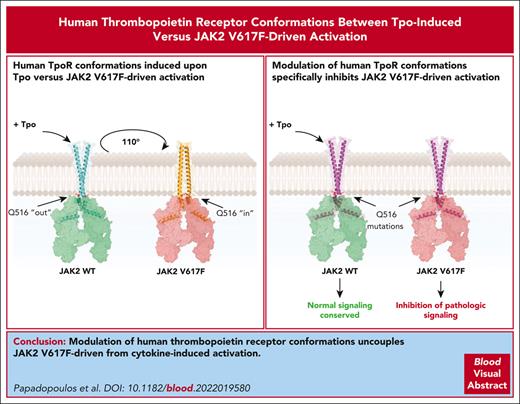
Key Points
JAK2 V617F and Tpo induce dimerization of hTpoR in different dimeric conformations.
Modulation of hTpoR allows specific inhibition of JAK2 V617F–driven signaling.
Abstract
The thrombopoietin receptor (TpoR) plays a central role in myeloproliferative neoplasms (MPNs). Mutations in JAK2, calreticulin, or TpoR itself drive the constitutive activation of TpoR and uncontrolled proliferation and differentiation of hematopoietic stem cells and progenitors. The JAK2 V617F mutation is responsible for most MPNs, and all driver mutants induce pathologic TpoR activation. Existing therapeutic strategies have focused on JAK2 kinase inhibitors that are unable to differentiate between the mutated MPN clone and healthy cells. Surprisingly, the targeting of TpoR itself has remained poorly explored despite its central role in pathology. Here, we performed a comprehensive characterization of human TpoR activation under physiological and pathological conditions, focusing on the JAK2 V617F mutant. Using a system of controlled dimerization of the transmembrane and cytosolic domains of TpoR, we discovered that human TpoR (hTpoR) adopts different dimeric conformations upon Tpo-induced vs JAK2 V617F–mediated activation. We identified the amino acids and specific dimeric conformation of hTpoR responsible for activation in complex with JAK2 V617F and confirmed our findings in the full-length receptor context in hematopoietic cell lines and primary bone marrow cells. Remarkably, we found that the modulation of hTpoR conformations by point mutations allowed for specific inhibition of JAK2 V617F–driven activation without affecting Tpo-induced signaling. Our results demonstrate that modulation of the hTpoR conformation is a viable therapeutic strategy for JAK2 V617F–positive MPNs and set the path for novel drug development by identifying precise residues of hTpoR involved in JAK2 V617F–specific activation.
Introduction
The thrombopoietin receptor (TpoR/MPL) is the master regulator of megakaryocytosis1,2 and hematopoietic stem cell (HSC) quiescence, proliferation, and differentiation.3-6 Like other type 1 cytokine receptors, TpoR activation is induced by its homodimerization7 in a productive conformation.8,9 Under normal conditions, the binding of 1 molecule of Tpo to 2 molecules of TpoR10 induces the close apposition of the 2 monomers and their homodimerization at the cell surface.7 Productive dimerization of TpoR subunits, in turn, brings appended JAK2 kinases into a conformation that enables their transphosphorylation and consecutive activation of downstream signaling pathways.7,9,10
Dysregulation of TpoR signaling is a major driving factor of myeloproliferative neoplasms (MPNs)11-13 and bone marrow failure.14 Consequently, identifying how TpoR dimerization mediates downstream signaling under physiological and pathological conditions is critical for identifying novel therapeutic options. However, the careful characterization of the conformational requirements of TpoR activation has, so far, been limited to the study of murine TpoR (mTpoR), in a physiological context,9 or human TpoR (hTpoR), focusing on the transmembrane segment inserted in an artificial TOXCAT (ToxR-chloramphenical acetyltransferase) construct without JAK2 binding.15 Because of major differences in both amino acid sequences and activation mechanisms,9,15,16 studies on mTpoR cannot be transposed onto its human homolog. Recent studies showed that diabodies or DARPins (designed ankyrin repeat proteins) acting as cytokine mimetics can differentially modulate the signaling of the erythropoietin receptor (EpoR) by fine-tuning the intermonomeric distance and/or their relative orientations.17,18 Diabodies can similarly modulate the downstream signaling of hTpoR, but their mechanisms of action have not been solved.19 Surprisingly, there has been, so far, no detailed characterization of the conformational dynamics of hTpoR under pathological conditions.
The JAK2 V617F mutation is the most frequent cause of MPNs and is rarely found in other hematological malignancies.20-23 It induces constitutive dimerization and activation of TpoR and other cytokine receptors,7,24 leading to the uncontrolled proliferation and differentiation of HSCs and progenitors. Current therapeutic strategies have focused on kinase inhibitors that nonselectively target both wild-type (WT) and mutant JAK2, which results in serious side effects.25 Furthermore, recent reports suggest that JAK2 inhibitors mediate the selection of clones with ras mutations, leading to an increased risk of transformation.26 Here, we defined the conformational landscapes of hTpoR activation in the presence of JAK2 WT and JAK2 V617F. Our work identified major differences between the activation patterns of mTpoR and hTpoR but, most importantly, between hTpoR when it is activated by Tpo vs by JAK2 V617F. Building upon these findings, we showed that the inhibition or promotion of specific interfaces by point mutations allows for the uncoupling of JAK2 V617F–driven signaling from Tpo-induced activation of hTpoR, both in cell lines and primary bone marrow cells (BMCs).
Methods
Details of the experimental protocols are provided in supplemental Methods, available on the Blood website.
CFU
Lineage-negative BMCs from MPL knockout (KO) JAK2 WT/WT or WT/V617F mice were retrovirally transduced with hTpoR WT or mutants. Colony-forming unit megakaryocytes (CFU-Mks) were generated with 15 000 cells using MegaCult-C following the manufacturer’s instructions (StemCell technologies). Single-cell CFU were generated from sorted lin–Sca1+Kit+ (LSK) cells stably transduced with hTpoR WT or mutants in MethoCult (M3234) supplemented with 20 ng/mL murine stem cell factor with or without 50 ng/mL hTpo. Classical CFU was performed similarly from 5000 LSKs but with a complete cytokine cocktail (with or without Tpo), following the manufacturer’s instructions (StemCell technologies).
Phospho-flow cytometry
Cells (TF-1 or Ba/F3, BALB/c mouse factor dependent 3 cells) were starved for 4 hours or incubated with hTpo (25 ng/mL) and processed as described.27 Staining was performed with PE (phycoerythrin)-coupled anti-pSTAT5 (SRBCZE, eBioscience), PE-cyanine 7 (PE-Cy7)–coupled anti-pSTAT5 (LUVNKLA, eBioscience), and allophycocyanin (APC)-coupled anti-pSTAT1 (REA159, Miltenyi Biotec), following the manufacturer’s instructions. Acquisition was performed on a full-spectrum Cytek Aurora flow cytometer.
Proliferation assays and CellTiter-Glo
For proliferation assays, 250 000 cells were washed and seeded in 10 mL RPMI and 10% fetal bovine serum without cytokine and counted each day using a Coulter automated cell counter in triplicate. For CellTiter-Glo assays, cells were processed similarly and plated at a concentration of 2500 per well in 96 wells plate. The luminescence was measured following the manufacturer’s instructions (Promega).
Results
Dimeric conformations in hTpoR regulate downstream signaling differently than in mTpoR
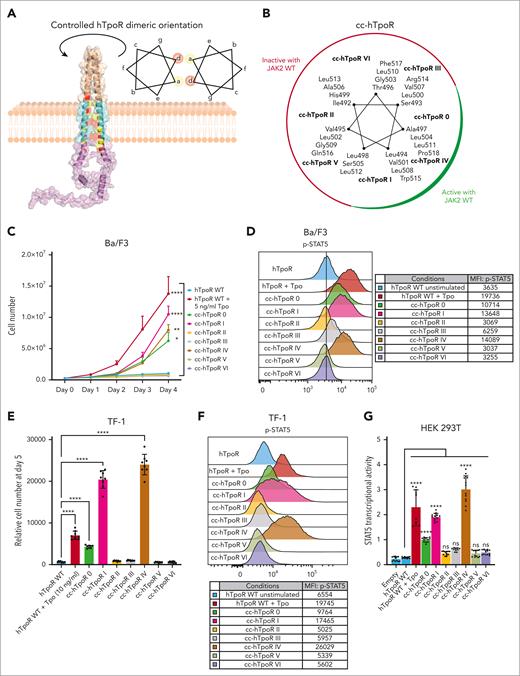
To study the conformational landscapes of hTpoR dimerization, we used an approach previously validated in different receptors.9,28,29 The extracellular domain (ECD) of hTpoR was replaced by a dimeric coiled coil (cc) composed of the Put3 dimerization domain. By engineering the junction between the cc domain and hTpoR transmembrane domain (TMD), dimerization of hTpoR TMD is tightly controlled. We generated 7 cc hTpoR fusion proteins (cc-hTpoR 0 to VI), allowing for the individual study of each of the 7 possible dimeric orientations of hTpoR, in which amino acids at positions a and d of a heptad repeat face each other (Figure 1A-B; supplemental Figure 1A). The cc-hTpoR fusion proteins were transduced into murine Ba/F3 cells and sorted for equal level of green fluorescent protein (GFP) reporter (supplemental Figure 1B,D). Of the 7 possible dimeric interfaces of hTpoR, only 3 induced signaling activity capable of promoting the cytokine-independent growth of Ba/F3 hematopoietic cells (Figure 1C) and significant STAT5 phosphorylation (Figure 1D). These results were confirmed in human TF-1 hematopoietic cells (Figure 1E-F; supplemental Figure 1C,E) and in HEK293T using a STAT5 transcriptional activity assay (Figure 1G; supplemental Figure 1F). Similar to cc-mTpoR9 and other coiled-coil constructs,28,29 protein expression varied between orientations despite equal transcription but did not correlate with activation, yet all fusion proteins were expressed (supplemental Figure 1B-F). The 3 active conformations (interfaces 0, I, and IV) were all positioned in the same broad interface (Figure 1B) of the transmembrane (TM) α-helix that is centered on Trp515 and is required to maintain an inactive state under basal conditions.12,30,31 This pattern of activation contrasted with that previously obtained for mTpoR, in which 6 out of 7 dimeric conformations induced significant signaling activity.9 The differences between mTpoR and hTpoR were dependent on the His499 and Gly503 residues of hTpoR, corresponding respectively to Leu492 and Ser496 in mTpoR (supplemental Figure 2A-D).
Active and inactive conformations of human TpoR. (A) Illustration of the Put3-hTpoR TM-ICD fusion protein allowing controlled dimerization of hTpoR TM interfaces. The illustration represents the Put3 dimerization motif (wheat) fused to hTpoR TM (cyan) and ICD (magenta). Positions a and b are colored in yellow and red, respectively. The structure was generated using AlphaFold 2.0.32 The helical wheel diagram represents the amino acid in each of the seven possible positions, denoted as a to g. The amino acids in a and d are at the interface. TM, transmembrane; ICD, intracellular domain. (B) Helical wheel diagram of cc-hTpoR constructs with amino acids in the interface for each of the 7 cc-hTpoR. The active (green) and inactive (red) interfaces are highlighted. (C) Proliferation assay of Ba/F3 cells stably transduced with the indicated constructs. Values represent the mean (±SD) of 3 independent experiments performed in triplicate (N = 3, n = 9). (D) Representative flow cytometry measurement of STAT5 phosphorylation in Ba/F3 cells expressing the indicated constructs. Mean fluorescence intensity (MFI) is indicated. (E) Luminescent viability assay (CellTiter-Glo) used to measure proliferation of TF-1 cells stably transduced with indicated constructs. Values represent the mean (±SD) of 8 biological replicates (n = 8). (F) Representative flow cytometry measurement of STAT5 phosphorylation in TF-1 cells expressing the indicated constructs. Mean fluorescence intensity (MFI) is indicated. (G) STAT5 transcriptional activity was measured by a luciferase assay in HEK293T transiently transfected with hJAK2 WT and the indicated constructs. Values represent the mean (±SD) of 4 independent experiments performed in triplicate. (C,E,G) Statistics: two-way analysis of variance followed by the SIDAK multiple comparison test. ns, nonsignificant. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
Active and inactive conformations of human TpoR. (A) Illustration of the Put3-hTpoR TM-ICD fusion protein allowing controlled dimerization of hTpoR TM interfaces. The illustration represents the Put3 dimerization motif (wheat) fused to hTpoR TM (cyan) and ICD (magenta). Positions a and b are colored in yellow and red, respectively. The structure was generated using AlphaFold 2.0.32 The helical wheel diagram represents the amino acid in each of the seven possible positions, denoted as a to g. The amino acids in a and d are at the interface. TM, transmembrane; ICD, intracellular domain. (B) Helical wheel diagram of cc-hTpoR constructs with amino acids in the interface for each of the 7 cc-hTpoR. The active (green) and inactive (red) interfaces are highlighted. (C) Proliferation assay of Ba/F3 cells stably transduced with the indicated constructs. Values represent the mean (±SD) of 3 independent experiments performed in triplicate (N = 3, n = 9). (D) Representative flow cytometry measurement of STAT5 phosphorylation in Ba/F3 cells expressing the indicated constructs. Mean fluorescence intensity (MFI) is indicated. (E) Luminescent viability assay (CellTiter-Glo) used to measure proliferation of TF-1 cells stably transduced with indicated constructs. Values represent the mean (±SD) of 8 biological replicates (n = 8). (F) Representative flow cytometry measurement of STAT5 phosphorylation in TF-1 cells expressing the indicated constructs. Mean fluorescence intensity (MFI) is indicated. (G) STAT5 transcriptional activity was measured by a luciferase assay in HEK293T transiently transfected with hJAK2 WT and the indicated constructs. Values represent the mean (±SD) of 4 independent experiments performed in triplicate. (C,E,G) Statistics: two-way analysis of variance followed by the SIDAK multiple comparison test. ns, nonsignificant. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
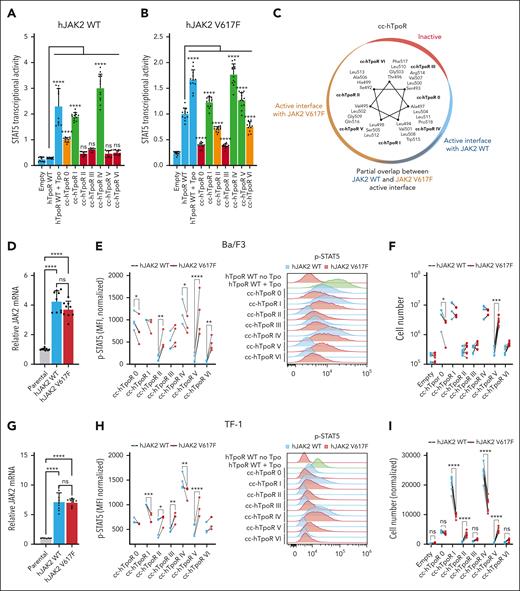
JAK2 WT and JAK2 V617F require different conformations of hTpoR dimers for activity
Unlike Tpo, which induces dimerization of TpoR via the ECD, the JAK2 V617F mutant induces inside-out (from the intracellular domain) dimerization of TpoR through the transdimerization of JAK2 molecules by their pseudokinase domain.7,24 We hypothesized that this differential mechanism of activation could translate into different active and inactive conformations of hTpoR. To test this hypothesis, we measured STAT5 transcriptional activity induced by each of the cc-hTpoR with JAK2 WT or V617F. The active interface of hTpoR was shifted 110° clockwise in the presence of JAK2 V617F, with the strongest activation with cc-hTpoR I, IV, and V, partial activation with cc-hTpoR II and VI, and loss of activation for interface 0 (Figure 2A-C; supplemental Figure 3A). To validate these results, JAK2 WT and V617F were stably expressed in Ba/F3 and TF-1 cells. The ratio of exogenous (WT or V617F) vs endogenous (WT) JAK2 was ∼1:3 in Ba/F3 and ∼1:7 in TF-1 (Figure 2D,G). Cytokine-independent proliferation of hematopoietic cells overexpressing JAK2 WT was similar to that observed with only endogenous JAK2, confirming that overexpression did not alter the observed phenotype with the endogenous level of JAK2 (supplemental Figure 3B). Next, we compared the ability of each of the 7 cc-hTpoR fusion proteins to induce cytokine-independent proliferation and STAT5 phosphorylation in Ba/F3 and TF-1 cells in the presence of JAK2 WT or JAK2 V617F. In both cell lines, the interface allowing the cytokine-independent proliferation and STAT5 phosphorylation was shifted clockwise in the presence of JAK2 V617F, with an expression profile similar to that of JAK2 WT (supplemental Figure 3C-D). In comparison with cells expressing only JAK2 WT, cells expressing JAK2 V617F as well induced lower STAT5 phosphorylation in the conformations imposed by cc-hTpoR 0, I, and IV and the strongest increase in STAT5 phosphorylation was at the interface imposed by cc-hTpoR V and II (Figure 2E,H). This translated into increased autonomous cell proliferation in the presence of JAK2 V617F upon signaling induced by interfaces V and II (significantly for TF-1) and decreased proliferation when the hTpoR interface was shifted toward interface 0 (significantly for Ba/F3 cells), I, or IV (significantly for TF-1; Figure 2F,I). Interestingly, TF-1 cells that expressed the highest level of JAK2 V617F displayed the strongest reduction of activity in the presence of cc-hTpoR I and IV, suggesting that activation induced by these interfaces in Ba/F3 cells may occur via endogenous JAK2 WT rather than exogenous JAK2 V617F.
Active conformations of hTpoR differ in presence of JAK2 WT and JAK2 V617F. (A-B) STAT5 transcriptional activity measured by luciferase assay in HEK293T transiently transfected with hJAK2 WT (A) or hJAK2 V617F (B) and the indicated constructs. Values represent the mean (±SD) of 4 independent experiments performed in triplicate. The active interfaces are shown in green, inactive interfaces in red, and partially active interfaces in orange. Controls (empty vector and hTpoR WT) are shown in blue. (C) Helical wheel diagram of cc-hTpoR constructs with amino acids at the interface for each of the 7 cc-hTpoR. The gradient of active orientations for JAK2 V617F (brown) and JAK2 WT (blue) is illustrated. The inactive interface is shown in red. (D) Relative messenger RNA (mRNA) of hJAK2 WT, hJAK2 V617F, and endogenous mJAK2 WT of Ba/F3 cells. Values represent the mean (±SD) mRNA levels quantified by qPCR from 3 independent experiments performed in triplicate (N = 3, n = 9). (E) Left: STAT5 phosphorylation in Ba/F3 cells expressing either hJAK2 WT or hJAK2 V617F together with indicated constructs. Values represent the mean fluorescence intensity (MFI) of 3 to 4 independent experiments. Right: Representative flow cytometry measurement of STAT5 phosphorylation in Ba/F3 cells expressing hJAK2 WT (blue) or hJAK2 V617F (red) and the indicated constructs. (F) Proliferation assay of Ba/F3 cells stably transduced with hJAK2 WT (blue) or hJAK2 V617F (red) and the indicated constructs. Values represent the mean (±SD) of 3 independent experiments performed in triplicate (N = 3, n = 9). (G) Relative mRNA of hJAK2 WT, hJAK2 V617F, and endogenous hJAK2 WT of TF-1 cells. Values represent the mean (±SD) mRNA levels quantified by qPCR from 3 independent experiments performed in triplicate (N = 3, n = 9). (H) Left: STAT5 phosphorylation in TF-1 cells expressing either hJAK2 WT or hJAK2 V617F together with the indicated constructs. Values represent mean fluorescence intensities (MFI) of 3 to 4 independent experiments. Right: Representative flow cytometry measurement of STAT5 phosphorylation in TF-1 cells expressing hJAK2 WT (blue) or hJAK2 V617F (red) and the indicated constructs. (I) Luminescent viability assay (CellTiter-Glo) was used to measure the proliferation of TF-1 cells stably transduced with hJAK2 WT (blue) or hJAK2 V617F (red) with the indicated constructs. Values represent the mean (±SD) of 8 biological replicates (n = 8). (A-B, D-I) Statistics: two-way analysis of variance followed by the SIDAK multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Active conformations of hTpoR differ in presence of JAK2 WT and JAK2 V617F. (A-B) STAT5 transcriptional activity measured by luciferase assay in HEK293T transiently transfected with hJAK2 WT (A) or hJAK2 V617F (B) and the indicated constructs. Values represent the mean (±SD) of 4 independent experiments performed in triplicate. The active interfaces are shown in green, inactive interfaces in red, and partially active interfaces in orange. Controls (empty vector and hTpoR WT) are shown in blue. (C) Helical wheel diagram of cc-hTpoR constructs with amino acids at the interface for each of the 7 cc-hTpoR. The gradient of active orientations for JAK2 V617F (brown) and JAK2 WT (blue) is illustrated. The inactive interface is shown in red. (D) Relative messenger RNA (mRNA) of hJAK2 WT, hJAK2 V617F, and endogenous mJAK2 WT of Ba/F3 cells. Values represent the mean (±SD) mRNA levels quantified by qPCR from 3 independent experiments performed in triplicate (N = 3, n = 9). (E) Left: STAT5 phosphorylation in Ba/F3 cells expressing either hJAK2 WT or hJAK2 V617F together with indicated constructs. Values represent the mean fluorescence intensity (MFI) of 3 to 4 independent experiments. Right: Representative flow cytometry measurement of STAT5 phosphorylation in Ba/F3 cells expressing hJAK2 WT (blue) or hJAK2 V617F (red) and the indicated constructs. (F) Proliferation assay of Ba/F3 cells stably transduced with hJAK2 WT (blue) or hJAK2 V617F (red) and the indicated constructs. Values represent the mean (±SD) of 3 independent experiments performed in triplicate (N = 3, n = 9). (G) Relative mRNA of hJAK2 WT, hJAK2 V617F, and endogenous hJAK2 WT of TF-1 cells. Values represent the mean (±SD) mRNA levels quantified by qPCR from 3 independent experiments performed in triplicate (N = 3, n = 9). (H) Left: STAT5 phosphorylation in TF-1 cells expressing either hJAK2 WT or hJAK2 V617F together with the indicated constructs. Values represent mean fluorescence intensities (MFI) of 3 to 4 independent experiments. Right: Representative flow cytometry measurement of STAT5 phosphorylation in TF-1 cells expressing hJAK2 WT (blue) or hJAK2 V617F (red) and the indicated constructs. (I) Luminescent viability assay (CellTiter-Glo) was used to measure the proliferation of TF-1 cells stably transduced with hJAK2 WT (blue) or hJAK2 V617F (red) with the indicated constructs. Values represent the mean (±SD) of 8 biological replicates (n = 8). (A-B, D-I) Statistics: two-way analysis of variance followed by the SIDAK multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
These discrepancies between JAK2 WT and V617F-driven activation of TpoR were specific to the human homolog, and complete replacement of the murine TMD by human TMD was required to recover these differences in mTpoR (supplemental Figure 3E,G).
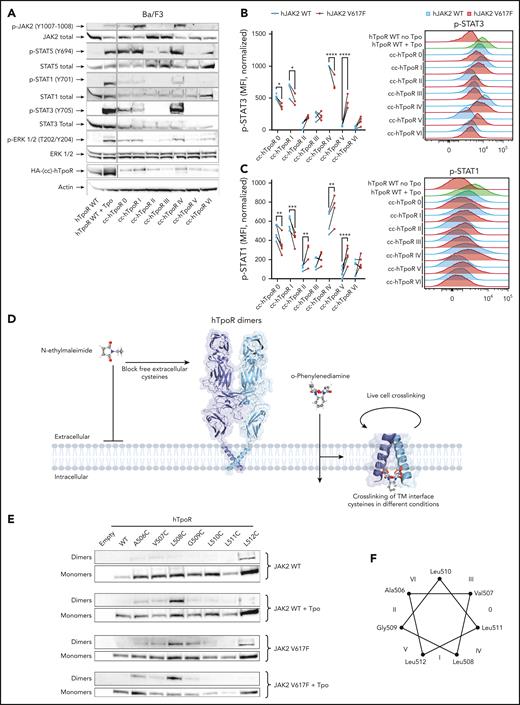
Downstream pathways activated by hTpoR dimeric interfaces with JAK2 WT and JAK2 V617F
In addition to STAT5, the STAT1/3 and MAPK pathways are induced upon TpoR activation. A careful study of the relationship between signaling and dimeric conformations was previously conducted for mTpoR9 and mEpoR,29 and a similar mechanism was recently suggested for hTpoR19 and hEpoR17,18 but without the identification of a relationship that may exist between a particular pathway and specific dimeric conformations. Given that hTpoR activation was less permissive than that of mTpoR, we asked whether a similar modulation of downstream signaling pathways was true in the human context. We measured the signaling induced by each of the 7 cc-hTpoRs in Ba/F3 cells expressing only endogenous JAK2 WT. Unlike mTpoR, in which specific conformations favored one pathway over another, the relative activation of downstream pathways was homogeneous for hTpoR, except for STAT3, which was prominently more phosphorylated by cc-hTpoR IV (Figure 3A). Next, we quantified STAT3 and STAT1 phosphorylation in Ba/F3 cells stably expressing JAK2 WT or V617F using flow cytometry. Similar to STAT5 (Figure 2E,H), STAT3 phosphorylation was reduced in conformations imposed by cc-hTpoR 0, I, and IV in the presence of JAK2 V617F and increased in conformations imposed by cc-hTpoR V, with a nonsignificant increase in conformations II and VI (Figure 3B). Remarkably, although the changes in STAT1 phosphorylation between JAK2 WT and JAK2 V617F reproduced those observed for STAT3 and STAT5 at interfaces 0, I, II, and V, the conformation represented by cc-hTpoR IV displayed an increased STAT1 phosphorylation with JAK2 V617F (Figure 3C). This indicated that JAK2 V617F and JAK2 WT require distinct conformational landscapes of TpoR dimers for activation, but that STAT1 activation follows a slightly distinct pattern of activation in the presence of JAK2 V617F.
Modulation of downstream pathways and dimerization of full length hTpoR by JAK2 WT and JAK2 V617F. (A) Representative western blotting of phosphorylation pattern of major signaling pathways from Ba/F3 stably expressing indicated constructs. (B) Left: STAT3 phosphorylation in Ba/F3 cells expressing either hJAK2 WT or hJAK2 V617F together with indicated constructs. Values represent mean fluorescence intensity (MFI) of 3 to 4 independent experiments. Right: Representative flow cytometry measurement of STAT3 phosphorylation in Ba/F3 cells expressing hJAK2 WT (blue) or hJAK2 V617F (red) and the indicated constructs. (C) Left: STAT1 phosphorylation in Ba/F3 cells expressing either hJAK2 WT or hJAK2 V617F together with the indicated constructs. Values represent the mean fluorescence intensity (MFI) of 4 to 5 independent experiments. Right: Representative flow cytometry measurement of STAT1 phosphorylation in Ba/F3 cells expressing hJAK2 WT (blue) or hJAK2 V617F (red) and the indicated constructs. (D) Illustration of the live-cell crosslinking assay. Dimerization of human TpoR was assessed by introducing cysteine point mutations in the singleton of human TpoR, which contains the entire extracellular domain. Pretreatment with N-ethylmaleimide (NEM) prevents nonspecific crosslinking of extracellular cysteines and all intracellular cysteines are removed by truncation of the intracellular domain after the JAK2 binding domain. The membrane-permeable cysteine crosslinker o-phenylenediamine (o-PDM) has a 4 Ä arm-length and allows the stable crosslinking of cysteines at the interface. (E) Representative western blot in denaturing and reducing conditions of monomers (bottom) and crosslinked dimers (top) of hTpoR WT and cysteine mutants in the presence of JAK2 WT with and without Tpo, or JAK2 V617F with or without Tpo. (F) Helical wheel diagram illustrating the position of each amino acid mutated to cysteines 1 by 1. Interfaces represented by each of the 7 cc-hTpoR are shown with roman numbers from 0 to VI.
Modulation of downstream pathways and dimerization of full length hTpoR by JAK2 WT and JAK2 V617F. (A) Representative western blotting of phosphorylation pattern of major signaling pathways from Ba/F3 stably expressing indicated constructs. (B) Left: STAT3 phosphorylation in Ba/F3 cells expressing either hJAK2 WT or hJAK2 V617F together with indicated constructs. Values represent mean fluorescence intensity (MFI) of 3 to 4 independent experiments. Right: Representative flow cytometry measurement of STAT3 phosphorylation in Ba/F3 cells expressing hJAK2 WT (blue) or hJAK2 V617F (red) and the indicated constructs. (C) Left: STAT1 phosphorylation in Ba/F3 cells expressing either hJAK2 WT or hJAK2 V617F together with the indicated constructs. Values represent the mean fluorescence intensity (MFI) of 4 to 5 independent experiments. Right: Representative flow cytometry measurement of STAT1 phosphorylation in Ba/F3 cells expressing hJAK2 WT (blue) or hJAK2 V617F (red) and the indicated constructs. (D) Illustration of the live-cell crosslinking assay. Dimerization of human TpoR was assessed by introducing cysteine point mutations in the singleton of human TpoR, which contains the entire extracellular domain. Pretreatment with N-ethylmaleimide (NEM) prevents nonspecific crosslinking of extracellular cysteines and all intracellular cysteines are removed by truncation of the intracellular domain after the JAK2 binding domain. The membrane-permeable cysteine crosslinker o-phenylenediamine (o-PDM) has a 4 Ä arm-length and allows the stable crosslinking of cysteines at the interface. (E) Representative western blot in denaturing and reducing conditions of monomers (bottom) and crosslinked dimers (top) of hTpoR WT and cysteine mutants in the presence of JAK2 WT with and without Tpo, or JAK2 V617F with or without Tpo. (F) Helical wheel diagram illustrating the position of each amino acid mutated to cysteines 1 by 1. Interfaces represented by each of the 7 cc-hTpoR are shown with roman numbers from 0 to VI.
Interestingly, nuclear magnetic resonance (NMR) studies have revealed that the activation of hTpoR by eltrombopag or W515X mutations induces the unfolding of the α-helix at the transmembrane-intracellular (TM-IC) junction around Q516.33,34 Using AlphaFold 2.0,32 we generated predictions for each of the 7 cc-hTpoR categorized as inactive (II, III, and VI), active (I and IV), and special interfaces (0 and V) based on their ability to drive downstream signaling in the presence of JAK2 WT or V617F. All inactive interfaces in the presence of JAK2 WT or V617F were predicted as parallel helices without “kinks” in the α-helices and with His499 either directly in the inner face of the helix (positions a and d; supplemental Figure 4) or at position g or “side in.” This is remarkable because the inactive conformation of hTpoR was suggested to involve the presence of the human-specific His499 at the interface of the receptor dimer.15,33 In contrast, interfaces active with both WT and mutant JAK2 had His499 either in position f (“His out”) or b (“side out”), reflecting a thermodynamically unstable conformation in the absence of Tpo. Furthermore, both interfaces were predicted to induce a kink or unfolding of the α-helix at the TM-IC junction, as experimentally shown.34 Finally, special interfaces displayed a mixed pattern with His499 either at position c (side out, interface 0) or e (side in, interface V) and a more restricted kink at the TM-IC junction.
Activation of the full-length WT hTpoR differs between Tpo and JAK2 V617F
The study of cc-hTpoR fusion proteins allowed for the precise characterization of activation mechanisms in the presence of both JAK2 WT and V617F. However, this may not perfectly mimic the situation in which the full-length hTpoR is activated by either Tpo or mutant JAK2. To validate our results, we designed a live-cell, cysteine-specific crosslinking assay to determine the dimerization interfaces of full-length hTpoR upon activation. The procedure described in Figure 3D allows for the specific crosslinking of cysteines introduced in a singleton from position 506 to 512 of hTpoR to span 2 turns of the TM α-helix. Upon stimulation, dimerization of hTpoR allows for the crosslinking of cysteines that are in the active interface, and western blotting of crosslinked dimers then informs on the interface that is adopted upon activation. In the absence of stimulation, low levels of dimerization were observed for the A506C, V507C, and L512C mutants surrounding inactive interfaces II or V and III or VI. Upon Tpo stimulation, strong dimerization occurred for hTpoR L508C (Figure 3E-F), corresponding to the junction between interfaces I and IV that are active with JAK2 WT. Remarkably, cytokine-independent dimerization by JAK2 V617F resulted in the dimerization of both L508C and G509C, with an increase also in L512C dimerization compared with that induced by JAK2 WT with Tpo (Figure 3E-F). Together with the above results, this indicated that JAK2 V617F induces dimerization of hTpoR in an interface that lies between interfaces V and I, thus, 110° clockwise from that induced by Tpo. The addition of Tpo in the presence of JAK2 V617F resulted in the complete recovery of the Tpo-induced interface, indicating that Tpo can circumvent JAK2 V617F–induced activation to promote a conformation that resembles the conformation induced by cc-hTpoR I and IV.
Modulation of the hTpoR conformation allows for the specific inhibition of JAK2 V617F–driven signaling
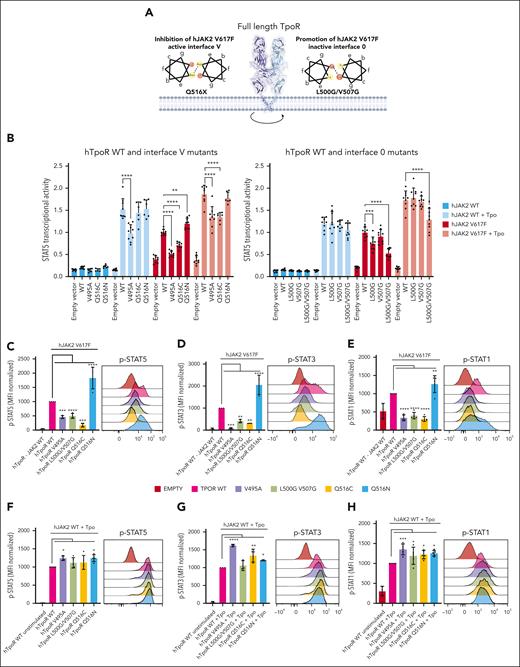
The observation that hTpoR adopts distinct dimeric interfaces upon Tpo-induced and JAK2 V617F–driven dimerization suggests the possibility of specific inhibition of pathologic signaling via the modulation of hTpoR conformations. In the context of cc-hTpoR fusion proteins, 3 dimeric interfaces (I, IV, and V) supported strong activation by JAK2 V617F, and 2 of these (I and IV) were active with JAK2 WT. Therefore, we hypothesized that JAK2 V617F induces dimerization of hTpoR preferentially at an interface comparable with that of cc-hTpoR V and that interface 0 may prevent JAK2 V617F–induced TpoR activation. To confirm these results and assess whether modulation of hTpoR conformation could specifically prevent JAK2 V617F–induced activation, we defined 2 alternative strategies (Figure 4A). First, we aimed at disturbing the dimeric interface V required for JAK2 V617F activation. We targeted Q516, which lies at the junction between the JAK2 V617F specifically active interface (V) and partially active interface (II). Given the property of glutamine (like that of asparagine) to form hydrogen bonds when facing each other, we predicted that the mutation of Q516 would disturb JAK2 V617F–driven dimerization at interface V without disturbing Tpo-dependent activity, which requires interfaces I and IV (Figures 1 and 2). Our results indicated that Q516X mutations systematically reduced JAK2 V617F–driven activity (Figure 4B, left; supplemental Figure 5A), whereas most mutations barely disturbed Tpo-induced signaling and surface localization (supplemental Figure 5B). Conversely, mutation of Q516 to asparagine (Q516N), which displays a similar property but with a longer arm length, thus more prone to reciprocal hydrogen bonding, resulted in increased activation with JAK2 V617F but not with Tpo (Figure 4B; supplemental Figure 5A). This mutant exhibited decreased cell-surface localization, yet it was hyperactive with JAK2 V617F. To further confirm the role of interface V, we introduced the V495A mutation that lies between interfaces V and II but upstream of Q516 in the TMD. The V495A mutation resulted in the loss of activation by JAK2 V617F while remaining active upon Tpo stimulation (Figure 4B, right), albeit at a lower level than the WT because of lower surface localization (supplemental Figure 5C). In the second strategy, we aimed at promoting the formation of hTpoR dimers at interface 0, which was specifically inactive with JAK2 V617F and slightly active with JAK2 WT. We used the property of glycine residues to form so-called glycine-zippers when placed in tandem at the same interface of an α-helix.35 This would lead to a close apposition of the membrane helices due to the small size of Gly residues. We introduced the L500G/V507G double mutation to favor dimerization around the interface 0 or III. Single mutations (L500G and V507G) resulted in the absence or minor reduction in JAK2 V617F–driven activity. In contrast, the double L500G/V507G mutant strongly inhibited JAK2 V617F–driven activation without perturbing Tpo-dependent signaling (Figure 4B, right) or cell-surface localization (supplemental Figure 5D).
Specific inhibition of JAK2 V617F signaling by modulation of hTpoR conformation. (A) Illustration of 2 strategies used to specifically inhibit JAK2 V617F-driven activation of hTpoR. Left: Helical wheel diagram positioned at interface V. Asparagine and glutamine residues facing each other in an α-helix have the property to form hydrogen bonds to stabilize the interface in which they lie. Mutation of Q516 to another residue (except asparagine) is predicted to disrupt the JAK2-V617F–specific active interface. Right: Glycines positioned at regular positions on the same face of an α-helix form the so-called glycine zipper, which can stabilize a specific interface. (B) STAT5 transcriptional activity measured by luciferase assay of HEK293T transiently transfected with hJAK2 WT or V617F and indicated hTpoR WT or mutants. Values represent the mean (±SD) of 3 independent experiments performed in triplicate. Each dot represents a replicate. (C-H) Left: STAT1/3/5 phosphorylation in Ba/F3 cells expressing either hJAK2 V617F (C-E) or hJAK2 WT (F-H) together with the indicated hTpoR mutants. Values represent the mean fluorescence intensities (MFI) of 3 to 4 independent experiments. Right: Representative flow cytometry measurement of STAT1/3/5 phosphorylation in Ba/F3 cells expressing hJAK2 V617F (C-E) or hJAK2 WT (F-H) and hTpoR mutants. (I-J) Proliferation assay of Ba/F3 cells stably transduced with hJAK2 V617F (I) or hJAK2 WT (J) and indicated as hTpoR WT or mutants. Values represent the mean (±SD) of 3 independent experiments performed in triplicate (N = 3, n = 9). (K) Flow cytometry analysis of cell surface expression of HA-tagged hTpoR WT and indicated mutants on Ba/F3 stably expressing hJAK2 WT or V617F and indicated hTpoR WT and mutants. (L) Representative western blot of HA-hTpoR WT and mutants stably expressed in Ba/F3 cells. (M-N) Luminescent viability assay (CellTiter-Glo) used to measure proliferation of TF-1 cells stably transduced with hJAK2 V617F (M) or hJAK2 WT (N) with the indicated hTpoR mutants. Values represent mean (±SD) of 8 biological replicates (n = 8). (B-K, M-N) Two-way analysis of variance followed by SIDAK multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Specific inhibition of JAK2 V617F signaling by modulation of hTpoR conformation. (A) Illustration of 2 strategies used to specifically inhibit JAK2 V617F-driven activation of hTpoR. Left: Helical wheel diagram positioned at interface V. Asparagine and glutamine residues facing each other in an α-helix have the property to form hydrogen bonds to stabilize the interface in which they lie. Mutation of Q516 to another residue (except asparagine) is predicted to disrupt the JAK2-V617F–specific active interface. Right: Glycines positioned at regular positions on the same face of an α-helix form the so-called glycine zipper, which can stabilize a specific interface. (B) STAT5 transcriptional activity measured by luciferase assay of HEK293T transiently transfected with hJAK2 WT or V617F and indicated hTpoR WT or mutants. Values represent the mean (±SD) of 3 independent experiments performed in triplicate. Each dot represents a replicate. (C-H) Left: STAT1/3/5 phosphorylation in Ba/F3 cells expressing either hJAK2 V617F (C-E) or hJAK2 WT (F-H) together with the indicated hTpoR mutants. Values represent the mean fluorescence intensities (MFI) of 3 to 4 independent experiments. Right: Representative flow cytometry measurement of STAT1/3/5 phosphorylation in Ba/F3 cells expressing hJAK2 V617F (C-E) or hJAK2 WT (F-H) and hTpoR mutants. (I-J) Proliferation assay of Ba/F3 cells stably transduced with hJAK2 V617F (I) or hJAK2 WT (J) and indicated as hTpoR WT or mutants. Values represent the mean (±SD) of 3 independent experiments performed in triplicate (N = 3, n = 9). (K) Flow cytometry analysis of cell surface expression of HA-tagged hTpoR WT and indicated mutants on Ba/F3 stably expressing hJAK2 WT or V617F and indicated hTpoR WT and mutants. (L) Representative western blot of HA-hTpoR WT and mutants stably expressed in Ba/F3 cells. (M-N) Luminescent viability assay (CellTiter-Glo) used to measure proliferation of TF-1 cells stably transduced with hJAK2 V617F (M) or hJAK2 WT (N) with the indicated hTpoR mutants. Values represent mean (±SD) of 8 biological replicates (n = 8). (B-K, M-N) Two-way analysis of variance followed by SIDAK multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
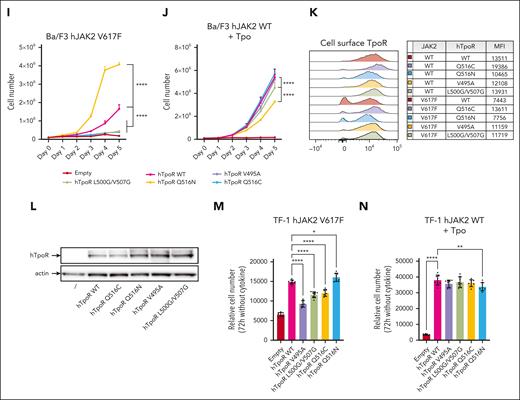
hTpoR WT or mutants were then transduced into Ba/F3 cells stably expressing either JAK2 WT or V617F, as shown in Figure 2, and we measured STAT1/3/5 phosphorylation and Tpo-independent proliferation. Consistent with the aforementioned results, the ability of JAK2 V617F to induce phosphorylation and activation of STATs and cytokine-independent proliferation was prevented by either favoring interface 0 (hTpoR L500G/V507G mutant) or disfavoring interface V (V495A and Q516C mutants) but was increased in the Q516N mutant (Figure 4C-E,I). In contrast, Tpo stimulation induced phosphorylation and activation of STATs similarly in all mutants (Figure 4F-H). Tpo-dependent proliferation was also similar between hTpoR WT and the Q516C and L500G/V507G mutants and slightly reduced for the V495A and Q516N mutants, in agreement with their lower surface localization (Figure 4J-K). Remarkably, mutants that were defective in activation by JAK2 V617F also displayed reduced internalization in the presence of JAK2 V617F (Figure 4K), confirming the inability of mutant JAK2 to activate these receptors.36 Importantly, the expression of these mutants was similar or even increased compared with that of hTpoR WT (Figure 4L), and the results were validated in human TF-1 cell lines in a cytokine-independent proliferation assay (Figure 4M-N). Surprisingly, these 2 strategies did not prevent JAK2 V617F–dependent dimerization of hTpoR (supplemental Figure 5E-F), suggesting that simple modulation of hTpoR conformation, rather than disruption of hTpoR dimers, is sufficient to inhibit JAK2 V617F–driven activation of hTpoR.
Specific inhibition of JAK2 V617F activation by the modulation of hTpoR in primary BMCs
To validate our findings in primary BMCs, we used Mpl KO mice2,37 crossed with vav-creJAK2 V617F mice that express 1 copy of JAK2 WT and 1 copy of JAK2 V617F (heterozygous) under its endogenous promoter in hematopoietic cells. This was done to prevent the unwanted effect of endogenous mTpoR/MPL. The lineage-negative fraction of BMCs from Mpl KO JAK2 WT/WT or Mpl KO JAK2 WT/V617F mice was isolated and retrovirally transduced to restore the expression of hTpoR WT or mutants. To assess the ability of JAK2 V617F and Tpo to activate the hTpoR mutants, we first performed megakaryocyte colony-forming assays in the presence or absence of Tpo.
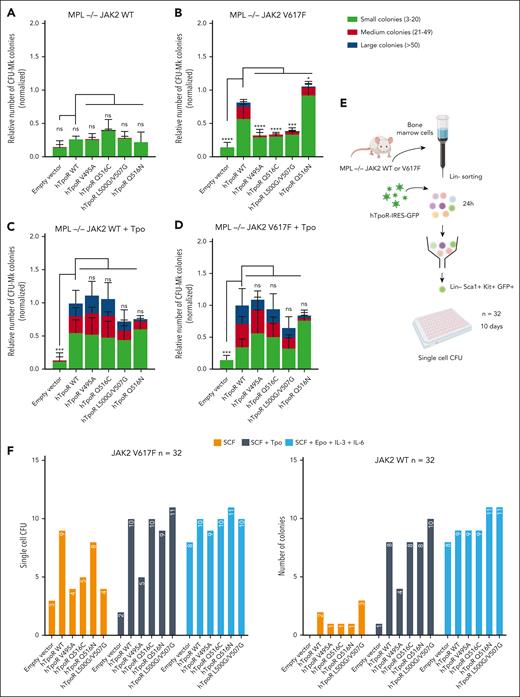
In Mpl KO BMCs expressing JAK2 V617F, restoration of hTpoR WT resulted in a significant increase in megakaryocyte colony formation, including very large colonies (>50 cells). Comparatively, mutants disfavoring interface V (hTpoR V495A and Q516C) or favoring interface 0 (hTpoR L500G/V507G) inhibited colony growth, especially of large colonies, when compared with hTpoR WT (Figure 5A-B). In contrast, the introduction of the Q516N mutation resulted in increased formation of CFU-Mk in the presence of JAK2 V617F. Importantly, stimulation with Tpo resulted in an overall similar megakaryocyte colony formation for hTpoR WT and mutants (Figure 5C-D). Then, we evaluated the ability of hTpoR mutants to inhibit the hematopoietic stem and progenitor cell propagation induced by JAK2 V617F or Tpo. Retrovirally transduced (GFP-positive) LSK cells were single-cell sorted by flow cytometry, and colony formation was assessed with or without Tpo or complete cytokine cocktail (without Tpo) (Figure 5E). In the absence of Tpo, colony formation was reduced for all inhibitory mutants compared with that for hTpoR WT or Q516N with an MPL KO JAK2 V617F background. Similar numbers of colonies were observed between hTpoR WT and mutants in the presence of Tpo, except for hTpoR V495A, which already displayed a lower response to Tpo in other assays, and no difference was detected with a complete CFU cytokine cocktail (Figure 5F). It is noteworthy that the presence of hTpoR did not cause a significant lineage shift in a classical CFU assay with a complete cytokine cocktail with or without Tpo, except for the increase in CFU-Mk colonies already observed in the CFU-Mk–specific assay (supplemental Figure 6).
Specific inhibition of JAK2 V617F-driven activation by modulation of hTpoR conformations in primary BMCs. (A-D) Colony-forming unit megakaryocytes (CFU-Mk) assays. Lineage-negative bone marrow cells were isolated from Mpl KO JAK2 WT/WT or JAK2 WT/V617F mice (n = 4) and retrovirally transduced with an empty vector, hTpoR WT, or the indicated mutants. One day after infection, the cells were plated in a semisolid collagen-based megakaryocyte culture medium with lipids supplemented with 10 ng/mL of mIL-3 and 20 ng/mL of hIL-6. In the condition with Tpo (C-D), 50 ng/mL of hTpo was added. Megakaryocytes were stained with acetylthiocholine iodide after 7 days and counted blindly with an inverted microscope. The colonies were separated into small (3-20 cells), medium (21-49 cells), and large (>50 cells). Values represent the mean (±SD) of 4 independent experiments with 2 different transductions. Statistics: two-way analysis of variance followed by the SIDAK multiple comparison test. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗P < .05. (E) Illustration of the single-cell colony assay experimental protocol. Lineage-negative bone marrow cells were isolated from Mpl KO JAK2 WT or JAK2 V617F mice and retrovirally transduced with hTpoR-IRES-GFP or an empty vector. Twenty-four hours after infection, stably transduced LSK cells were sorted by flow cytometry and plated at 1 cell per well (n = 32 per condition) in 96-well plate with MethoCult medium with SCF alone, SCF + Tpo, or SCF + Epo + IL-6 + IL-6. The presence of CFU was assessed after 10 days. (F) Number of single-cell colony-forming unit from LSK cells expressing indicated constructs in Mpl KO JAK2 WT or Mpl KO JAK2 V617F background.
Specific inhibition of JAK2 V617F-driven activation by modulation of hTpoR conformations in primary BMCs. (A-D) Colony-forming unit megakaryocytes (CFU-Mk) assays. Lineage-negative bone marrow cells were isolated from Mpl KO JAK2 WT/WT or JAK2 WT/V617F mice (n = 4) and retrovirally transduced with an empty vector, hTpoR WT, or the indicated mutants. One day after infection, the cells were plated in a semisolid collagen-based megakaryocyte culture medium with lipids supplemented with 10 ng/mL of mIL-3 and 20 ng/mL of hIL-6. In the condition with Tpo (C-D), 50 ng/mL of hTpo was added. Megakaryocytes were stained with acetylthiocholine iodide after 7 days and counted blindly with an inverted microscope. The colonies were separated into small (3-20 cells), medium (21-49 cells), and large (>50 cells). Values represent the mean (±SD) of 4 independent experiments with 2 different transductions. Statistics: two-way analysis of variance followed by the SIDAK multiple comparison test. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗P < .05. (E) Illustration of the single-cell colony assay experimental protocol. Lineage-negative bone marrow cells were isolated from Mpl KO JAK2 WT or JAK2 V617F mice and retrovirally transduced with hTpoR-IRES-GFP or an empty vector. Twenty-four hours after infection, stably transduced LSK cells were sorted by flow cytometry and plated at 1 cell per well (n = 32 per condition) in 96-well plate with MethoCult medium with SCF alone, SCF + Tpo, or SCF + Epo + IL-6 + IL-6. The presence of CFU was assessed after 10 days. (F) Number of single-cell colony-forming unit from LSK cells expressing indicated constructs in Mpl KO JAK2 WT or Mpl KO JAK2 V617F background.
Together, these results indicate that modulation of the hTpoR conformation, here via point mutations, is a viable strategy to specifically inhibit JAK2 V617F–driven activation in primary cells.
Discussion
We reported that the conformation of hTpoR dimers was differently modulated by physiologic (JAK2 WT) vs pathologic signaling by JAK2 V617F. This finding led us to show that several strategies involving mutations in key residues of the hTpoR transmembrane and cytosolic juxtamembrane regions effectively uncoupled JAK2 V617F–driven from Tpo-induced hTpoR activation. Current treatment strategies for JAK2 V617F–positive MPNs are dominated by JAK2 inhibitors, such as ruxolitinib or fedratinib. However, these inhibitors lack specificity as they do not differentiate between WT and mutant JAK2, leading to deleterious side effects and a lack of curative potential.25 Our results suggest that specific inhibition of JAK2 V617F–driven signaling is feasible by tuning the precise dimeric conformation adopted by hTpoR and that such a strategy would spare physiologic TpoR-JAK2 signaling. Here, this was achieved by specific point mutations, and further studies should focus on identifying drugs or small molecules that can achieve the same results. Modulation of the hTpoR conformation was previously achieved by the small molecule eltrombopag, which allows for the activation of hTpoR by binding to His499 and stabilization of an active conformation.16,33 Our results suggest that a similar strategy could be pivotal in identifying novel therapeutic options that specifically inhibit and potentially eradicate the JAK2 V617F clone.
An alternative strategy would be to modulate the hTpoR conformation from the ECD. Encouragingly, for EpoR and TpoR, extracellular ligands have been engineered that bind to the ECDs and can form complexes that place receptor monomers at different distances or impose different conformations, tuning the receptors to induce subtly different signaling activities.17-19 Notably, certain diabodies act for both EpoR and TpoR as weak agonists for physiologic signaling via JAK2 WT and antagonists, including with JAK2 V617F, via TpoR.19 For EpoR, structural work indicated that this is due to a longer intermonomeric distance,18 but the mechanisms of action were not solved for TpoR. By identifying the specific conformations of hTpoR induced by JAK2 V617F and defining how these differ from Tpo-induced activation, our work could lead to the rational design of novel molecules specifically targeting JAK2 V617F–bound hTpoR. Interestingly, our finding that STAT1 follows a slightly different pattern of activation with JAK2 V617F hints that such molecules could potentially be used to inhibit STAT3/5 signaling while preserving STAT1 priming by JAK2 V617F expressing cells, which could be useful in the context of IFN-α treatment.38,39
Our results also indicated that mTpoR and hTpoR exhibit significant differences with respect to their signaling via JAK2 WT and JAK2 V617F. These findings impact various mouse models of MPNs, such as JAK2 V617F or calreticulin (CALR) knockin mutants. For both types of models, the phenotype of the disease differs from that of human MPN. For example, JAK2 V617F induces essential thrombocythemia, polycythemia vera, and myelofibrosis, whereas in most mouse models, knockin of JAK2 V617F induces polycythemia vera and later myelofibrosis.40-42 In the case of knockin models of CALR mutants, only essential thrombocythemia is obtained and not myelofibrosis, unless for CALR del52 homozygous mice, whereas in humans, the mutation is generally heterozygous.40,43-45 We suggest that this may be due to the differential activation of mTpoR and hTpoR. Complete hTpoR knockin is not possible in mice because of the suboptimal response of hTpoR to mTpo.46 By identifying the precise residues that are responsible for the discrepancies between mTpoR and hTpoR, our results suggest the design of a humanized TpoR mouse model that would be pivotal for MPN research and preclinical studies.
From a structural perspective, our work couples the changes in the secondary structure around the TMD observed upon hTpoR activation33,47 with the modulation of the hTpoR dimeric conformation. Overall, our system of engineered dimers, combined with live-cell crosslinking, allowed us to link dimeric orientation with biological and pathological activation as well as with the structure around the TMD, owing to the very good concordance between AlphaFold 2.0 predictions and solid-state NMR data.34
Our study provides the proof of principle that hTpoR conformations can be manipulated to specifically inhibit JAK2 V617F–driven signaling and identify the molecular basis for novel therapeutic options in JAK2 V617F–positive MPNs.
Acknowledgments
The authors thank Lidvine Genet and Céline Mouton for expert technical support and Nicolas Dauguet for flow cytometry assistance.
This work was supported by the Ludwig Institute for Cancer Research, Fonds de la Recherche Scientifique, Fonds National de la Recherche Scientifiques, Fondation contre le Cancer, Salus Sanguinis and Fondation “Les avions de Sébastien,” projects Action de recherché concertée 16/21-073, WELBIO F 44/8/5–MCF/UIG–10955, Belgium (S.N.C.), a Fonds Spécial pour la Recherche PhD Fellowship from the Université catholique de Louvain (N.P.), and an Aspirant PhD Fellowship from FRS-FNRS, Belgium (N.P.).
Authorship
Contribution: N.P. conceived and designed the study, created constructs, performed biochemical and functional experiments in all cell lines and primary cells, and analyzed the data; A.P. created the constructs and performed biochemical and functional experiments in HEK293T; G.L. created cysteine mutants of hTpoR; A.N. provided support for the ex vivo experiments; J.S. created the initial cc-mTpoR constructs; N.P. and S.N.C. wrote the manuscript and interpreted data; and S.N.C. supervised the study and provided funding.
Conflict-of-interest disclosure: S.N.C. is a cofounder of MyeloPro GmbH. The remaining authors declare no competing financial interests.
Correspondence: Stefan N. Constantinescu, de Duve Institute, Université Catholique de Louvain, Ave Hippocrate, 74 UCL 74-4, 1200 Brussels, Belgium; e-mail: stefan.constantinescu@uclouvain.be.
References
Author notes
Information notes about experimental procedures and data are available upon request from the corresponding author, Stefan N. Constantinescu (stefan.constantinescu@uclouvain.be).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal