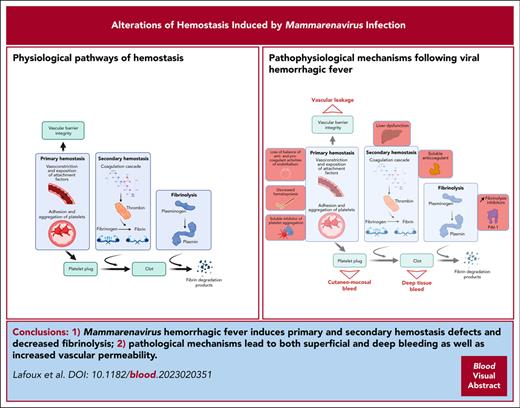
Key Point
Lethal arenavirus infection is associated with severe hemorrhagic signs.
Primary and secondary hemostasis and vascular integrity are impaired.
Abstract
Viral hemorrhagic fevers (HF) are a group of acute febrile diseases with high mortality rates. Although hemostatic dysfunction appears to be a major determinant of the severity of the disease, it is still unclear what pathogenic mechanisms lead to it. In clinical studies it is found that arenaviruses, such as Lassa, Machupo, and Guanarito viruses cause HF that vary in symptoms and biological alterations. In this study we aimed to characterize the hemostatic dysfunction induced by arenaviral HF to determine its implication in the severity of the disease and to elucidate the origin of this syndrome. We found that lethal infection with Machupo, Guanarito, and Lassa viruses is associated with cutaneomucosal, cerebral, digestive, and pulmonary hemorrhages. The affected animals developed a severe alteration of the coagulation system, which was concomitant with acute hepatitis, minor deficit of hepatic factor synthesis, presence of a plasmatic inhibitor of coagulation, and dysfunction of the fibrinolytic system. Despite signs of increased vascular permeability, endothelial cell infection was not a determinant factor of the hemorrhagic syndrome. There were also alterations of the primary hemostasis during lethal infection, with moderate to severe thrombocytopenia and platelet dysfunction. Finally, we show that lethal infection is accompanied by a reduced hematopoietic potential of the bone marrow. This study provides an unprecedented characterization of the hemostasis defects induced by several highly pathogenic arenaviruses.
Introduction
The Arenaviridae family comprises several highly pathogenic viruses responsible for hemorrhagic fevers (HF) with high lethality rates.1-3 Neither vaccine nor treatment has yet been licensed for these diseases.4 Pathogenic arenaviruses belong to 2 serotypes, old-world (OW) arenaviruses, such as Lassa (LASV), which is endemic to West Africa, and new-world (NW) arenaviruses, such as Machupo (MACV) or Guanarito (GTOV) viruses, which cause epidemics in South America.
Severe arenaviral HF causes neurological and hemorrhagic symptoms that ultimately lead to multiorgan failure, shock syndrome, and death. The pathophysiology of these diseases is still largely unknown because of the difficulty of accessing human samples and the necessity of working under biosafety level 4 (BSL-4) conditions. As highlighted in recent publications,5-7 the only animal models that reliably reproduce clinical features of human disease are nonhuman primates (NHP), which further restricts the number of facilities that can perform such studies.
The hemorrhagic syndrome is a major determinant of the severity of the disease in infected patients. Indeed, clinical bleeding is the symptom most predictive of a death in patients with Lassa fever.8 In addition, clinical studies have highlighted biomarkers of dysregulated coagulation as strongly predictive of a fatal outcome.9,10 Thrombocytopenia and platelet dysfunction were demonstrated in fatal cases caused by different arenaviral species, with variable degrees of severity.9-12 Coagulation tests of activated partial thromboplastin time (aPTT), prothrombin time (PT), and thrombin time (TT) were inconstantly affected in NHP and clinical studies on MACV, Junin virus (JUNV), and LASV.13-15
Infection of vascular endothelial cells (vEC) by arenaviruses is suspected to play a major role in the increased vascular permeability.16,17 Indeed, vEC can be productively infected in vitro by LASV and JUNV.18,19 These models revealed that infection of vEC induced cellular dysfunction, which is characterized by overexpression of vasoactive agents and adhesion molecules.18,20 Two studies showed that infection of vEC by JUNV and Pichinde virus (PICV) induced an increase in permeability of vascular monolayers through adherens junction disruption.21,22
The description and pathophysiology of this complex biological and clinical syndrome is still very fragmented. Notably, no comparative in vivo study has ever been done to clarify the link between this hemostasis defect and the severity of the disease or to compare its extent between arenaviral species. In this study, we took advantage of a large cohort of animals, which were infected by several arenaviruses and longitudinally monitored in comparable manners to propose a comprehensive analysis of this hemorrhagic syndrome. This animal model reliably reproduced alterations observed in patients and allowed us to study every aspect previously mentioned in the literature to identify differences between viral species and severity factors.
Methods
Study design
All biological samples used for this work were generated during anterior animal studies. The data from a total of 6 separate cohorts of cynomolgus macaques were used. Animals were challenged with viruses diluted between 1000 and 3000 focus-forming units (FFU)/mL of phosphate-buffered saline and mock-infected animals were injected with 0.5 mL of phosphate-buffered saline. These studies were conducted in a BSL-4 laboratory (Laboratoire P4 Jean Mérieux, Lyon, France). Details of the follow-up and specimens taken can be found in the original articles.5,6,23,24 Humane end points to the protocol included reaching the maximum clinical score, severe hypothermia, body temperature >41.5°C for 3 days, coma, or seizure. All animal studies have been validated by regional ethical committees.5,6,23,24
Viruses
LASV strains AV and Josiah, MACV strain Carvallo, and GTOV strain INH-95551 were produced in VeroE6 cells cultured in Dulbecco’s modified Eagle medium. Supernatants were harvested, titrated, and frozen at −80°C.
Hematology and platelet function tests
Blood collected in EDTA tubes was used to obtain blood cell counts on an MS9 automate (Melet Schloesing Laboratoires). Impedance platelet aggregometry was measured with a Multiplate Analyzer (Roche Diagnostics). In brief, platelet aggregation in the test plasma was stimulated by following 3 different agonists: adenosine 5′-diphosphate (ADP; 6.5 μM), ristocetin (RISTO; 0.8 mg/mL), and collagen (COL; 3.2 μg/mL).
Coagulation tests and coagulation factors assays
For coagulation tests, such as aPTT, PT, TT, and dilute Russell’s viper venom time (dRVVT) test as well as fibrinogen and D-dimer quantifications, plasma was used on a Helena C-4 coagulometer (Helena Biosciences) according to the manufacturer’s instructions. Coagulation factors were quantified by proxy of their coagulative potential in cryopreserved plasma from 3.2% sodium citrate tubes for animals infected with LASV AV and LASV Josiah and from EDTA tubes for animals infected with MACV and GTOV. For mixing studies and dRVVT, the control plasma was a pool of 3 mock-infected animals.
Colony-forming cell (CFC) assays
Bone marrow mononuclear cells were isolated by centrifugation from aspirates taken at the autopsy. CD34+ cells were immunomagnetically separated on MACS columns (Miltenyi Biotec) and cultured in semisolid medium for 10 days at a density of 3.3 × 103 cells/mL in triplicates. Colony numbers were visually assessed under a light microscope.
Histology
Organs were embedded in paraffin. Sections of 3-μm thickness were cut to be stained with hematoxylin and eosin. In situ hybridization (ISH) was performed with RNAscope (Bio Techne) multiplex kit. Probes were designed to target the S segment of the relevant arenavirus. Immunofluorescence (IF) was done with the following primary antibodies: mouse anti-tissue factor (TF) (clone TF9-10H10), rabbit anti-CD31 (clone EP3095), and mouse anti-fibrin (clone 59D8). Detection of viral genome and target proteins was performed with Opal fluorophores (Akoya) and counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Images were captured on a LSM980 microscope using the ZEN software (Zeiss). For quantifications, whole-slide images were captured with a Yokogawa CQ1 microscope and analyzed with QuPath software.
Human umbilical endothelial vein cells (HUVEC) and VeroE6 replication kinetics
HUVEC were obtained from 3 donors and cultured using the E cell growth medium 2 (Promo Cell). Vero E6 cells were used as positive control for infection and grown in Dulbecco’s modified Eagle medium. Cells were infected at a multiplicity of infection of 0.01, and supernatants were harvested at 0, 24, 48, 72, and 96 hours after infection. Titrations were performed on VeroE6 cells.
Plasminogen activator inhibitor 1 (PAI-1) enzyme-linked immunosorbent assay
Cryopreserved plasma sampled at 12 days post infection (DPI) was analyzed using a PAI-1 monkey enzyme-linked immunosorbent assay kit from ThermoFisher according to the manufacturer’s instructions and read by chemiluminescence on a Tecan Infinite 200 Pro.
Western blots
Lung samples of animals infected with MACV, GTOV, or mock-infected were lysed in radioimmunoprecipitation assay (RIPA) buffer (10 mg of tissue for 100 μL of buffer) using a Qiagen TissueLyser II. Cryopreserved plasma sampled at 12 DPI was depleted of albumin and immunoglobulins using HighSelect columns (ThermoFisher). Samples were separated in sodium dodecyl sulfate polyacrylamide gel electrophoresis (4%-15%) and transferred to a nitrocellulose membrane. Because experimental conditions can modify the overall concentration of proteins in the plasma (through liver deficiency as previously observed5) and in the lung (through alveolar edema), we did not normalize sample loading according to protein quantity but rather using a constant weight by volume ratio for the lung and a constant volume of plasma. Target proteins were detected with the following primary antibodies: TF9-10H10 anti-TF, R&D #AF-2974 anti-tissue factor pathway inhibitor (TFPI), Abcam #ab49735 anti-PF4, Invitrogen #PA5-87043 anti-CD62P, EPR24639-3 anti-ICAM1, and EPR5047 anti-VCAM1. Species-specific secondary antibodies conjugated with horseradish peroxidase were used, and staining was performed with Bio-Rad Clarity Max substrate and imaged on an ImageQuant LAS4000. Relative protein levels were evaluated by measuring signal intensities on ImageJ and comparing them with the mean of the 3 mock animals.
Transcriptome
For both RNA sequencing data sets, bioinformatics analysis was performed using the RNA sequencing pipeline from RNAflow (https://gitlab.pasteur.fr/hub/rnaflow). Reads were cleaned of adapter sequences and low-quality sequences using cutadapt. STAR, with default parameters, was used for alignment against the reference genome. Reads were assigned to genes using featureCounts from the Subreads package. Differential analysis was performed using the DESeq2 R package to identify genes for which the expression profiles were significantly different among each pair of biological conditions.
Statistics
An unpaired, nonparametric 2-tailed Mann-Whitney t test was used to compare coagulation parameters, coagulation factor levels, infectious titers in HUVEC, platelet counts, platelet aggregation levels, and PAI-1 levels between 2 groups, and an unpaired, parametric Welch t test was used to compare colony numbers for CFC assays. For the gene set–related heatmaps, variable stabilizing transformation (VST) transformed data were centered to the Mock and scaled to the variance of each data set. Then, these normalized data sets were aggregated by condition to obtain 1 single value by condition and time point. The sex of the animals was not adjusted for because of the statistical confounding effects of the experimental conditions of interest. For the correlation analysis, area under the curve was calculated with a baseline of 0 for interferon α1 and of 281 G/L (the mean of all animals at 0 DPI) for platelets, and Pearson correlation coefficients were calculated.
Results
A total of 39 cynomolgus macaques were inoculated with pathogenic arenaviruses in a BSL-4 laboratory. Two strains of the OW arenavirus LASV, Josiah and AV, were used to challenge 15 and 9 monkeys, respectively, whereas 2 species of NW arenaviruses, MACV and GTOV were inoculated to 6 and 3 animals, respectively (supplemental Table 1; available on the Blood website). Three animals served as mock-infected controls.
Infected animals presented a transient leukopenia affecting lymphocytes, monocytes, and neutrophils and moderate anemia (supplemental Table 2). After a period of nonspecific febrile syndrome, arenavirus-infected animals started recovering, whereas others displayed hemorrhagic symptoms, more commonly in MACV- and GTOV-infected macaques (83% and 100%) than among LASV Josiah animals (55%). Only superficial bleeding, such as epistaxis and petechiae were noted for the latter, while MACV- and GTOV-infected groups developed digestive tract hemorrhages manifested by melena and hematochezia. All animals infected with LASV Josiah or MACV and 1 GTOV animal were euthanized between 11 and 18 DPI because they reached the clinical end point. One GTOV and all LASV AV–infected animals started recovering after 18 to 20 DPI, whereas the third GTOV-infected animal displayed symptoms until the end of the procedure.
Necropsies revealed gross pathological lesions in animals that succumbed to infection (supplemental Table 1 and supplemental Figure 1). Macaques fatally infected with LASV Josiah, MACV, and GTOV presented hemorrhages in the lungs, gastrointestinal tract, and intracranial space. All animals presented peritoneal effusions, indicating increased vascular permeability.
Organs harvested at autopsy were stained in hematoxylin and eosin and examined microscopically to determine the localization and the eventual cause of bleeding.
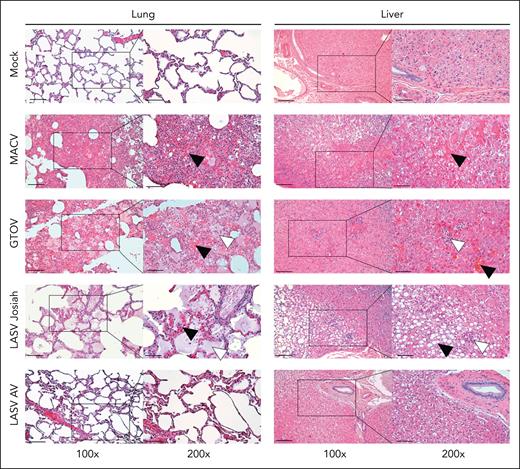
The lungs of animals infected with MACV, GTOV, and LASV Josiah presented histopathologic features of acute lung injury (Figure 1, left column). Parietal thickening owing to immune cells infiltration was found in lethally infected animals. These animals had areas of hemorrhage in the lung parenchyma. A large number of hemosiderin-laden macrophages was present, indicating an active hemophagocytosis. Alveolar edema was also observed, predominantly in LASV Josiah–infected animals. The absence of capillary congestion did not point toward cardiogenic edema but rather increased vascular permeability.
Histopathological changes associated with hemostasis defects in the organs of infected monkeys. Samples harvested during necropsy of animals infected with MACV, GTOV, LASV Josiah, LASV AV, or mock-infected were stained using hematoxylin-eosin coloration. Black squares correspond to the area imaged in the inset on the right. Left column: lungs show varying degrees of hemorrhage, alveolar edema, and interstitial infiltration. Black arrowheads indicate areas of hemorrhage and white arrowheads indicate alveolar edema. Right column: hepatic parenchyma injury includes macrovesicular steatosis, focal necrosis and peri-portal mononuclear infiltrates. Black arrowheads indicate areas of hepatocellular degeneration and necrosis; white arrowheads indicate inflammatory infiltrates. Images are representative of observations made in 3 different animals for each condition. Left column scale bar = 100 μm; right column scale bar = 50 μm.
Histopathological changes associated with hemostasis defects in the organs of infected monkeys. Samples harvested during necropsy of animals infected with MACV, GTOV, LASV Josiah, LASV AV, or mock-infected were stained using hematoxylin-eosin coloration. Black squares correspond to the area imaged in the inset on the right. Left column: lungs show varying degrees of hemorrhage, alveolar edema, and interstitial infiltration. Black arrowheads indicate areas of hemorrhage and white arrowheads indicate alveolar edema. Right column: hepatic parenchyma injury includes macrovesicular steatosis, focal necrosis and peri-portal mononuclear infiltrates. Black arrowheads indicate areas of hepatocellular degeneration and necrosis; white arrowheads indicate inflammatory infiltrates. Images are representative of observations made in 3 different animals for each condition. Left column scale bar = 100 μm; right column scale bar = 50 μm.
The most frequent lesions across all lethally infected animals were found in the liver, evocating an acute viral hepatitis (Figure 1, right column). The presence of periportal mononuclear cells infiltrates was noted in all studied animals, with LASV Josiah and MACV animals presenting the most severe form. Hepatocellular damage was also overt in various extent among groups. GTOV-infected animals presented the mildest form, with the presence of swollen hepatocytes and diffuse councilman apoptotic bodies. One LASV Josiah and 2 MACV-infected monkeys had severe lesions with numerous apoptotic bodies, focal necrosis of hepatocytes, and ballooning degeneration of large areas. Lobular organization was perturbed among all groups, manifested by congested sinusoids and loss of visible demarcation among lobules. All LASV AV–infected animals were exempt from any pathological change at the time of euthanasia.
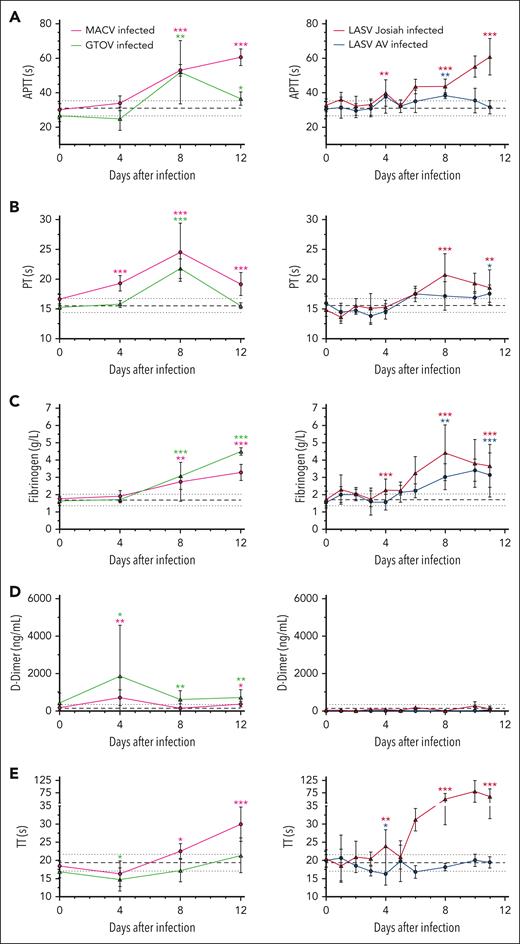
We investigated the coagulation parameters of infected cynomolgus macaques to determine whether clinical bleeding was related to specific alterations of hemostasis. The aPTT was significantly prolonged for MACV and GTOV animals at 8 DPI and continued increasing until death for MACV while it returned to almost normal values for GTOV at 12 DPI (Figure 2A). For LASV Josiah–infected monkeys, aPTT prolongation started as soon as at 4 DPI and continuously increased until death while it stayed close to normal for LASV AV–infected animals. PT was prolonged in MACV-infected animals from 4 DPI to 12 DPI with a peak at 8 DPI, time point at which the PT of GTOV animals was also prolonged before going back toward normal values (Figure 2B). Among the LASV Josiah group, the increase was slightly slower but followed a similar pattern with a peak at 8 DPI whereas LASV AV–infected animals remained normal throughout the study.
Coagulation system defects associated with hemorrhagic fever-causing arenaviruses. (A) aPTT expressed in seconds. (B) PT expressed in seconds. (C) Fibrinogen concentration in g/L. (D) D-dimer levels in ng/mL. (E) Thrombin time expressed in seconds. Mean values for infected animals (MACV in magenta, GTOV in green, LASV AV in blue, and LASV Josiah in red) are presented with error bars for SEM. The base value for each parameter is presented as the mean of all values from 3 mock-infected animals and the value at the day of challenge of 24 animals. The continuous black line represents the mean and dotted line the SEM. Asterisks indicate a statistically significant difference with the base level according to a 2-tailed Mann-Whitney test (∗P < .05; ∗∗P < .005; ∗∗∗P < .0005). Tests were done for values at 4, 8, 11, and 12 DPI. Number of animals: 3 MACV-infected, 3 GTOV-infected animals, 12 LASV Josiah-infected and 6 LASV AV-infected animals. SEM, standard error of the mean.
Coagulation system defects associated with hemorrhagic fever-causing arenaviruses. (A) aPTT expressed in seconds. (B) PT expressed in seconds. (C) Fibrinogen concentration in g/L. (D) D-dimer levels in ng/mL. (E) Thrombin time expressed in seconds. Mean values for infected animals (MACV in magenta, GTOV in green, LASV AV in blue, and LASV Josiah in red) are presented with error bars for SEM. The base value for each parameter is presented as the mean of all values from 3 mock-infected animals and the value at the day of challenge of 24 animals. The continuous black line represents the mean and dotted line the SEM. Asterisks indicate a statistically significant difference with the base level according to a 2-tailed Mann-Whitney test (∗P < .05; ∗∗P < .005; ∗∗∗P < .0005). Tests were done for values at 4, 8, 11, and 12 DPI. Number of animals: 3 MACV-infected, 3 GTOV-infected animals, 12 LASV Josiah-infected and 6 LASV AV-infected animals. SEM, standard error of the mean.
Plasma fibrinogen levels steadily increased starting at 4 DPI and until 12 DPI in all groups (Figure 2C). D-dimers levels remained low except for 2 outlier animals in MACV and GTOV groups that were transiently increased but came back to normal by 8 DPI (Figure 2D). In MACV- but not GTOV-infected animals, TT showed a late prolongation at 12 DPI, whereas in LASV Josiah animals it was strongly elevated from 8 DPI (Figure 2E).
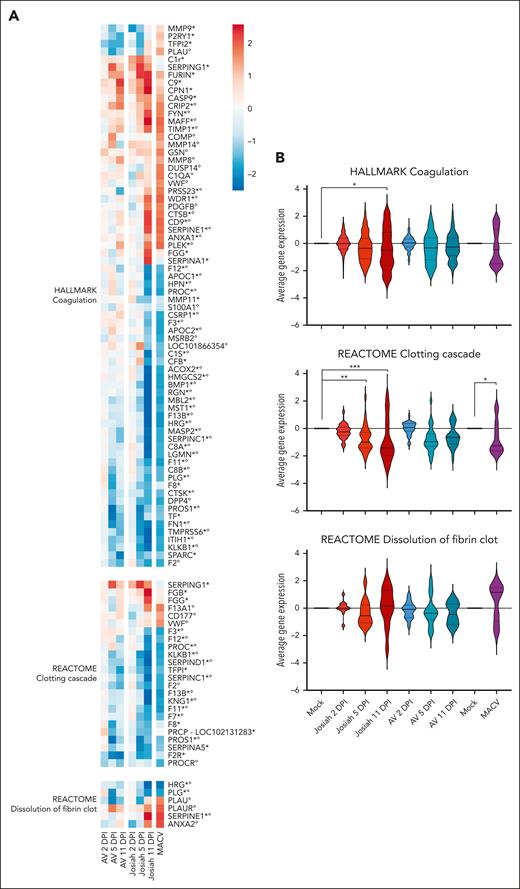
To determine whether the alterations of coagulation could be explained by a dysfunction of hepatocytes during infection, we conducted a transcriptomic analysis of liver cells from animals infected with LASV Josiah, LASV AV, and MACV. Total RNA was extracted from organs harvested at 3 sequential points of euthanasia, 2, 5, and 11 DPI for the 2 LASV strains or when animals reached the clinical end point for MACV. The expression of 3 publicly available gene sets was compared with the gene sets of the mock-infected animals (Figure 3A). Gene expression of factor XII (FXII), FXI, and FVII was significantly downregulated in Josiah and MACV–infected animals at 11 DPI, whereas FV, FIX, and FX showed little to no change (supplemental Figure 2). Consistent with the observed hyperfibrinogemia, the genes encoding 2 of the fibrinogen subunits, FGB and FGG, were upregulated in the Josiah group at 11 DPI. The KLKB1 gene coding for plasma kallikrein and the KNG1 gene coding for kininogen, 2 procoagulant proteins were also downregulated during lethal infection. The fibrinolysis system appeared to be negatively affected in animals developing a severe disease. Indeed, the expression of key inhibitors of fibrinolysis SERPINE1 (also known as PAI-1), TIMP1, and HRG was increased in Josiah animals at 11 DPI and MACV at the time of death whereas plasminogen (PLG), which encodes for plasminogen was decreased. The overall expression level of these gene sets was compared between infected and mock-infected groups (Figure 3B). The expression of the coagulation gene set was significantly lower in the Josiah 5 DPI group and the clotting cascade gene set was downregulated in Josiah 5 DPI, Josiah 11 DPI, and MACV groups, indicating that hepatocyte dysfunction in lethal infection can play a role in dysregulated hemostasis.
Significant transcriptomic changes associated with hemostasis in the liver of macaques infected with LASV Josiah, LASV AV, and MACV. (A) Transcriptomic analysis of liver cells at 2, 5 or 11 DPI for AV and Josiah or at time of death for MACV. The heatmaps represent the gene expression in each pathway. Only statistically differentially expressed genes (for Josiah vs mock, Josiah vs AV, or MACV vs mock comparisons) are included. The color of the heatmaps represents the mock–related scaled normalized counts averaged for each group and time point (n = 3 for each time point and infection status and n = 6 for MACV). Gene names were annotated regarding their differential expression status: asterisk refers to genes significant in the mock-AV-Josiah data set whereas degree symbols refer to genes significant in the Machupo data set. (B) Violin plots comparing the global expression variation relative to the mock. The overall gene sets over- or under-expression was tested with a 1-way mixed analysis of variance and P-values were corrected with the Tukey multiple comparison test.
Significant transcriptomic changes associated with hemostasis in the liver of macaques infected with LASV Josiah, LASV AV, and MACV. (A) Transcriptomic analysis of liver cells at 2, 5 or 11 DPI for AV and Josiah or at time of death for MACV. The heatmaps represent the gene expression in each pathway. Only statistically differentially expressed genes (for Josiah vs mock, Josiah vs AV, or MACV vs mock comparisons) are included. The color of the heatmaps represents the mock–related scaled normalized counts averaged for each group and time point (n = 3 for each time point and infection status and n = 6 for MACV). Gene names were annotated regarding their differential expression status: asterisk refers to genes significant in the mock-AV-Josiah data set whereas degree symbols refer to genes significant in the Machupo data set. (B) Violin plots comparing the global expression variation relative to the mock. The overall gene sets over- or under-expression was tested with a 1-way mixed analysis of variance and P-values were corrected with the Tukey multiple comparison test.
We measured the activity of coagulation factors to determine if secondary hemostasis defects could be due to a decreased coagulation factor synthesis (Figure 4A-B). During the course of the disease, the activity of FXI, FIX, FVIII, and FVII decreased for MACV and GTOV animals. LASV-infected animals also presented alterations of FXII, FXI, FVIII, and FVII but did not strongly differ between AV and Josiah strains. Importantly, all factors remained higher than 60% of their normal activity.
Alterations of coagulation factor activity after arenavirus-induced HF. (A) Factors of the intrinsic pathway of coagulation: factors XII, XI, IX, and VIII. (B) Factors of the extrinsic and common pathway: factors II, V, VII, and X. Tests were performed using EDTA plasma for GTOV and MACV and citrate plasma for LASV Josiah and AV. The legend is as for Figure 2. The base level is calculated as the mean of all values from 3 mock-infected animals and the value at the day of challenge of 6 infected animals. Three animals of each infection status were included.
Alterations of coagulation factor activity after arenavirus-induced HF. (A) Factors of the intrinsic pathway of coagulation: factors XII, XI, IX, and VIII. (B) Factors of the extrinsic and common pathway: factors II, V, VII, and X. Tests were performed using EDTA plasma for GTOV and MACV and citrate plasma for LASV Josiah and AV. The legend is as for Figure 2. The base level is calculated as the mean of all values from 3 mock-infected animals and the value at the day of challenge of 6 infected animals. Three animals of each infection status were included.
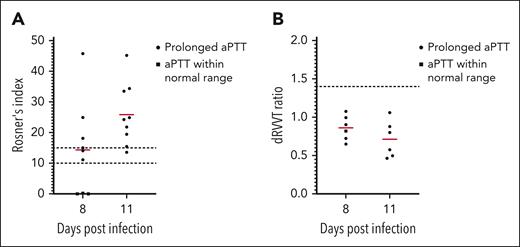
To investigate the anticoagulant activity of infected plasma, we performed a mixing test on plasma from Josiah-infected animals at 8 and 11 DPI (Figure 5A). The Rosner index was calculated for each sample to determine if the mix of the infected plasma and the control plasma had a corrected coagulation time. At 8 DPI, 6 animals out of 9 had a prolonged aPTT, and all of them had a Rosner index >10, indicating the presence of a plasmatic inhibitor of coagulation. At 11 DPI, all 9 animals had both prolonged aPTT and a Rosner >10. Because lupus anticoagulants (LA) are commonly observed in viral infections,25 we performed a dRVVT test on these plasma (Figure 5B). None of the 18 samples tested had a prolonged dRVVT, indicating that LA was not the cause of the observed coagulopathy.
Presence of a circulating inhibitor of coagulation in the plasma of LASV Josiah animals. (A) Plasma from LASV Josiah-infected animals at 8 or 11 DPI was mixed 50:50 with a pool of 3 mock-infected animals. The aPTT of the test plasma, the control plasma, and the mixed plasma were measure to calculate the Rosner index as follows: RI = (aPTT of mix – aPTT of control)/aPTT of test. Squares represent the samples for which the aPTT of the test plasma was within a 95% confidence interval of the mean of mocks and circles the samples with a prolonged aPTT. Red bars represent the mean of each time point. Dashed lines highlight the scores of 10 and 15, between which the result is evocative of an inhibitor of coagulation but inconclusive and above which the test is considered positive with certainty. (B) dRVVT of LASV Josiah-infected animals at 8 or 11 DPI. Legend is as previously, except for the dashed line that indicates the score of 1.5, which is the threshold of positivity for lupus anticoagulant.
Presence of a circulating inhibitor of coagulation in the plasma of LASV Josiah animals. (A) Plasma from LASV Josiah-infected animals at 8 or 11 DPI was mixed 50:50 with a pool of 3 mock-infected animals. The aPTT of the test plasma, the control plasma, and the mixed plasma were measure to calculate the Rosner index as follows: RI = (aPTT of mix – aPTT of control)/aPTT of test. Squares represent the samples for which the aPTT of the test plasma was within a 95% confidence interval of the mean of mocks and circles the samples with a prolonged aPTT. Red bars represent the mean of each time point. Dashed lines highlight the scores of 10 and 15, between which the result is evocative of an inhibitor of coagulation but inconclusive and above which the test is considered positive with certainty. (B) dRVVT of LASV Josiah-infected animals at 8 or 11 DPI. Legend is as previously, except for the dashed line that indicates the score of 1.5, which is the threshold of positivity for lupus anticoagulant.
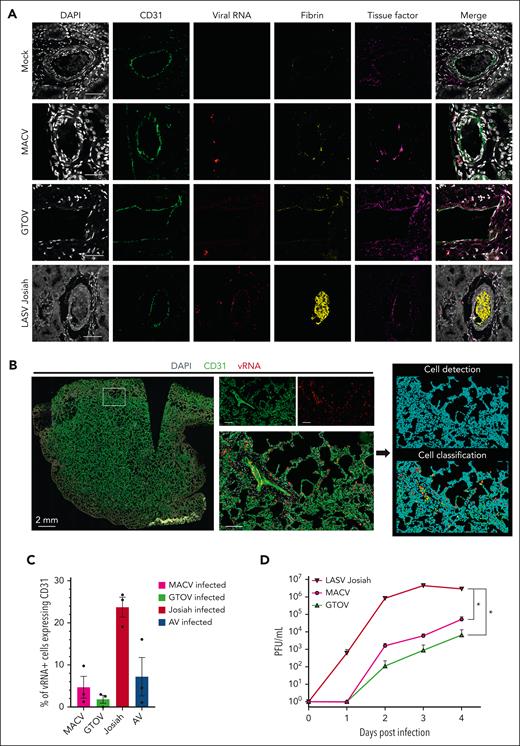
To determine if direct infection of endothelial cells was responsible for the observed increase in vascular permeability, we performed multiplexed IF on the tissues of infected animals (Figure 6A). We used RNA ISH to detect viral genome and IF for the endothelial cell marker CD31, fibrin and TF. Colocalization of ISH and CD31 was observed in LASV Josiah but not GTOV- or MACV-infected animals (Figure 6A; supplemental Figure 3A), and TF staining was more pronounced on the vessel walls in animals infected with MACV, GTOV, and LASV Josiah. To quantitatively measure vEC infection, we performed whole-organ confocal scanning of the lungs, individual cell detection, and classification according to CD31 and/or vRNA positivity (Figure 6B), which confirmed that LASV Josiah had more tropism for vEC than for the other viral strains (Figure 6C). In the kidneys and lungs of Josiah animals, we found a large number of fibrin-obstructed capillaries and areas of fibrin deposits along the alveolar walls. We measured the plasma levels of PAI-1 at the peak of the disease and found significant differences to the mock condition for animals infected by LASV Josiah or MACV. We used another IF panel coupling ISH, TF, and the monocytic marker CD68 (supplemental Figure 3B) which showed that monocytes containing viral RNA of LASV Josiah, MACV, and GTOV displayed abnormal TF expression. Western blot analysis of lung homogenates (supplemental Figure 3D-E) revealed increased levels of TF in all infected conditions except with GTOV and increased TFPI in all conditions compared to the mock. We also studied the replication of LASV Josiah, MACV, and GTOV in HUVEC (Figure 6D). LASV Josiah had a faster growth curve and reached a higher peak at 3 DPI than MACV and GTOV which reached their peak at 4 DPI. Notably, there was a total absence of GTOV replication in 2 of the 3 tested donors of HUVEC. To study vEC activation, we measured ICAM-1 and VCAM-1 levels in lung samples (supplemental Figure 3F-G), which showed increased expression after viral challenge especially in Josiah and MACV groups. These results indicate that tropism of arenaviruses for vEC is not the main cause of the observed hemorrhagic tendency, at least in the case of NW arenaviruses, MACV and GTOV.
Differences in tropism for endothelial cells between hemorrhagic fever-causing arenaviruses. (A) Multiplex immunofluorescence and ISH staining of kidney sections from infected or mock-infected animals. Single channel images of DAPI, CD31 (vascular endothelial cells), fibrin, viral RNA, and TF are shown as well as their merge. Images are representative of observations made in 3 separate animals for each condition. Scale bar corresponds to 50 μm. (B) Whole-slide images obtained by confocal scanner. Three slices in the Z dimension were transformed by maximum intensity projection. Left image shows a whole lung from a LASV Josiah-infected animal with the 3 staining merged. The 3 images in the center correspond to the white inset and allow appreciation of the staining localization at a cellular level. It includes a medium caliber vessel surrounded by alveoli. Scale bars = 50 μm. The 2 images on the right are from QuPath software and illustrate the process of cell detection based on DAPI staining and cell classification based on CD31 and vRNA positivity. Color-coding: blue = unclassified; green = CD31+ vRNA–; yellow = CD31+ vRNA+; red = CD31– vRNA+. (C) Quantification of the classification obtained in the previous analysis. Three organs for each condition were quantified. (D) Infectious particles titers in the supernatant of HUVEC infected with 3 different hemorrhagic fever-causing arenaviruses, harvested at 5 different time points after infection. Error bars correspond to the standard error of the mean of 3 replicates, asterisks signal a statistically significant difference between titers at 4 DPI according to a two-tailed Mann-Whitney test (P < .05).
Differences in tropism for endothelial cells between hemorrhagic fever-causing arenaviruses. (A) Multiplex immunofluorescence and ISH staining of kidney sections from infected or mock-infected animals. Single channel images of DAPI, CD31 (vascular endothelial cells), fibrin, viral RNA, and TF are shown as well as their merge. Images are representative of observations made in 3 separate animals for each condition. Scale bar corresponds to 50 μm. (B) Whole-slide images obtained by confocal scanner. Three slices in the Z dimension were transformed by maximum intensity projection. Left image shows a whole lung from a LASV Josiah-infected animal with the 3 staining merged. The 3 images in the center correspond to the white inset and allow appreciation of the staining localization at a cellular level. It includes a medium caliber vessel surrounded by alveoli. Scale bars = 50 μm. The 2 images on the right are from QuPath software and illustrate the process of cell detection based on DAPI staining and cell classification based on CD31 and vRNA positivity. Color-coding: blue = unclassified; green = CD31+ vRNA–; yellow = CD31+ vRNA+; red = CD31– vRNA+. (C) Quantification of the classification obtained in the previous analysis. Three organs for each condition were quantified. (D) Infectious particles titers in the supernatant of HUVEC infected with 3 different hemorrhagic fever-causing arenaviruses, harvested at 5 different time points after infection. Error bars correspond to the standard error of the mean of 3 replicates, asterisks signal a statistically significant difference between titers at 4 DPI according to a two-tailed Mann-Whitney test (P < .05).
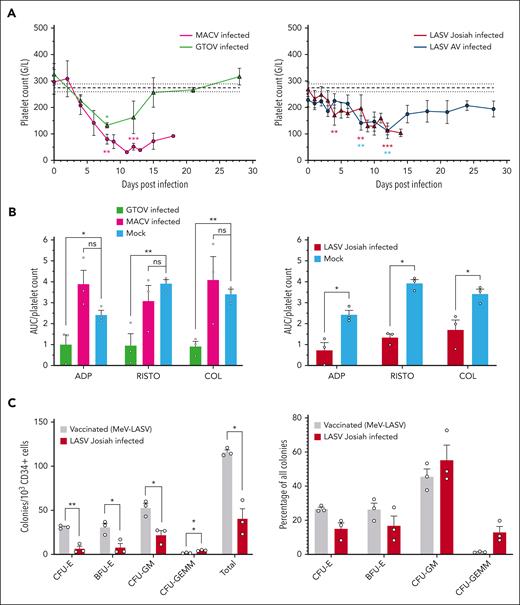
To compare the kinetics and amplitude of thrombocytopenia among different arenaviral HF, we measured platelet counts after challenge. GTOV-infected animals had a transient and moderate thrombocytopenia, peaking at 8 DPI (Figure 7A). On the other hand, the MACV group presented a more severe form, reaching its lowest at 11 DPI with a mean of 31 G/L. LASV AV– and Josiah-infected monkeys had similar patterns up to 12 DPI when the AV group started to recover, and Josiah kept decreasing until death (Figure 7A). Because high levels of type-I interferon (IFN) can cause thrombocytopenia,26 we performed a correlation analysis between IFN-α1 levels and platelet counts indiscriminately of viral species but found no significant correlation (supplemental Figure 4A).
Variable levels of thrombocytopenia and platelet dysfunction during infection with highly pathogenic arenaviruses. (A) Platelet counts expressed in giga cells/L. The base level is calculated as the mean of all values from 3 mock-infected animals and the value at the day of challenge of 53 animals. The rest of the legend is as previously described. (B) Platelet function evaluated through platelet aggregation normalized by platelet count. Platelet aggregation was measured in response to 3 classical platelet agonists: ADP, RISTO, and COL. Tests were performed at the day of euthanasia and raw values were normalized by the platelet count on the corresponding time point. Bars and error bars represent the mean and SEM of each condition, individual values appear as circles. Asterisks indicate a statistically significant difference with the base level according to a 2-tailed Mann-Whitney test (∗P < .05; ∗∗P < .01; ∗∗∗P < .005). (C) Number and relative frequency of colonies from each myeloid lineage obtained after a 10-day culture of CD34+ hematopoietic cells from vaccinated and unvaccinated animals challenged with LASV Josiah animals. Bone marrow specimens from 3 animals in each condition were used and cultures were carried out in triplicates. Dots correspond to the mean of technical triplicates, bars to the mean of 3 animals and error bars to their standard error of the mean. Asterisks indicate a statistically significant difference with the base level according to a Welch t test (∗P < .05; ∗∗P < .01).
Variable levels of thrombocytopenia and platelet dysfunction during infection with highly pathogenic arenaviruses. (A) Platelet counts expressed in giga cells/L. The base level is calculated as the mean of all values from 3 mock-infected animals and the value at the day of challenge of 53 animals. The rest of the legend is as previously described. (B) Platelet function evaluated through platelet aggregation normalized by platelet count. Platelet aggregation was measured in response to 3 classical platelet agonists: ADP, RISTO, and COL. Tests were performed at the day of euthanasia and raw values were normalized by the platelet count on the corresponding time point. Bars and error bars represent the mean and SEM of each condition, individual values appear as circles. Asterisks indicate a statistically significant difference with the base level according to a 2-tailed Mann-Whitney test (∗P < .05; ∗∗P < .01; ∗∗∗P < .005). (C) Number and relative frequency of colonies from each myeloid lineage obtained after a 10-day culture of CD34+ hematopoietic cells from vaccinated and unvaccinated animals challenged with LASV Josiah animals. Bone marrow specimens from 3 animals in each condition were used and cultures were carried out in triplicates. Dots correspond to the mean of technical triplicates, bars to the mean of 3 animals and error bars to their standard error of the mean. Asterisks indicate a statistically significant difference with the base level according to a Welch t test (∗P < .05; ∗∗P < .01).
To test platelet function, we measured aggregation in response to different agonists (Figure 7B). LASV Josiah and GTOV but not MACV-infected animals presented lower ratios of aggregation per platelet than noninfected controls in response to ADP, RISTO, and COL. In addition, we found largely increased levels of soluble PF4 in the plasma of animals infected with Josiah and GTOV compared to that in the mock (supplemental Figures 4B-C).
To determine if thrombocytopenia might be due to a defect in hematopoiesis, we isolated CD34+ hematopoietic cells from the bone marrow of animals infected with LASV Josiah whether or not previously vaccinated with MeV-LASV. This vaccine protected 100% of animals against a lethal challenge from LASV Josiah with minimal and transient clinical signs.24 We performed CFC assays on these samples and quantified lineage-restricted progenitors after 10 days of culture (Figure 7C). There was an overall decrease in the ability of CD34+ cells from nonvaccinated animals to differentiate into hematopoietic progenitors, except for their most immature form, colony-forming unit granulocyte erythrocyte monocyte megakaryocyte (CFU-GEMM). The erythroid lineage was particularly affected, whereas monocytic-granulocytic lineages were slightly increased in relative frequency. This indicates that lethal LASV Josiah infection is associated with defective hematopoietic differentiation and proliferation.
Discussion
Despite their clear implication in disease severity, the understanding of hemostasis defects associated with arenaviral HF remained unclear. In this study, we present the first comparative analysis of hemostatic disorders induced by 4 highly pathogenic arenaviruses in a NHP model.
Following the principle of reducing the number of animals used to reach a scientific aim, we took advantage of nonvaccinated animals that were initially part of multiple vaccine studies. This design introduces heterogeneity in the material available for each group. To minimize the bias associated with separate animal experiments, we matched infected groups to relevant control groups for which we had comparable samples in all experiments, allowing robust comparisons.
Our model of infection recapitulated all the hematological features observed in clinical studies, such as thrombocytopenia, leukopenia, platelet dysfunction, clotting-time prolongation, high PAI-1 levels, and hemorrhagic symptoms.8,10-12,27-30 The differences in the severity of these features between the AV and Josiah strains also recapitulate the discrepancies observed between patients with severe and mild Lassa fever.
Because the cutaneomucosal bleeding observed in patients is usually limited, it was uncertain whether the hemorrhagic syndrome could really be implicated in the disease severity. To our knowledge, we identify for the first time that arenaviral infection can induce cerebral and cerebellar hemorrhages. These hemorrhages can cause coma and death by infarction or compression of the central nervous system. Increased permeability highlighted by peritoneal effusions and pulmonary edema could also play a role in the terminal shock syndrome by inducing fluid balance dysregulation, tissue hypoxia, and multiorgan failure as observed in fatal cases.31
We set out to determine the underlying causes of hemorrhages by first investigating the coagulation system. Clotting times were prolonged in all lethally infected animals despite an absence of consumption coagulopathy through disseminated intravascular coagulation (DIC), as evidenced by elevated fibrinogen, close to normal levels of coagulation factors and the absence of D-dimers. This is in accordance with data obtained in infected patients in whom thrombocytopenia is moderate and levels of fibrin degradation products are not correlated with outcome.9,29 Because this coagulopathy was accompanied by severe tissue damage, infiltration of immune cells, degeneration of hepatocytes in the liver, and elevated hepatic enzymes,5,6 we performed a transcriptomic analysis of liver cells to investigate a potential link. In lethally infected monkeys, there was indeed a downregulation of coagulation factors transcription as well as that of coagulation system proteins, such as kininogen and kallikrein and an upregulation of inhibitors of fibrinolysis. Despite these indications of liver damage involvement in the coagulopathy, the activity of coagulation factors in the plasma was only moderately lowered and did not reach levels that could cause clotting-time prolongation or bleeding on its own. Consequently, we performed plasma-mixing experiments that showed the presence of a circulating inhibitor of coagulation in the plasma of animals infected with Josiah. We could not identify this inhibitor but we proposed several possible causes, such as (1) an endogenous heparin-like substance overexpressed during the infection or (2) a secreted viral protein, such as GP1.32,33 Interestingly, it has been reported that platelet dysfunction may also be mediated by a soluble factor, which could be the same as the one we identified.11,12 If so, antagonizing the activity of this factor would have a great therapeutic potential.
Our histological analysis showed that infection with LASV Josiah resulted in fibrin deposition in the lungs and kidneys. Because vessel walls and infected monocytes displayed increased staining for TF antigen, we suspected that resulted from a local thrombogenic environment, but TF or TFPI levels measured in the lungs using Western blot were not correlated with the presence of fibrin. Topologically, TF staining on vessel walls was not predictive of fibrin deposition on the surrounding tissue. Plasma levels of the antifibrinolytic protein PAI-1 were not higher in LASV Josiah–infected animals either. Other actors, such as the complement and kinin systems, may be activated by local inflammation and result in a loss of balance between procoagulant and fibrinolytic activities.
Many studies hypothesized that direct infection of vEC was the cause of increased vascular permeability. We show that NW arenaviruses MACV and GTOV, which caused peritoneal effusions and lung edema, have very little in vivo tropism for endothelial cells and that they replicate less efficiently than LASV Josiah in human primary endothelial cells. This indicates that tropism for vEC is not the determining factor in the increased vascular permeability caused by these viruses, although this may be implicated in the case of LASV Josiah. On the other hand, it is possible that the release of inflammatory mediators, such as interleukin 6 and tumor necrosis factor α (which is described in this model5) by activated immune cells causes vEC activation as highlighted by ICAM-1 and VCAM-1 upregulation. In addition, activated EC are known to produce increased levels of PAI-1,34 which is a feature of infection by LASV Josiah and MACV. This activation can result in a loss-of-barrier integrity, which leads to vascular leakage and increased leukocyte extravasation resulting in leukopenia and immune-cell infiltration, both of which were observed in our model. Importantly, this process is not dependent on direct infection of vEC but rather on the immunological state of the host, explaining the presence of these signs in animals infected with NW arenaviruses.
We show that arenaviral HF is accompanied by a central hematopoiesis defect that could be responsible for the observed thrombocytopenia. Moreover, studies of the lymphocytic choriomeningitis virus showed that IFN signaling may induce a deficit in platelet production and serotonin release during infection.35,36 This mechanism could also be suspected for HF-causing arenaviruses because they induce a strong release of IFN in the bloodstream,37 but our correlation analysis showed no significant link between IFN-α1 levels and platelet counts. In addition to low platelet counts, animals infected with GTOV and LASV Josiah but not with MACV presented an acquired platelet dysfunction. As previously mentioned, evidence shows that this platelet dysfunction may be mediated by a soluble factor in the plasma of the infected host.11,12,27 Notably, most described inhibitors of platelet aggregation are not able to antagonize ADP, RISTO, and COL–induced responses simultaneously. Our study suggests 2 nonexclusive mechanisms for this syndrome, (1) activated vEC release pathological levels of ADPases, prostacyclin, and nitric oxide that have antiaggregatory effects and (2) overt platelet activation suggested by high levels of soluble PF4 results in platelet exhaustion as described elsewhere.38-40
Finally, some aspects of the pathology, such as increased PAI-1 expression, fibrin deposition, low FVII levels, and expression of TF on monocytes that are reminiscent of sepsis-associated hemostatic disorders, which could represent an incentive to translating some therapeutic interventions already used successfully in patients with sepsis.41
To summarize, we provide evidence that during arenaviral infection, potentially life-threatening bleeding are associated with hepatic failure and coagulopathy mediated by a circulating inhibitor of coagulation. These symptoms do not appear to be related to vEC infection but rather to endothelial activation. Simultaneously, decreased hematopoietic activity, thrombocytopenia, and platelet aggregation defects impair primary hemostasis, participating in the hemorrhagic syndrome. These findings will allow better understanding of hemorrhagic fever syndromes caused by arenaviruses but also viruses, such as Ebola, dengue and yellow fever viruses. It also provides a framework to test potential therapeutics and countermeasures to this syndrome.
Acknowledgments
The authors thank S. Prost (Commissariat à l'Energie Atomique (CEA), Université Paris Sud 11, INSERM U1184, Infectious Diseases Models for Innovative Therapies Department (IDMIT), Fontenay-aux-Roses, France) for his advice and help in studying hematopoiesis. We thank G. Jouvion (Ecole Nationale Vétérinaire d'Alfort, Unité d'Histologie et d'Anatomie Pathologique, Maisons-Alfort, France) for advice on anatomic pathology. The authors are grateful to K. Noy (Unité de Biologie des Infections Virales Emergentes [UBIVE]) for his assistance in the writing process. The authors thank J. Brocard, E. Chatre, and E. Caracas Bobocioiu (Plateau Technique Imagerie/Microscopie, SFR Biosciences, UAR3444/Centre National de la Recherche Scientifique, US8/Inserm, ENS de Lyon, Université Claude Bernard Lyon I) for their assistance in the development of spectral confocal microscopy. We thank Q. Pascal and S. Luccantoni (CEA, Université Paris Sud 11, INSERM U1184, IDMIT, Fontenay-aux-Roses, France) for their advice on tissue processing and staining. The authors are grateful to B. Renaudin (UBIVE) for her administrative support.
This study was funded by the Délégation Générale pour l'Armement (Agence Nationale de la Recherche - Accompagnement Spécifique des Travaux de Recherches et d’Innovation Défense, ANR-ASTRID 2014, France) and by a grant from the “Grand Projet Fédérateur de Vaccinologie” of the Institut Pasteur obtained by S. Baize.
Authorship
Contribution: S. Baize, S.R., M. Mateo, and N.B. designed the animal experiments; M. Moroso, L.B., O.L., O.J., S. Barron, A.V., A.D., M.D., and F.J. handled the animal care and autopsies; N.B., J.H., G.F., and B.L. processed the tissues; B.L. did the H&E staining and imaging, multiplex immunofluorescent staining and imaging and colony-forming cell assays; C.P., V.B.-C., and S.R. did the coagulation and platelet function tests; C.P. and B.L. performed the coagulation factors tests; E.P. and N.P. did the transcriptomic analysis; G.F. did the experiments on human umbilical endothelial vein cells and extractions of RNA for Machupo virus–infected animals; N.B., C.P., S.R., M. Mateo, V.B.-C., A.J., C.G., and X.C. participated in bone marrow specimens processing and hematologic counts; B.L. analyzed the data; S. Baize and B.L. wrote the manuscript; H.R. and C.N. reviewed the manuscript; and S. Baize supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sylvain Baize, Unité de Biologie des Infections Virales Emergentes, Institut Pasteur, 21 Ave Tony Garnier, 69007 Lyon, France; e-mail: sylvain.baize@pasteur.fr.
References
Author notes
Transcriptomic data are available on Zenodo (https://doi.org/10.5281/zenodo.8398455).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal