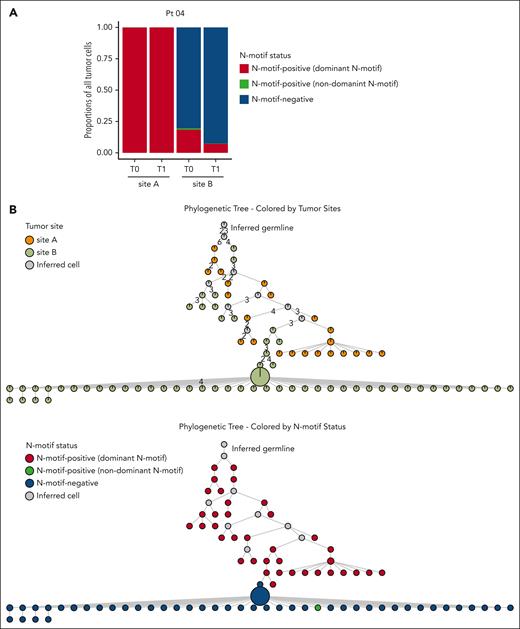
N-motif status also differed among tumor sites in patient 04 (Figure 1). We detected N-motif–positive cells in all tumor sites, but in site B, these accounted for only 20% of all tumor cells at T0 and 8% at T1 (Figure 3A; supplemental Table 4). Analyzing the inferred phylogenetic tree of this patient, including all tumor sites (Figure 3B, top; supplemental Figure 5, top), 2 N-motifs, IGH VDJ: NIT (FR2) and IGK VJ: NYS (CDR3), could be detected at both sites, indicating that these N-motifs were acquired as an early event before divergent evolution (Figure 3B, bottom; supplemental Figure 5, bottom). However, at site B, this N-motif–positive population was overtaken by an N-motif–negative population (Figure 3B, bottom; supplemental Figures 5, bottom, and 6A). Further analysis showed that the N-motif–negative subpopulations at site B did not only lose their IGH VDJ N-motif through SHM but also lost an acquired N-motif in their light chains because of a κ-to-λ light chain switch (supplemental Figure 6B; supplemental Table 3) as the subsequently acquired λ chain contained no acquired N-motifs. Of note, flow cytometry analysis confirmed our finding of sizable separate κ- and λ-restricted populations in this patient (supplemental Figure 7A-B). Collectively, these results suggest that other acquired features can outcompete the selective advantage imparted by lectin-induced BCR signaling.
N-motif–positive cells can be outcompeted by N-motif–negative cells. (A) Stacked bar plot showing proportions of N-motif–positive tumor cells with the N-motif of the dominant tumor clone (dominant N-motif, red), N-motif–positive tumor cells with a different acquired N-motif (with a different amino acid sequence and/or location) compared with that of the dominant tumor clone (nondominant N-motif, green), and N-motif–negative tumor cells (blue) across multiple tumor sites and time points for patient 04. N-motif–positive cells can harbor N-motifs in the heavy and/or light chains. (B) Phylogenetic trees inferred for patient 04 by using the patient’s single-cell heavy chain BCR sequences. Only tumor subclones with at least 18 cells are included. Each circle displays a specific tumor subclone, with the size of circle representing the number of tumor cells within each subclone. Subclones are colored according to the tumor site (top) and N-motif status (bottom). N-motif–positive subclones are divided into subclones with N-motifs of the dominant tumor clone (dominant N-motif, red) and cells with a different acquired N-motifs (nondominant N-motif, green) compared with that of the dominant clone. N-motif–positive cells harbor N-motifs in the heavy and/or light chains. Numbers on branches indicate that >1 mutation separates 1 subclone from the other. Gray circles represent inferred tumor subclones.
N-motif–positive cells can be outcompeted by N-motif–negative cells. (A) Stacked bar plot showing proportions of N-motif–positive tumor cells with the N-motif of the dominant tumor clone (dominant N-motif, red), N-motif–positive tumor cells with a different acquired N-motif (with a different amino acid sequence and/or location) compared with that of the dominant tumor clone (nondominant N-motif, green), and N-motif–negative tumor cells (blue) across multiple tumor sites and time points for patient 04. N-motif–positive cells can harbor N-motifs in the heavy and/or light chains. (B) Phylogenetic trees inferred for patient 04 by using the patient’s single-cell heavy chain BCR sequences. Only tumor subclones with at least 18 cells are included. Each circle displays a specific tumor subclone, with the size of circle representing the number of tumor cells within each subclone. Subclones are colored according to the tumor site (top) and N-motif status (bottom). N-motif–positive subclones are divided into subclones with N-motifs of the dominant tumor clone (dominant N-motif, red) and cells with a different acquired N-motifs (nondominant N-motif, green) compared with that of the dominant clone. N-motif–positive cells harbor N-motifs in the heavy and/or light chains. Numbers on branches indicate that >1 mutation separates 1 subclone from the other. Gray circles represent inferred tumor subclones.
Biological differences between N-motif–positive and –negative tumor cells
Observing that both N-motif–positive and –negative cells can be selected and expanded during FL evolution, we next aimed to elucidate the underlying biological differences between these 2 groups of tumor cells by comparing the phenotypes of N-motif–positive and –negative cells from the same tumors. Unique single-cell barcodes allowed us to integrate the scBCR and scRNA data of patients 04 and 07 (supplemental Figure 8A). Using this combined BCR/transcriptome data set, we first searched for genes differentially expressed between N-motif–positive and N-motif–negative tumor cells before treatment (T0) within each patient. Among the significantly differentially expressed genes in both patients, we detected higher expression of genes related to the BCR pathway (CD79B, BTK, and BANK1) and to interactions with the tumor microenvironment (IFNGR2, IFITM2, and REL) in the N-motif–positive cells, whereas N-motif–negative cells revealed higher expression of genes associated with metabolic processes (LDHA, COX5A, and FABP5; Figure 4A). Next, we scored individual tumor cells from each patient for these pathways and identified pathways that were significantly differentially expressed between N-motif–positive and –negative tumor cells in both patients. We confirmed that N-motif–positive cells exhibited a higher expression of the BCR pathway, interferon response and tumor necrosis factor pathways, whereas cells without N-motifs had high expression levels of metabolic pathways, including oxidative phosphorylation and glycolysis (Figure 4B). Investigating pathway activity in N-motif–positive and –negative tumor cells at T1 (supplemental Figure 8B), we observed similar biological differences between N-motif–positive and –negative tumor cells despite intervening therapy. These results suggest that the phenotypic differences we observed in N-motif–positive vs –negative tumor cells are relatively stable over time.
Biological differences between N-motif–positive and –negative tumor cells. (A) Volcano plot displaying differentially expressed genes between T0 tumor cells with and without N-motifs separately for patients 04 (left) and 07 (right). Genes enriched in N-motif–positive tumor cells are colored red, and those enriched in N-motif–negative cells, blue. Selected genes are highlighted. (B) Heat map exhibiting enriched pathways in N-motif–positive and N-motif–negative T0 tumor cells from patient 04 and 07. P values were calculated using Wilcoxon rank sum tests with false discovery rate: ∗P < .05; ∗∗P < 1 × 10e−10; ∗∗∗P < 1 × 10e−25; ∗∗∗∗P < 1 × 10e−50. (C) Table showing single-cell complementary DNA–based detected numbers of mutated vs all cells for each reported mutation for the N-motif–positive and N-motif–negative tumor cells for patients 04 and 07 using long-read sequencing. T cells from the same samples served as control. 1Mutation results in a premature termination codon. 2Mutation results in a frameshift. AA, amino acid; CDS, identification of coding sequences; del, deletion; fs, frameshift; IFN-γ, interferon gamma; TGFβ, transforming growth factor β; TNFA, tumor necrosis factor α; Pt, patient.
Biological differences between N-motif–positive and –negative tumor cells. (A) Volcano plot displaying differentially expressed genes between T0 tumor cells with and without N-motifs separately for patients 04 (left) and 07 (right). Genes enriched in N-motif–positive tumor cells are colored red, and those enriched in N-motif–negative cells, blue. Selected genes are highlighted. (B) Heat map exhibiting enriched pathways in N-motif–positive and N-motif–negative T0 tumor cells from patient 04 and 07. P values were calculated using Wilcoxon rank sum tests with false discovery rate: ∗P < .05; ∗∗P < 1 × 10e−10; ∗∗∗P < 1 × 10e−25; ∗∗∗∗P < 1 × 10e−50. (C) Table showing single-cell complementary DNA–based detected numbers of mutated vs all cells for each reported mutation for the N-motif–positive and N-motif–negative tumor cells for patients 04 and 07 using long-read sequencing. T cells from the same samples served as control. 1Mutation results in a premature termination codon. 2Mutation results in a frameshift. AA, amino acid; CDS, identification of coding sequences; del, deletion; fs, frameshift; IFN-γ, interferon gamma; TGFβ, transforming growth factor β; TNFA, tumor necrosis factor α; Pt, patient.
We sought to determine whether phenotypic differences between N-motif–positive and N-motif–negative tumor cells in patients 04 and 07 could be due to differences in cancer gene mutations. For both patients, we had available results from targeted hybrid-capture DNA sequencing of 164 genes commonly mutated in hematologic malignancies performed on tumors resected before this study. We conducted long-read sequencing on available pretreatment single-cell complementary DNA for both patients, as previously described.31 Cell barcodes of N-motif–positive and –negative tumor cells as well as T cells identified in the original short read sequencing data were considered for downstream analysis (supplemental Table 5). We assessed the number of mutated vs all cells for each reported mutation for the N-motif–positive and –negative tumor cells for each patient (Figure 4C). For all evaluable variants, there were no differences in the prevalence between N-motif–positive and N-motif–negative cells.
Discussion
By analyzing paired heavy and light chain sequences of >300 000 single tumor cells from 17 patients, including cells from different tumor sites and time points, our study provides a comprehensive and dynamic assessment of BCR characteristics in FL over time. Both by serial tumor sampling and building BCR sequence evolutionary trees, our study demonstrated that although most patients acquired at least 1 N-motif as an early and stable event, there are exceptional patient cases: 2 patients gained no detectable N-motifs in the heavy or light chain at any site or time point and another 2 patients had differences in the prevalence of N-motifs among synchronously sampled tumor sites, demonstrating that N-motif–negative tumor cells could be selected and expanded preferentially. This suggested that other features may allow N-motif–negative cells to overcome the selective advantage held by N-motif–positive cells in FL. Indeed, we discovered that N-motif–negative tumor cells have higher activity of pathways associated with metabolism and confirmed that N-motif–positive tumor cells display increased BCR pathway activity.
In line with previous studies reporting acquisition of N-motifs in 75% to 90% of FL cases,4,8 77% of our patients with FL harbored at least 1 acquired N-motif in the IGH and/or IGK/L V(D)J region. Acquired N-motifs were detectable across distinct tumor sites and over time, providing further evidence that the acquisition of N-motifs is an important event in FL pathogenesis and probably occurs in clonal precursor cells before subclonal divergence.16 We confirm that the majority of FL tumor cells maintain the original acquired N-motifs in their heavy and light chains despite ongoing SHM that might have been expected to alter those previously acquired N-motifs.
Analyzing distinct synchronously sampled tumor sites within each patient, we demonstrate that the prevalence of N-motif–positive and –negative tumor cells can differ remarkably between sites. In 1 case, tumor cells at 1 site gained an N-motif after dissemination to distinct anatomic sites, whereas the majority of tumor cells from the other site acquired none, despite comparable SHM rates at these sites. Both subpopulations, N-motif–positive and –negative, expanded over time, and little cell migration was observed between the 2 sites, independent of their N-motif status. Additionally, clinical tumor progression in this patient arose from an N-motif–negative subclone, providing evidence that expansion of N-motif–negative subpopulations can also underlie tumor progression. In a second patient, both tumor sites contained N-motif–containing clones, but at 1 tumor site, those cells were outcompeted by N-motif–negative subpopulations. These 2 cases demonstrate that other acquired features can overcome the selective advantage of N-linked glycosylation.
A strength of this study was the use of integrated scBCR and scRNA data, which provided a reliable and unique approach to understand underlying biological differences between N-motif–positive and –negative tumor cells within a patient. N-motif–positive tumor cells appeared to interact highly with their tumor microenvironment, reflected in an upregulation of BCR pathway, likely in an antigen-independent manner through mannose-lectin binding, tumor necrosis factor signaling and inflammatory responses, whereas N-motif–negative tumor cells appeared to be dependent on tumor-intrinsic metabolic pathways, such as glycolysis and oxidative phosphorylation. Interestingly, a previous FL study showed that gene sets associated with glutamatergic signaling and enzymes involved in glucose and glutamine metabolism are enriched in patients with FL who relapsed early.32 These findings are significant because they suggest that upregulation of other, for example metabolic, pathways can be an alternative to activation of the lectin-mediated BCR pathway in providing selective survival advantage to lymphoma cells. This may serve as a basis for resistance to BCR pathway inhibitors in mature B-cell malignancies, a hypothesis that merits further testing. Although our study provides a comprehensive and detailed picture of the clinical course of our patients, its scope is limited to 17 patient cases, which constrains our ability to examine more tumor samples with primarily N-motif–negative tumor cells. In the future, the N-motif status of patients could be considered in FL studies to help define the frequency of patients without acquired N-motifs and further dissect the genetic, transcriptional, and functional differences between N-motif–positive and –negative cells. Moreover, finding discordant N-motif status patterns among tumor sites provides further evidence that a single tumor biopsy specimen might not capture the full scope of a patient’s disease18 and that strategies such as multisite sampling or circulating tumor DNA33 should be considered to assess tumor heterogeneity within a patient.
In summary, by integrating phylogenetic and transcriptomic analyses at the single-cell level, we observed that the acquisition of N-motifs is an early and stable event in many patients with FL, but selection and expansion of N-motif–negative cells can occur, and N-motif–negative cells can also thrive, via the activation of alternative pathways. Our findings underscore the molecular intratumoral heterogeneity of FL and shed light on evolutionary paths FL tumors can take.
Acknowledgments
The authors thank members of the Levy and Ji laboratories for critical discussion. The authors thank Alyssa Kanegai, Rachel Greenstein, Destiny Philips, Summer Guo, and particularly Etelka Gabriel for assistance with sample acquisition. The graphical abstract was created with BioRender.com.
This work was supported by National Institutes of Health, National Cancer Institute grant R35 CA197353 (R.L.), Leukemia and Lymphoma Society grant TRP 6539-18 (R.L.), and the Hoogland Lymphoma Research Fund. T.S. was supported at different times by National Institutes of Health, National Cancer Institute grant K08CA252637. S.H. was supported by a Mildred Scheel postdoctoral fellowship of the German Cancer Aid (70113507). Additional support came from National Institutes of Health, National Human Genome Research Institute grant R01 HG006137 (H.P.J. and S.M.G.).
Authorship
Contribution: S.H., T.S., and R.L. designed the study. S.H., T.S, and A.S. performed single-cell experiments; S.H., T.S., G.D., A.S., and S.M.G. processed and analyzed single-cell RNA-seq and BCR-seq data; D.K.C. performed and analyzed flow cytometry experiments; S.M.G. and T.C. processed and analyzed single-cell long-read sequencing data; B.M., S.R.L., and M.G.O. performed the fine needle aspirates and collected and reviewed clinical samples; S.H., T.S., G.D., D.K.C., A.S., S.M.G., H.P.J., and R.L. interpreted the data; and S.H., T.S., and R.L. wrote the manuscript with input from all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Levy, Center for Clinical Science Research, Stanford University, 269 Campus Dr, Room 1105, Stanford, CA 94305; email: levy@stanford.edu.
References
Author notes
∗T.S. and R.L. contributed equally to this work.
scRNA-seq data are deposited in the National Center for Biotechnology Information dbGaP (study accession phs002188).
scRNA-seq data from 10 patients have been included in a previous study (PMCID: PMC8160505).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal