Key Points
Allogeneic HCT quickly and durably resolved disease manifestations in patients with CGD, with excellent survival.
HCT should be considered early for CGD before comorbidities affect the performance status.
Abstract
Chronic granulomatous disease (CGD) is a primary immunodeficiency characterized by life-threatening infections and inflammatory conditions. Hematopoietic cell transplantation (HCT) is the definitive treatment for CGD, but questions remain regarding patient selection and impact of active disease on transplant outcomes. We performed a multi-institutional retrospective and prospective study of 391 patients with CGD treated either conventionally (non-HCT) enrolled from 2004 to 2018 or with HCT from 1996 to 2018. Median follow-up after HCT was 3.7 years with a 3-year overall survival of 82% and event-free survival of 69%. In a multivariate analysis, a Lansky/Karnofsky score <90 and use of HLA-mismatched donors negatively affected survival. Age, genotype, and oxidase status did not affect outcomes. Before HCT, patients had higher infection density, higher frequency of noninfectious lung and liver diseases, and more steroid use than conventionally treated patients; however, these issues did not adversely affect HCT survival. Presence of pre-HCT inflammatory conditions was associated with chronic graft-versus-host disease. Graft failure or receipt of a second HCT occurred in 17.6% of the patients and was associated with melphalan-based conditioning and/or early mixed chimerism. At 3 to 5 years after HCT, patients had improved growth and nutrition, resolved infections and inflammatory disease, and lower rates of antimicrobial prophylaxis or corticosteroid use compared with both their baseline and those of conventionally treated patients. HCT leads to durable resolution of CGD symptoms and lowers the burden of the disease. Patients with active infection or inflammation are candidates for transplants; HCT should be considered before the development of comorbidities that could affect performance status. This trial was registered at www.clinicaltrials.gov as #NCT02082353.
Introduction
Chronic granulomatous disease (CGD) is an inborn error of immunity caused by mutations in genes that encode components or regulators of the reduced NADP oxidase complex, resulting in defective reactive oxidase production and impaired microbial killing. Patients with CGD generally suffer from early-onset life-threatening bacterial and fungal infections, immune dysregulation, inflammation, autoimmunity, and poor growth.1
CGD is inherited in an X-linked or autosomal recessive manner. The incidence is ∼1:200 000 in the United States.2 Despite antimicrobial prophylaxis, patients develop severe infections.3 Inflammatory disease is also common, affecting ∼50% of patients, and it can be progressive and difficult to control.4-6 Early mortality remains high, with 50% survival by the fourth decade of life.2,7,8 In addition, morbidity experienced by patients negatively affects the quality of life.9
Allogeneic hematopoietic cell transplantation (HCT) is currently the only proven therapy for CGD and has been successful in resolving infections and inflammatory disease.10-16 Despite well-described survival outcomes, patient factors that predict best HCT outcomes are still lacking. It is unknown whether having residual (oxidase-positive) vs absent (oxidase-null) reduced NADP oxidase function affects HCT outcomes, and the impact of infection and inflammatory disease on transplant outcomes remains poorly characterized. Importantly, comparisons of clinical status and disease manifestations between HCT-treated patients and conventionally treated (CT) patients are also lacking.
The Primary Immune Deficiency Treatment Consortium (PIDTC) was established in 2009 to conduct multi-institutional observational studies of treatments for rare primary immunodeficiency diseases, including CGD, in the United States and Canada.17 Here, we report the detailed outcomes of a large multicenter cohort of 391 patients with CGD treated with either conventional medical therapy (non-HCT) or with allogeneic HCT at 44 PIDTC centers. We demonstrate that HCT compared with conventional medical therapy resolves infections and inflammation within the first year without later recurrence, oxidase status and genotype do not affect outcomes after HCT, and preexisting infections or inflammation do not adversely affect HCT survival.
Materials and methods
PIDTC Protocol 6903 (NCT02082353) is a multicenter observational natural history study evaluating the clinical outcomes of patients with CGD born in 1988 or later and treated either conventionally (ie, non-HCT) or with allogeneic HCT between 1995 and 2018. The protocol was approved by the institutional review boards of each participating center. All patient participants and their guardians provided consent and assent for PIDTC 6903 according to the Declaration of Helsinki.
Patients
An eligibility panel evaluated clinical and laboratory data for each enrollee, and patients were deemed eligible if they had the following: (1) a decrease in neutrophil function and/or abnormal genotype and (2) clinical or family history consistent with CGD. Patient neutrophil function was classified as oxidase-null or oxidase-positive. Oxidase-null was defined as having a dihydrorhodamine (DHR) assay stimulation index ≤ 2.5, DHR mean fluorescence intensity < 225 arbitrary units, neutrophil oxidase production ≤ 3% of the normal control for laboratory, or ferricytochrome C reduction assay with O2– <2.3 nmol per 106 cells per hour. Oxidase-positive was defined as having results below the normal control level but above these cutoffs. For patients without functional assay data, absence of detectable protein on western blot or presence of a genetic mutation predicted to lead to absent oxidase production were also classified as oxidase-null.
The CT group who were treated with standard antimicrobial therapy and surgical management, as clinically indicated, were enrolled as a retrospective cohort between 2004 and 2018. The CT group was used as a comparator group in our analysis but was not prospectively followed up because of study limitations. The HCT group was studied either retrospectively or prospectively. Patients who received transplantation before the study opening in January 2014 were enrolled in the retrospective HCT cohort and, in general, patients who received transplantation in January 2014 or later were enrolled in the prospective HCT cohort and followed up longitudinally. Retrospective HCT-treated patients were unable to cross over into the prospective HCT-treated cohort. Clinical and laboratory data were collected for the year before enrollment for the CT group and for the year before HCT and at 1, 2, and 3 to 5 years after HCT in the HCT-treated cohort. Autoimmune and inflammatory diseases were diagnosed clinically, and the degree of control (active or controlled with medications) was assessed by the treating physicians. Infections were defined as clinically significant if they required hospitalization and/or a minimum of 16 days of antimicrobial therapy and were categorized as CGD-related based on the known susceptibility of the organism in CGD (supplemental Table 1, available on the Blood website).
Transplant characteristics
HCT data for the first or only allogeneic HCT, including donor relation, HLA concordance, graft source, graft manipulation and cell doses, conditioning regimen, and acute graft-versus-host disease (aGVHD) prophylaxis were recorded. Donor and recipient HLA match and relationship categories used for this study included matched related donor (MRD), mismatched related donor (MMRD), matched unrelated donor (MUD), and mismatched unrelated donor (MMUD).
Transplant outcomes
Neutrophil recovery was defined as the first of 3 consecutive days with a peripheral blood absolute neutrophil count >0.5 × 109/L. Platelet recovery was defined as the first day with a platelet count >20 × 109/L without transfusion support for 7 consecutive days. Donor and recipient chimerism data, including whole blood (WB) and lineage-specific chimerism, were collected. The percent of cells with normal oxidative burst (%DHR+) based on the DHR assay was used as a surrogate for donor myeloid chimerism when myeloid chimerism based on XY fluorescence in situ hybridization or short tandem repeat analysis was not available. Graft failure (GF) was defined as failure to achieve or maintain ≥10% donor cells in the myeloid lineage and/or ≥10%DHR+ neutrophils or receipt of second transplant with chemotherapy conditioning. Mixed chimerism was defined as 10% to 94% donor cells within any lineage and/or 10% to 94%DHR+ within the myeloid lineage. Data regarding HCT complications and survival were also collected. aGVHD was graded based on consensus criteria.18 Chronic GVHD (cGVHD) was reported as limited or extensive.19
Statistical analysis
Binary and categorical variables were summarized based on the frequency (%), and continuous variables were summarized based on the median with range. Categorical data were compared using the χ2 test, and numerical data were compared using the Kruskal-Wallis test, except for height and weight z scores, which were compared using the t test, and infection densities (number of infections per year of exposure time), which were compared using Poisson regression. Comparisons between the conventional arm and the 3- to 5-year post-HCT visit were adjusted for birth year and oxidase stratum, using a regression model for height and weight z scores, Cochran-Mantel-Haenszel test for binary outcomes, and Poisson regression for infection densities. Longitudinal analyses of outcomes among survivors at each timepoint were conducted using generalized estimating equations, with an independent working correlation matrix to handle correlation between the same individuals assessed over time. Binary outcomes were recorded using a logit link function and binomial model, infection densities were recorded using a log link function and Poisson model, and height and weight z scores were recorded using an identity link function and normal model. Cumulative incidence curves were generated for grade 2 to 4 and grade 3 to 4 aGVHD and for cGVHD, with death as a competing risk. Data of patients who underwent second transplantation were censored at the time of the second transplantation for analysis of GVHD. Kaplan-Meier survival curves were generated for overall (OS) and event-free survival (EFS), for which an event was defined as GF, receipt of a subsequent transplant, or death. Groups were compared using the log-rank test. Multivariate analyses were performed using Cox proportional hazards regression modeling. Forward, stepwise variable selection was used to identify predictors of each outcome. The proportional hazards assumption was assessed through graphical approaches and time-dependent covariates, and no violations were identified. The following variables were considered for each model: baseline oxidase status, genotype, Lansky/Karnofsky performance status, history of CGD-related or any infection, history of inflammatory disease, history of noninfectious pulmonary abnormalities, history of surgical resection, use of interferon gamma (IFN-γ), C-reactive protein level, platelet count, age at HCT, donor type, stem cell source, use of serotherapy, GVHD prophylaxis, and conditioning regimen intensity. A P value < .05 was considered statistically significant.
Results
Patient characteristics
A total of 391 patients were enrolled: 151 CT patients and 240 HCT-treated patients (Table 1). The median year of enrollment for the CT group was 2017 (2004-2018), and the median year of HCT for the HCT-treated group was 2013 (1996-2018). The HCT-treated cohort included both retrospective (n = 172) and prospective (n = 68) patients, who, for the purposes of this analysis, were grouped together (n = 240). CT patients were retrospectively enrolled and not prospectively followed up. The median age of CT patients at the time of enrollment was 15.7 years (range, 0.3-29.9 years), and the median age of HCT-treated patients at the time of HCT was 5 years (range, 0.3-28.1 years). Twenty-six HCT-treated patients (10.8%) were aged ≥18 years. Most patients were male in both groups. Pathogenic variants in CYBB (encoding gp91phox) causing X-linked CGD were most common, followed by NCF1 (p47phox) in both groups (supplemental Table 2). Patients with X-linked CGD were most likely to undergo HCT (73%; P = .035). Oxidase-null patients were more likely to have variants in CYBB (P < .001), were not segregated based on race (P = .891), and more commonly underwent transplantation (P = .046). Caucasian (68.4%) and Asian (60%) patients were twice as likely to undergo HCT as African American/Black (30%) patients (P < .001; Table 1). Rates of HCT increased after the year 2000 (P < .001).
Demographics of CT patients and HCT-treated patients
| Characteristic . | Total N = 391 . | Retrospective CT patients∗ n = 151 . | Retrospective or prospective HCT† n = 240 . | P value . |
|---|---|---|---|---|
| Age, median y (range)‡ | 9.45 (0.32-29.85) | 15.7 (0.3-29.9) | 5 (0.3-28.1) | <.001 |
| Sex,n (%) | .261 | |||
| Male | 336 (85.9) | 126 (83.4) | 210 (87.5) | |
| Female | 55 (14.1) | 25 (16.6) | 30 (12.5) | |
| Race, n (%) | <.001 | |||
| American Indian/Alaska native | 1 (0.4) | 1 (0.6) | 0 | |
| Asian | 20 (5.1) | 8 (5.3) | 12 (5) | |
| Black/African American | 47 (12) | 33 (21.9) | 14 (5.8) | |
| Caucasian | 275 (70.3) | 87 (57.6) | 188 (78.4) | |
| Unknown | 48 (12.2) | 22 (14.6) | 26 (10.8) | |
| Ethnicity n (%) | .784 | |||
| Hispanic/Latino/Spanish | 58 (14.8) | 22 (14.6) | 36 (15) | |
| Not Hispanic/Latino/Spanish | 291 (74.5) | 116 (76.8) | 175 (72.9) | |
| Unknown | 42 (10.7) | 13 (8.6) | 29 (12.1) | |
| Genotype (%) | .035 | |||
| CYBB (gp91phox) | 270 (69) | 94 (62.3) | 176 (73.3) | |
| CYBA (p22phox) | 20 (5.2) | 9 (6) | 11 (4.6) | |
| NCF1 (p47phox) | 59 (15.1) | 34 (22.5) | 25 (10.4) | |
| NCF2 (p67phox) | 17 (4.3) | 5 (3.3) | 12 (5) | |
| NCF4 (p40phox) | 2 (0.5) | 0 | 2 (0.8) | |
| Unknown | 23 (5.9) | 9 (6) | 14 (5.9) | |
| Oxidase status (%) | .046 | |||
| Null | 231 (59.1) | 85 (56.3) | 146 (60.8) | |
| Positive | 110 (28.1) | 52 (34.4) | 58 (24.2) | |
| Unknown | 50 (12.8) | 14 (9.3) | 36 (15) | |
| Birth y n (%) | <.001 | |||
| 1988-1993 | 68 (17.4) | 40 (26.5) | 28 (11.7) | |
| 1994-1999 | 73 (18.7) | 32 (21.2) | 41 (17.1) | |
| 2000-2005 | 99 (25.3) | 38 (25.2) | 61 (25.4) | |
| 2006-2011 | 89 (22.8) | 31 (20.5) | 58 (24.2) | |
| 2012-2017 | 62 (15.9) | 10 (6.6) | 52 (21.7) |
| Characteristic . | Total N = 391 . | Retrospective CT patients∗ n = 151 . | Retrospective or prospective HCT† n = 240 . | P value . |
|---|---|---|---|---|
| Age, median y (range)‡ | 9.45 (0.32-29.85) | 15.7 (0.3-29.9) | 5 (0.3-28.1) | <.001 |
| Sex,n (%) | .261 | |||
| Male | 336 (85.9) | 126 (83.4) | 210 (87.5) | |
| Female | 55 (14.1) | 25 (16.6) | 30 (12.5) | |
| Race, n (%) | <.001 | |||
| American Indian/Alaska native | 1 (0.4) | 1 (0.6) | 0 | |
| Asian | 20 (5.1) | 8 (5.3) | 12 (5) | |
| Black/African American | 47 (12) | 33 (21.9) | 14 (5.8) | |
| Caucasian | 275 (70.3) | 87 (57.6) | 188 (78.4) | |
| Unknown | 48 (12.2) | 22 (14.6) | 26 (10.8) | |
| Ethnicity n (%) | .784 | |||
| Hispanic/Latino/Spanish | 58 (14.8) | 22 (14.6) | 36 (15) | |
| Not Hispanic/Latino/Spanish | 291 (74.5) | 116 (76.8) | 175 (72.9) | |
| Unknown | 42 (10.7) | 13 (8.6) | 29 (12.1) | |
| Genotype (%) | .035 | |||
| CYBB (gp91phox) | 270 (69) | 94 (62.3) | 176 (73.3) | |
| CYBA (p22phox) | 20 (5.2) | 9 (6) | 11 (4.6) | |
| NCF1 (p47phox) | 59 (15.1) | 34 (22.5) | 25 (10.4) | |
| NCF2 (p67phox) | 17 (4.3) | 5 (3.3) | 12 (5) | |
| NCF4 (p40phox) | 2 (0.5) | 0 | 2 (0.8) | |
| Unknown | 23 (5.9) | 9 (6) | 14 (5.9) | |
| Oxidase status (%) | .046 | |||
| Null | 231 (59.1) | 85 (56.3) | 146 (60.8) | |
| Positive | 110 (28.1) | 52 (34.4) | 58 (24.2) | |
| Unknown | 50 (12.8) | 14 (9.3) | 36 (15) | |
| Birth y n (%) | <.001 | |||
| 1988-1993 | 68 (17.4) | 40 (26.5) | 28 (11.7) | |
| 1994-1999 | 73 (18.7) | 32 (21.2) | 41 (17.1) | |
| 2000-2005 | 99 (25.3) | 38 (25.2) | 61 (25.4) | |
| 2006-2011 | 89 (22.8) | 31 (20.5) | 58 (24.2) | |
| 2012-2017 | 62 (15.9) | 10 (6.6) | 52 (21.7) |
Two patients included in the retrospective conventional arm ultimately underwent HCT. They were not included in the HCT-treated cohort.
There were no retrospective HCT-treated patients followed up prospectively.
Age for CT patients was at the time of enrollment. Age for HCT-treated patients was at the time of HCT.
Clinical manifestations of CT and HCT-treated patient cohorts at baseline
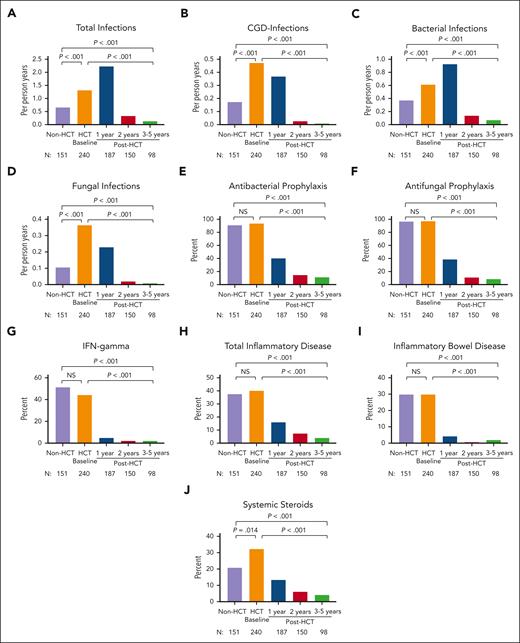
Total, bacterial, fungal, and CGD-related infection densities were significantly higher in HCT-treated patients at pre-HCT baseline than in CT patients (all, P < .001; Figure 1A-D; supplemental Table 3). Of note, 24% of HCT-treated patients had active infection at the time of HCT (supplemental Table 4), and 45% of those infections were fungal. Antibacterial and antifungal prophylaxis were used in >90% of both cohorts; subcutaneous IFN-γ was used in approximately half of each cohort (Figure 1E-G; supplemental Table 3).
Infection density, inflammatory disease, and treatment in CT (non-HCT) and HCT-treated patients. Frequency of total infections (A), CGD infections (B), bacterial infections (C), and fungal infections (D); the use of antibacterial prophylaxis (E), antifungal prophylaxis (F), and IFN-γ (G); the frequency of total inflammatory disease (H) and inflammatory bowel disease (I); and the use of systemic steroids (J) are shown in CT (non-HCT) and HCT-treated patients before HCT and at 1, 2, and 3 to 5 years after HCT. P values compare CT (non-HCT) time point with pre-HCT (HCT baseline), CT with post-HCT time point of 3 to 5 years, and pre-HCT with post-HCT time point of 3 to 5 years.
Infection density, inflammatory disease, and treatment in CT (non-HCT) and HCT-treated patients. Frequency of total infections (A), CGD infections (B), bacterial infections (C), and fungal infections (D); the use of antibacterial prophylaxis (E), antifungal prophylaxis (F), and IFN-γ (G); the frequency of total inflammatory disease (H) and inflammatory bowel disease (I); and the use of systemic steroids (J) are shown in CT (non-HCT) and HCT-treated patients before HCT and at 1, 2, and 3 to 5 years after HCT. P values compare CT (non-HCT) time point with pre-HCT (HCT baseline), CT with post-HCT time point of 3 to 5 years, and pre-HCT with post-HCT time point of 3 to 5 years.
CT and HCT-treated patients had similar rates of total autoinflammatory disease (37.5% vs 40.2%; P = .623) and inflammatory bowel disease (IBD) (29.6% vs 29.7%; P = .893; Figure 1H-I), whereas HCT-treated patients at baseline had higher rates of noninfectious liver disease (7.9% vs 2.6%; P = .031) and noninfectious pulmonary disease (18.6% vs 11.3%; P = .046) (supplemental Figure 1). The proportion of patients with active inflammatory disease was similar between both groups. A greater proportion of HCT-treated patients underwent surgical resection of any location (15.8% vs 4.6%; P < .001), particularly lung resection (9.6% vs 0%; P < .001), and were more likely to have received systemic corticosteroids (32.3% vs 20.8%; P = .014) in the year before undergoing HCT than non-HCT CT patients in the year before enrollment (Figure 1J).
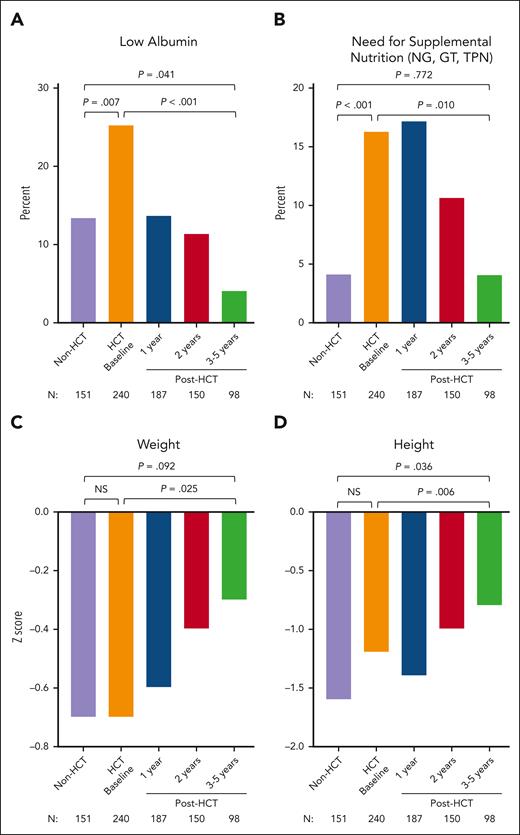
Both cohorts had lower than normal mean height and weight z scores at the time of enrollment (CT or pre-HCT baseline; Figure 2). At pre-HCT baseline, patients had worse overall nutritional status than CT patients, as measured based on low serum albumin levels (25.3% vs 13.4%; P = .007) and the need for supplemental nutrition (16.3% vs 4.1%; P < .001).
Nutrition and growth assessments in CT (non-HCT) and in HCT-treated patients. Frequency of low albumin level (A), need for supplemental nutrition via GT, NG tube, and TPN (B), weight z score (C), and height z scores (D) are shown in CT (non-HCT) and HCT-treated patients before HCT and 1, 2, and 3 to 5 years after HCT. P values compare CT (non-HCT) time point with pre-HCT (HCT baseline), CT with post-HCT time point of 3 to 5 years, and pre-HCT with post-HCT time point of 3 to 5 years. NG, nasogastric; GT, gastric tube; TPN, total parenteral nutrition NS, not significant.
Nutrition and growth assessments in CT (non-HCT) and in HCT-treated patients. Frequency of low albumin level (A), need for supplemental nutrition via GT, NG tube, and TPN (B), weight z score (C), and height z scores (D) are shown in CT (non-HCT) and HCT-treated patients before HCT and 1, 2, and 3 to 5 years after HCT. P values compare CT (non-HCT) time point with pre-HCT (HCT baseline), CT with post-HCT time point of 3 to 5 years, and pre-HCT with post-HCT time point of 3 to 5 years. NG, nasogastric; GT, gastric tube; TPN, total parenteral nutrition NS, not significant.
Lansky/Karnofsky performance scores were <90 in 11% of patients in both the CT and HCT-treated cohorts at baseline.
Transplant characteristics
Patients received transplantation between 1996 and 2018 (median year, 2013). Transplant characteristics are shown in Table 2 and supplemental Table 5. Most patients received MUD (n = 118; 49%) or MRD (n = 22; 25%) grafts. Graft source was bone marrow in 130 (54%), peripheral blood stem cells in 71 (30%), and umbilical cord blood (UCB) in 36 (15%) transplants. Three (2%) patients received UCB plus bone marrow grafts from HLA-matched siblings. Sixty-two patients (26%) received traditional busulfan and cyclophosphamide conditioning with or without additional agents. Remaining patients (n = 178) were treated with alternative conditioning regimens, including busulfan and fludarabine (n = 78) + thiotepa (n = 5) or busulfan and low dose TBI (n = 42), fludarabine and melphalan (n = 16) + thiotepa (n = 11), treosulfan and fludarabine (n = 7), or other regimens (n = 19). Most (n = 220; 92%) received serotherapy with their conditioning regimens. GVHD prophylaxis regimens were primarily calcineurin inhibitor-based (n = 180; 75%).
Transplant characteristics
| Characteristic . | Total N = 240 . |
|---|---|
| Age at HCT, median (range), y | 5.0 (0.3-28.1) |
| HCT (y), median, n (%) | 2013 |
| 1996-2000 | 9 (3.8) |
| 2001-2005 | 15 (6.3) |
| 2006-2010 | 43 (17.9) |
| 2011-2015 | 114 (47.5) |
| 2016-2018 | 59 (24.6) |
| Donor type, n (%) | |
| MSD/MRD | 60 (25.0) |
| MUD | 118 (49.2) |
| MMRD | 22 (9.2) |
| MMUD | 40 (16.7) |
| Donor gender, n (%) | |
| Female | 100 (41.7) |
| Male | 140 (58.3) |
| Stem cell source, n (%) | |
| Bone marrow | 130 (54.2) |
| Peripheral blood stem cells | 71 (29.6) |
| UCB | 36 (15.0) |
| Cord blood + bone marrow | 3 (1.3) |
| T-cell depletion, n (%) | |
| CD34+ selection | 9 (3.8) |
| TCRαβ+/CD19+ cell depletion | 3 (1.3) |
| CD3+/CD19+ cell depletion | 1 (0.4) |
| Conditioning regimen, n (%) | |
| Traditional Bu/Cy | 62 (25.8) |
| Bu/Cy | 33 (14) |
| Bu/Cy/Flu | 25 (10) |
| Bu/Cy/AraC or etoposide | 4 (1.7) |
| Alternative regimens | 178 (74.2) |
| Bu/Flu | 78 (32.5) |
| Bu/Flu/thiotepa | 5 (2.1) |
| Bu/TBI | 42 (17.5) |
| Bu | 5 (2.1) |
| Flu/Mel | 16 (6.7) |
| Flu/Mel/thiotepa | 11 (4.6) |
| Flu/Mel/Cy | 1 (0.4) |
| Flu/Mel/TBI | 1 (0.4) |
| Flu/Treo | 7 (3.0) |
| Flu/Treo/TBI | 1 (0.4) |
| Flu/Cy | 3 (1.3) |
| Flu/Cy/TBI | 2 (0.8) |
| Flu/TBI | 5 (2.1) |
| Flu/thiotepa | 1 (0.4) |
| Serotherapy, n (%) | |
| ATG | 105 (43.8) |
| Alemtuzumab | 115 (47.9) |
| None | 20 (8.3) |
| GVHD prophylaxis, n (%) | |
| CNI + MMF ± other | 80 (33.3) |
| CNI + MTX ± other | 52 (21.7) |
| CNI ± other | 48 (20.0) |
| PTCy ± other | 12 (5.0) |
| Sirolimus | 36 (15.0) |
| Other | 5 (2.1) |
| None | 7 (2.9) |
| Characteristic . | Total N = 240 . |
|---|---|
| Age at HCT, median (range), y | 5.0 (0.3-28.1) |
| HCT (y), median, n (%) | 2013 |
| 1996-2000 | 9 (3.8) |
| 2001-2005 | 15 (6.3) |
| 2006-2010 | 43 (17.9) |
| 2011-2015 | 114 (47.5) |
| 2016-2018 | 59 (24.6) |
| Donor type, n (%) | |
| MSD/MRD | 60 (25.0) |
| MUD | 118 (49.2) |
| MMRD | 22 (9.2) |
| MMUD | 40 (16.7) |
| Donor gender, n (%) | |
| Female | 100 (41.7) |
| Male | 140 (58.3) |
| Stem cell source, n (%) | |
| Bone marrow | 130 (54.2) |
| Peripheral blood stem cells | 71 (29.6) |
| UCB | 36 (15.0) |
| Cord blood + bone marrow | 3 (1.3) |
| T-cell depletion, n (%) | |
| CD34+ selection | 9 (3.8) |
| TCRαβ+/CD19+ cell depletion | 3 (1.3) |
| CD3+/CD19+ cell depletion | 1 (0.4) |
| Conditioning regimen, n (%) | |
| Traditional Bu/Cy | 62 (25.8) |
| Bu/Cy | 33 (14) |
| Bu/Cy/Flu | 25 (10) |
| Bu/Cy/AraC or etoposide | 4 (1.7) |
| Alternative regimens | 178 (74.2) |
| Bu/Flu | 78 (32.5) |
| Bu/Flu/thiotepa | 5 (2.1) |
| Bu/TBI | 42 (17.5) |
| Bu | 5 (2.1) |
| Flu/Mel | 16 (6.7) |
| Flu/Mel/thiotepa | 11 (4.6) |
| Flu/Mel/Cy | 1 (0.4) |
| Flu/Mel/TBI | 1 (0.4) |
| Flu/Treo | 7 (3.0) |
| Flu/Treo/TBI | 1 (0.4) |
| Flu/Cy | 3 (1.3) |
| Flu/Cy/TBI | 2 (0.8) |
| Flu/TBI | 5 (2.1) |
| Flu/thiotepa | 1 (0.4) |
| Serotherapy, n (%) | |
| ATG | 105 (43.8) |
| Alemtuzumab | 115 (47.9) |
| None | 20 (8.3) |
| GVHD prophylaxis, n (%) | |
| CNI + MMF ± other | 80 (33.3) |
| CNI + MTX ± other | 52 (21.7) |
| CNI ± other | 48 (20.0) |
| PTCy ± other | 12 (5.0) |
| Sirolimus | 36 (15.0) |
| Other | 5 (2.1) |
| None | 7 (2.9) |
Matching of MMRD and MMUD were as follows: MMRD: 3 of 8 (n = 2), 4 of 8 (n = 5), 5 of 6 (n = 1), 5 of 8 (n = 6), 6 of 8 (n = 1), 7 of 8 (n = 7) and MMUD: 4 of 6 (n = 2), 5 of 6 (n = 4), 5 of 8 (n = 4), 6 of 8 (n = 8), 7 of 8 (n = 22)
AraC, cytarabine; Bu, busulfan; CNI, calcineurin inhibitor; Flu, fludarabine; Mel, melphalan; MMF, mycophenolate mofetil; MTX, methotrexate; PTCy, posttransplant cyclophosphamide; RIC/RTC, reduced intensity conditioning/reduced toxicity conditioning; TBI, total body irradiation; Treo, treosulfan.
Neutrophil and platelet recovery, GF, and second transplants
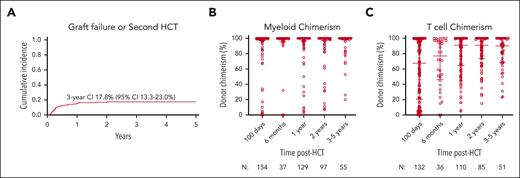
Neutrophil and platelet recovery occurred at a median of 19 (range, 4-86) and 23.5 (range, 1-403) days, respectively. The cumulative incidences of GF and/or second HCT by day +100 and 3 years were 8.9% (95% confidence interval [CI], 5.8-12.9) and 17.8% (95% CI, 13.3-23.0), respectively (Figure 3A). The cumulative incidence of receiving a second HCT was 13.2% (95% CI, 0.091-0.179) by 3 years. On multivariate analysis, melphalan-based regimens were associated with a significantly increased risk of GF/second HCT compared with busulfan-based regimens (hazard ratio [HR], 2.52; P = .012; Table 3). No other significant associations were noted.
GF and/or receipt of second HCT and donor chimerism with time after HCT. (A) The cumulative incidence of GF and/or receipt of second HCT with 95% CI and (B) myeloid and (C) T-cell donor chimerism after HCT with median and interquartile range are shown.
GF and/or receipt of second HCT and donor chimerism with time after HCT. (A) The cumulative incidence of GF and/or receipt of second HCT with 95% CI and (B) myeloid and (C) T-cell donor chimerism after HCT with median and interquartile range are shown.
Transplant factors based on multivariate analyses
| Parameter . | N . | HR (95% CI) . | P . |
|---|---|---|---|
| GF and/or second HCT | |||
| Conditioning intensity | .228∗ | ||
| Bu/Cy | 62 | 1.0 | |
| Alternative regimens | 177 | 1.60 (0.74-3.45) | .228 |
| Conditioning regimen | .029∗ | ||
| Busulfan-based | 192 | 1.0 | |
| Melphalan-based | 29 | 2.52 (1.23-5.18) | .012 |
| Other | 18 | 1.87 (0.74-4.72) | .187 |
| Grade 2-4 aGVHD | |||
| Serotherapy† | .004∗ | ||
| None | 20 | 1.0 | |
| ATG | 103 | 0.44 (0.17-1.11) | .083 |
| Alemtuzumab | 110 | 0.20 (0.07-0.54) | .002 |
| Donor type | .012∗ | ||
| MRD | 56 | 1.0 | |
| MMRD | 21 | 4.36 (1.18-16.1) | .027 |
| MUD | 117 | 5.27 (1.76-15.75) | .003 |
| MMUD | 39 | 6.87 (2.13-22.19) | .001 |
| Chronic GVHD | |||
| Inflammatory disease‡ | .037∗ | ||
| No | 132 | 1.0 | |
| Yes | 87 | 2.17 (1.06-4.43) | .037 |
| Pulmonary infection‡ | .006∗ | ||
| No | 139 | 1.0 | |
| Yes | 80 | 2.68 (1.33-5.42) | .006 |
| OS | |||
| Performance status | .004∗ | ||
| Lansky/Karnofsky ≥90 | 193 | 1.0 | |
| Lansky/Karnofsky <90 | 25 | .001 | |
| Unknown | 21 | 3.50 (1.63-7.50) | .735 |
| Donor HLA match | .012∗ | ||
| Matched (MRD + MUD) | 178 | 1.0 | |
| Mismatched (MMRD + MMUD) | 61 | 2.26 (1.19-4.28) | .012 |
| EFS§ | |||
| Performance status | <.001∗ | ||
| Lansky/Karnofsky ≥90 | 193 | 1.0 | |
| Lansky/Karnofsky <90 | 25 | 3.01 (1.66-5.46) | <.001 |
| Unknown | 21 | .636 | |
| Donor HLA match | .019∗ | ||
| Matched (MRD + MUD) | 178 | 1.0 | |
| Mismatched (MMRD + MMUD) | 61 | 1.81 (1.10-2.96) | .019 |
| Parameter . | N . | HR (95% CI) . | P . |
|---|---|---|---|
| GF and/or second HCT | |||
| Conditioning intensity | .228∗ | ||
| Bu/Cy | 62 | 1.0 | |
| Alternative regimens | 177 | 1.60 (0.74-3.45) | .228 |
| Conditioning regimen | .029∗ | ||
| Busulfan-based | 192 | 1.0 | |
| Melphalan-based | 29 | 2.52 (1.23-5.18) | .012 |
| Other | 18 | 1.87 (0.74-4.72) | .187 |
| Grade 2-4 aGVHD | |||
| Serotherapy† | .004∗ | ||
| None | 20 | 1.0 | |
| ATG | 103 | 0.44 (0.17-1.11) | .083 |
| Alemtuzumab | 110 | 0.20 (0.07-0.54) | .002 |
| Donor type | .012∗ | ||
| MRD | 56 | 1.0 | |
| MMRD | 21 | 4.36 (1.18-16.1) | .027 |
| MUD | 117 | 5.27 (1.76-15.75) | .003 |
| MMUD | 39 | 6.87 (2.13-22.19) | .001 |
| Chronic GVHD | |||
| Inflammatory disease‡ | .037∗ | ||
| No | 132 | 1.0 | |
| Yes | 87 | 2.17 (1.06-4.43) | .037 |
| Pulmonary infection‡ | .006∗ | ||
| No | 139 | 1.0 | |
| Yes | 80 | 2.68 (1.33-5.42) | .006 |
| OS | |||
| Performance status | .004∗ | ||
| Lansky/Karnofsky ≥90 | 193 | 1.0 | |
| Lansky/Karnofsky <90 | 25 | .001 | |
| Unknown | 21 | 3.50 (1.63-7.50) | .735 |
| Donor HLA match | .012∗ | ||
| Matched (MRD + MUD) | 178 | 1.0 | |
| Mismatched (MMRD + MMUD) | 61 | 2.26 (1.19-4.28) | .012 |
| EFS§ | |||
| Performance status | <.001∗ | ||
| Lansky/Karnofsky ≥90 | 193 | 1.0 | |
| Lansky/Karnofsky <90 | 25 | 3.01 (1.66-5.46) | <.001 |
| Unknown | 21 | .636 | |
| Donor HLA match | .019∗ | ||
| Matched (MRD + MUD) | 178 | 1.0 | |
| Mismatched (MMRD + MMUD) | 61 | 1.81 (1.10-2.96) | .019 |
Cy, cyclophosphamide.
Denotes overall P value, comparing all groups for that variable.
Pairwise comparison of ATG only vs alemtuzumab only: HR for Grade 2-4 aGVHD of 0.46 (0.23-0.91); P = .025. There was no effect on GF (P = .691).
History of inflammatory disease or pulmonary infection in the year prior to HCT regardless of control/resolution status
Events include GF, need for subsequent transplant, and death
Donor chimerism
Median WB donor chimerism was >95% at all time points, and median myeloid donor chimerism was 100% at all time points (Figure 3B). Median donor T-cell chimerism was 67.5% (range, 0%-100%) at 100 days after HCT and increased over time to 90% (range, 23%-100%) at 3 to 5 years after HCT (Figure 3C). Donor chimerism percentages in the B-cell and natural killer cell fractions mirrored those of the WB and myeloid compartment. Mixed donor and recipient myeloid chimerism on day +100 was significantly associated with later receipt of a second HCT. Only 1.1% (95% CI, 0.1-5.4) of patients with ≥95% myeloid chimerism at day +100 received a second HCT vs 28.3% (95% CI, 8-53) of patients with 11% to 94% myeloid chimerism (P < .001).
Stem cell boosts and donor lymphocyte infusions
Nine patients received stem cell boosts, and 7 patients received donor lymphocyte infusions after HCT. Indications were GF (n = 4), declining donor myeloid chimerism (n = 4), mixed donor chimerism (n = 4), poor graft function (n = 2), and infection (n = 1) or per protocol (n = 1). There was stabilization of donor chimerism in 4 patients, and improvement in graft function in 1. There was no response in 11 patients, death in 2, and a receipt of second HCT in 3.
aGVHD
The 180-day cumulative incidences of grades 2 to 4 and 3 to 4 aGVHD were 18.4% (95% CI, 13.6-23.7) and 6.2% (95% CI, 3.5-9.9), respectively (supplemental Figure 2). Patients who received serotherapy with their conditioning regimen had significantly lower rates of grade 2 to 4 aGVHD on multivariate analysis than patients who did not (antithymocyte globulin [ATG] HR, 0.44 [P = .083] and alemtuzumab HR, 0.20 [P = .002]; Table 3), and alemtuzumab was superior to ATG in preventing grade 2 to 4 aGVHD (HR, 0.46 [P = .025]). Grade 2 to 4 aGVHD was also significantly higher in patients who received grafts from HLA-mismatched and unrelated donors on multivariate analysis, with MMUD conferring the highest risk (MMRD HR, 4.36 [P = .027]; MUD HR, 5.27 [P = .003]; MMUD HR, 6.87 [P = .001]; Table 3).
cGVHD
The 2-year cumulative incidences of overall and extensive cGVHD were 19.1% (95% CI, 14.0-24.8) and 6.8% (95% CI, 4.1-11.3), respectively (supplemental Figure 2). History of inflammatory disease regardless of control (HR, 2.17; P = .037) or pulmonary infection (HR, 2.68; P = .006) in the year before HCT increased the risk of cGVHD.
Survival after transplant
A total of 198 HCT-treated patients were alive at a median follow-up of 3.7 years (interquartile range, 2.1-6.0). The estimated 3-year OS was 82.0% (95% CI, 76.2-86.5), and the estimated 3-year EFS was 69.1% (95% CI, 62.6-74.7) (Figure 4A). The most common causes of death were infection (n = 9), respiratory failure (n = 9), bleeding (n = 7), and multiorgan failure (n = 7). GVHD was the cause of death in 1 patient and contributed to the cause of death in 9 patients.
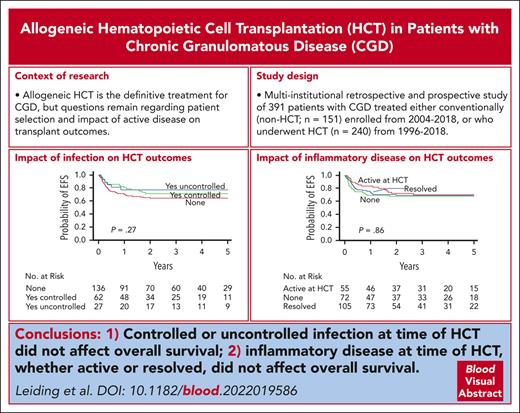
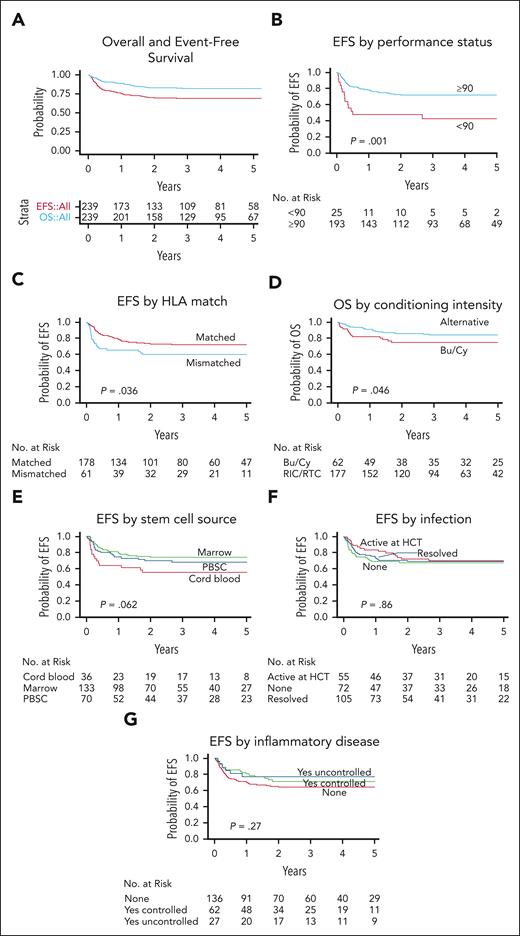
OS and EFS after HCT in 240 patients with CGD. The probability of OS and EFS after HCT with 95% CI (A) are shown. An event was defined as death, GF, or second HCT. The probability of EFS based on baseline performance status (B), EFS based on donor and recipient HLA match (C), OS based on conditioning intensity (D), EFS based on stem cell source (E), and EFS based on history of infection (F) and inflammatory disease (G) in the year before HCT and status at the time of HCT are shown. P values were obtained using log-rank test.
OS and EFS after HCT in 240 patients with CGD. The probability of OS and EFS after HCT with 95% CI (A) are shown. An event was defined as death, GF, or second HCT. The probability of EFS based on baseline performance status (B), EFS based on donor and recipient HLA match (C), OS based on conditioning intensity (D), EFS based on stem cell source (E), and EFS based on history of infection (F) and inflammatory disease (G) in the year before HCT and status at the time of HCT are shown. P values were obtained using log-rank test.
Lansky/Karnofsky performance status <90 at baseline was significantly associated with decreased OS on multivariate analysis, with an HR of 3.50 (P = .001; Table 3). Patients who received grafts from HLA-mismatched donors (ie, MMRD or MMUD) had significantly lower OS than patients who received grafts from HLA-matched donors (MRD or MUD; HR, 2.26; P = .012; Table 3). Traditional busulfan and cyclophosphamide–based conditioning was negatively associated with OS on univariate analysis compared with alternative conditioning regimens (Figure 4D; P = .047). However, the use of busulfan and cyclophosphamide–based conditioning segregated colinearly with donor and recipient HLA matches (ie, patients who received grafts from HLA-mismatched donors were more likely to receive busulfan and cyclophosphamide–based conditioning); therefore, conditioning regimens were not included in the final multivariate analysis model (supplemental Table 6).
Baseline Lansky/Karnofsky performance status <90 and the use of HLA-mismatched donor grafts were also significantly associated with decreased EFS on multivariate analysis with HRs of 3.01 (P < .001) and 1.81 (P = .019), respectively (Figure 4B-C; Table 3). There was a trend toward decreased EFS in patients who received UCB grafts (Figure 4E; P = .062). However, the use of UCB grafts segregated colinearly with the use of HLA-mismatched donors and was not included in the final multivariate analysis model.
Year of HCT, age at HCT (using both 5 and 18 years age as cutoffs), genotype, baseline oxidase status, history and status of infection, and history and status of autoimmune or inflammatory disease were not significantly associated with OS or EFS (Figure 4F-G; supplemental Figure 3).
Disease resolution after HCT
Total infection density was higher in the first year after HCT than at baseline, but CGD-related and fungal infections decreased significantly (Figure 1A-B; supplemental Table 7). Use of corticosteroids at the time of HCT was associated with overall (P = .012), bacterial (P = .035), viral (P = .012), and CGD-related (P = .008) infection densities at 1 year after HCT (supplemental Table 8). At 3 to 5 years after HCT, all infections, including CGD-related and fungal infections, were nearly eliminated (all P < .001), as was the use of antibacterial or antifungal prophylaxis or IFN-γ (Figure 1A-G; supplemental Table 9).
By 1 year after HCT, the total inflammatory disease (P < .001), IBD (P < .001), and noninfectious pulmonary disease (P = .015) had decreased significantly, and the use of systemic corticosteroids (P < .001) was also significantly lower (Figure 1H-J; supplemental Table 7). Use of corticosteroids at the time of HCT was associated with the presence of inflammatory disease 1 year after HCT (P = .009). Importantly, reduction in inflammatory disease burden was sustained (all, P < .001) at 3 to 5 years after HCT. Although the frequency of noninfectious liver disease was similar at baseline and during the first year after HCT but improved from 3 to 5 years after HCT, this did not reach statistical significance (P = .084). Consistent with the reduction in liver disease, the proportion of patients with elevated serum alkaline phosphatase (P = .005) and bilirubin levels (P < .001) decreased significantly at 3 to 5 years after HCT (supplemental Table 9). There was no increase after HCT in the incidence of other autoimmune/inflammatory conditions, including autoimmune cytopenias, arthritis, protein-losing enteropathy, immunoglobulin A nephropathy, systemic lupus erythematosus, antiphospholipid syndrome, or ophthalmic inflammation (data not shown).
Nutritional status significantly improved in all domains assessed at 3 to 5 years after HCT; the proportion of patients with low serum albumin levels (P < .001) or requiring supplemental nutrition (P = .010) decreased significantly, and weight (P = .025) and height (P = .006) z scores increased significantly (Figure 2; supplemental Table 9).
Non-HCT vs surviving HCT-treated patients at 3- to 5-year follow-up
CT patients were not prospectively followed up; thus, survival over time could not be compared between the 2 cohorts. Surviving HCT-treated patients at 3 to 5 years had significantly lower infection density in all domains (total, CGD-related, bacterial, and fungal) and lower rates of total inflammatory disease (4.1% vs 37.5%) and IBD (2% vs 29.8%) than CT patients (all, P < .001; Figures 1 and 2; supplemental Table 10). Less than 5% of patients who received transplantation required the use of antimicrobial prophylaxis, and 100% were free from systemic steroids at 3 to 5 years after HCT (all, P < .001).
Discussion
CGD results in infections, severe inflammatory and autoimmune complications, poor quality of life, and early mortality.1 Standard treatment primarily consists of prevention of infections with life-long antimicrobial prophylaxis and frequent surveillance for the development of infections or autoinflammation. Despite these efforts, life-threatening infections are still a major cause of morbidity and mortality.20 Autoinflammation can be systemic or organ specific, is progressive, and often requires life-long immunosuppression.4 Without definitive therapy, life expectancy is shortened to the fourth or fifth decade of life and is influenced by whether there is residual oxidase activity.21 We report the results of a large multicenter cohort of North American patients with CGD. CT patients and those who received transplantation received similar standard therapies: antimicrobial prophylaxis, nutritional support, and immunosuppression for the treatment of autoinflammation. Disease manifestations were present in both groups despite standard therapies with infections being more frequent in HCT patients at baseline, suggesting that the most severely affected patients underwent HCT. Importantly, our data demonstrate, as definitively as possible in the absence of a randomized trial, the favorable impact of HCT on reversal and prevention of disease manifestations compared with that in CT patients.
The OS and EFS of 82% and 69%, respectively, after HCT in this study were comparable with the survival rates of 86% and 76%, respectively, reported in a European cohort of 712 patients,11 published recently, and with those of several reports of smaller cohorts.13,14,22 Not surprisingly, patients with good baseline performance status (Lansky/Karnofsky ≥90) who received grafts from HLA-matched donors fared best. The use of busulfan/cyclophosphamide–based conditioning and UCB grafts were also associated with decreased OS and EFS, respectively. The European experience noted that patients aged ≥18 years had lower OS and a higher incidence of cGVHD than younger patients. Remarkably, age ≥18 years was not associated with adverse outcomes in our cohort. Pretransplant infection and inflammation have also been described, in some reports, to be associated with poorer post-HCT outcomes.11,14 However, a history of infection or inflammatory disease in the year before HCT did not negatively affect survival in this cohort. A Lansky/Karnofsky performance score <90 at baseline was, however, significantly associated with lower OS and EFS. These data indicate that specific disease complications do not affect survival after HCT, in general, but those complications that negatively affect the performance status of a patient do.
Despite the encouraging survival rates after HCT, we observed a high GF rate of 17.8% at 3 years, slightly greater than that observed in the European cohort.11 Early mixed myeloid chimerism on day 100 was found to be associated with GF in our cohort, and patients with mixed myeloid chimerism should be followed up closely. However, the number of patients with low stable mixed chimerism was insufficient to identify a predictive threshold, and there were patients who had stable mixed myeloid chimerism at 3 to 5 years after HCT. Melphalan-based conditioning was also associated with a higher risk of GF, and melphalan-based conditioning has previously been reported to result in high rates of mixed chimerism and requirement for additional hematopoietic cell products in patients with CGD.22 As such, our data suggest that busulfan is the preferred conditioning agent within transplant regimens. Because of a lack of pharmacokinetic data, we were unable to determine the optimal busulfan exposure to minimize toxicity while ensuring full and durable donor cell engraftment. The small number of patients who received treosulfan-based conditioning prevented analyses of the effect of treosulfan on GF. Notably, serotherapy, especially alemtuzumab, significantly decreased the risk of aGVHD without increasing the risk of GF.
Alemtuzumab conferred significantly greater protection against aGVHD than ATG and may be the serotherapy of choice for CGD. However, the timing of dosing and the role of alemtuzumab drug monitoring to optimize protection against GVHD while minimizing the risk of mixed chimerism and delayed immune reconstitution remains to be defined.23,24
The rates of severe aGVHD and extensive cGVHD were low in our cohort and on par with those previously reported.11,12 Notably, a history of inflammatory disease in the year before HCT was associated with an increased risk of cGVHD. Unfortunately, objective data on the severity and control of inflammatory disease in the year before or at the time of HCT were not collected, preventing additional associations from being made. Characterization of inflammatory disease with biomarkers and improved treatment strategies that may guide management strategy in patients with inflammatory disease are needed.
Patients who received transplantation had high rates of infection and inflammatory disease and poor growth at baseline, all hallmarks of CGD. Our data suggest that the burden of infections, inflammatory disease, and poor growth resolve quickly and durably in >90% of surviving patients who undergo HCT. CGD-related infections, colitis, and noninfectious pulmonary disease all decrease during the first year after HCT and were nearly eliminated at 3 to 5 years after HCT. Rates of liver disease also decreased with time after HCT. Antimicrobial prophylaxis and systemic steroids were discontinued at 3 to 5 years after HCT. Growth failure, likely a result of chronic infections and/or gastrointestinal disease, is a common feature in CGD; our findings indicate that HCT resolves the malnutrition and growth failure associated with CGD.
A distinguishing feature of this study is the comparison of clinical status of the non-HCT CT cohort with that of the HCT-treated cohort. Because CT patients were not prospectively followed up, survival and progression of disease could not be assessed or compared between the 2 cohorts. Despite this limitation, HCT-treated patients had a significantly higher burden of disease manifestations at pre-HCT baseline, indicating a more severe disease phenotype, and at 3 to 5 years after HCT, surviving HCT-treated patients had 80% lower rates of infection and 93% lower rates of inflammatory disease, >90% reduced use of antimicrobial prophylaxis, and no use of steroids; and showed improved growth compared with CT patients. Further, we did not observe a significant incidence of new-onset autoimmune or inflammatory disease after HCT in contrast to a recent publication.16 This observation supports the concept that autologous T cells or antigen-presenting cells that remain after submyeloablative conditioning are not likely to induce secondary autoimmunity.
Of note, despite oxidase-null mutations and clinical symptoms occurring equally across races, African American/Blacks underwent HCT at less than half the frequency as Caucasians and Asians. The reasons for this disparity are unclear but may include lack of donor availability, limited access to care, and socioeconomic factors; this warrants further investigation.25
HCT has traditionally been reserved for CGD patients with a history of severe infection. However, the resolution of disease manifestations suggests that all CGD patients, regardless of age, presence of inflammatory disease, or impaired growth, are expected to benefit from HCT. Notably, transplantation should be offered before the disease results in the impairment of the performance status of the patients. The association of preexisting inflammatory disease on transplantation outcomes, particularly cGVHD, requires further investigation. Although we have identified busulfan and serotherapy as important aspects of the conditioning regimen, there remains a need to further improve transplant approaches with a focus on determining the optimal conditioning regimen to allow for durable donor cell engraftment across a variety of donor/cell sources while minimizing toxicities.
Acknowledgments
The authors thank PIDTC Project Managers Kiana Soriano and Alison Yip for their dedicated work to advance the research goals of the consortium. The authors also thank all study coordinators at PIDTC sites for collection of clinical data from medical records and the clinical teams who provided medical care for patients. Finally, the authors acknowledge all the patients and families who have made this work possible.
This study was supported by the National Institutes of Health (NIH), Division of Allergy, Immunology and Transplantation, National Institute of Allergy and Infectious Diseases (NIAID), and the NIH, Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences (NCATS), Bethesda, MD: NIH NIAID grant U54AI082973 (multiple principal investigators [PIs]: J.M.P., C.C.D., and E.H.); NIH, National Institute of Neurological Disorders and Stroke grant U54NS064808 (PI: J. Krischer); and NIH, National Center for Advancing Translational Sciences (NCATS) grant U01TR001263 (PI: J. Krischer). The PIDTC is a part of the Rare Diseases Clinical Research Network of ORDR, NCATS. The collaborative work of the PIDTC with the Pediatric Transplantation and Cellular Therapy Consortium (PTCTC) is supported by the U54 grants listed, along with support of the PTCTC Operations Center by the St Baldrick's Foundation and NIH, National Heart, Lung, and Blood Institute (NHLBI) grant U10HL069254 (PI: M.M. Horowitz). Collaborative work of the PIDTC with the Center for International Blood and Marrow Transplant Research is supported by NIH, National Cancer Institute (NCI) grant U24CA076518 (PI: B.E. Shaw); NIH, NHLBI grant U01HL069294 (PI: M. M. Horowitz); by contracts HHSH250201200016C and HHSH234200637015C with the Health Resources and Services Administration (HRSA/DHHS); and by grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research. C.L.E. is supported by NIH, NCATS grants KL2TR002492 and UL1TR002494. L.D.N. is supported by NIH, NIAID, Division of Intramural Research, grant 1 ZIA AI001222-02. D.E.A. and S.-Y.P. are supported by funding from NIH, NCI, Intramural Research Program, Center for Cancer Research. H.L.M. is supported by NIH, NIAID, Division of Intramural Research, grant Z-A1000644. E.M.K. is supported by NIH, NIAID, Division of Intramural Research grant Z-A1000989.
The content and opinions expressed are solely the responsibility of the authors and do not represent the official policy or position of the NIAID, ORDR, NCATS, NIH, HRSA, or any other agency of the US government.
Authorship
Contribution: J.W.L. is the lead of the CGD PIDTC working group and contributed patients, designed the study, performed data analysis, and was primarily responsible for the writing of the manuscript; E.M.K. and H.L.M. are senior leaders of the CGD PIDTC working group, contributed patients, designed the study, performed data analysis, and were primarily responsible for the writing of the manuscript; D.E.A., S.P., R.A.M., S.K., K.M., D.C., S.J.S.L., E.G., E.L.F., and L.M.-F. are members of the CGD PIDTC working team and contributed patients, designed the study, performed data analysis, and cowrote and edited the manuscript; B.L. and R.W. provided all biostatistical support; D.G., V.K.P., J.R.H, F.T., L.M.B., J.B., N.K., J.D., O.W., M.K., B.R.O., J.J.B., A.R., H.C., G.D.E.C., L.R.F.S., C.M., M.T.V.L., L.C.Y., S. Chandrakasan, A.J., S.E.P., B.J.D.S., V.A., L.A.B., C.L.E., L.M.M., K.D., J.M., H.G.R., A.J.S., A.P.G., A.P.K., H.K.M., T.B.M., P.G., A.B., N.J.B., P.T., A.P., S. Chandra, H.A.-A., M.J.D., and O.B. provided patients, helped with data refining, and edited the manuscript; L.M.G., M.J.C., C.C.D., E.H., D.B.K., L.D.N., S.-Y.P., J.M.P., M.A.P., and T.R.T. are members of the PIDTC Steering Committee and contributed patients, designed the study, analyzed the data, and cowrote and edited the manuscript.
Conflict-of-interest disclosure: J.W.L. is an employee and shareholder of bluebird bio; a speaker and advisory board member of Horizon Therapeutics and Sobi; and a consultant for Prime Medicine. R.A.M. serves as a section editor for UpToDate and is an advisory board member of Horizon Therapeutics and Sobi. J.R.H. receives research support from Regeneron, CSL-Behring, ADMA, Enzyvant and author royalties from UpToDate. L.M.B. has received clinical trial support from Medac GmbH; is a member of the data safety monitoring board for a clinical trial with Jasper Therapeutics; and has served on an advisory board for Horizon Therapeutics. J.J.B. is an advisory board member of Horizon Therapeutics and Sobi. G.D.E.C. is a consultant for Miltenyi Biotec. L.R.F.S. serves as a consultant for Takeda, Grifols, and ADMA and as an advisory board member for Horizon, CSL-Behring, Enzyvant, and Incyte. S.E.P. receives support for the conduct of sponsored trials through Boston Children’s Hospital from AlloVir, Atara, and Jasper Therapeutics; is a consultant for CellEvolve, Smart Immune, and Regeneron; and has intellectual property related to the development of off-the-shelf viral-specific cytotoxic T lymphocytes, with all rights assigned to Memorial Sloan Kettering Cancer Center. C.C.D. is a consultant for Alexion and Jazz Pharmaceuticals. E.H. is an advisory board member of Octapharma, Takeda, Jasper, Therapeutics, and Rocket Pharma. M.A.P. is an advisory board member for Novartis, Gentibio, bluebird bio, Vertex, Medexus, and Equillium; and has received study support from Miltenyi Biotec and Adaptive. J.M.P. receives royalties from UpToDate and their spouse is an employee of Invitae. H.L.M. is part of a cooperative agreement between NIAID and Prime Medicine. The remaining authors declare no competing financial interests.
Correspondence: Jennifer W. Leiding, Johns Hopkins All Children’s Hospital, 600 Fifth St S, Suite 3200, St. Petersburg, FL 33701; e-mail: jleidin1@jhmi.edu; and Elizabeth M. Kang, Laboratory of Clinical Immunology and Microbiology, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Dr, Building 10-CRC, Room 6-3752, Bethesda, MD 20892; e-mail: ekang@niaid.nih.gov.
References
Author notes
Data are available on request from the corresponding author, Jennifer W. Leiding (jleidin1@jhmi.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal