Autologous CD19-chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment of pediatric relapsed/refractory B-cell precursor acute lymphoblastic leukemia (R/R BCP-ALL). In this issue of Blood, del Bufalo et al report their experience of using donor derived CAR-T cells in patients unsuitable for an autologous approach, opening up a potential alternative route to cure for such children.1
T cells from either the initial stem cell donor or, in exceptional cases, from a designated stem cell donor were transduced with a second-generation (4.1BB) CD19 CAR. The treatments were performed under hospital exemption. Eleven pediatric and young adult patients with R/R BCP-ALL after allogeneic hematopoietic stem cell transplantation (HSCT) and 2 patients with extremely refractory BCP-ALL with low CD3+ cells without prior allogeneic HSCT received allogeneic CAR-T cells. In all patients, expansion and persistence of the transfused CAR-T cells and complete remission with measurable residual disease negativity were achieved. Remarkably, not only was allogeneic CAR T-cell therapy effective, but it did not come at the cost of increased toxicity or increased risk for severe graft-versus-host disease (GVHD) compared with the rates observed in studies of autologous CAR T-cell therapy.
During recent decades, tremendous progress has been made in the treatment of children, adolescents, and young adults with BCP-ALL using multimodal chemotherapy protocols and allogeneic HSCT, providing cure in many cases.2,3 However, some patients remain refractory to induction therapy or relapse after HSCT. For these children, prognosis was poor, with survival not exceeding 10% to 30%.4,5 The introduction of autologous CD19-directed second-generation (4.1BB) CAR T-cell therapy in 2012 was game changer, bringing new hope for these previously incurable children and their families.6 Based on a global phase 2 study, second-generation CAR-T cells directed against CD19 were licensed for the treatment of patients with BCP-ALL who have refractory disease after second relapse or who relapse after allogeneic HSCT.7 The success of CAR-T cells is strongly influenced by their design: the optimization of costimulatory elements and vector composition are pivotal to achieving in vivo expansion and persistence of transfused CAR-T cells. The composition of each patient’s T-cell repertoire also substantially impacts on the success of this powerful therapy.
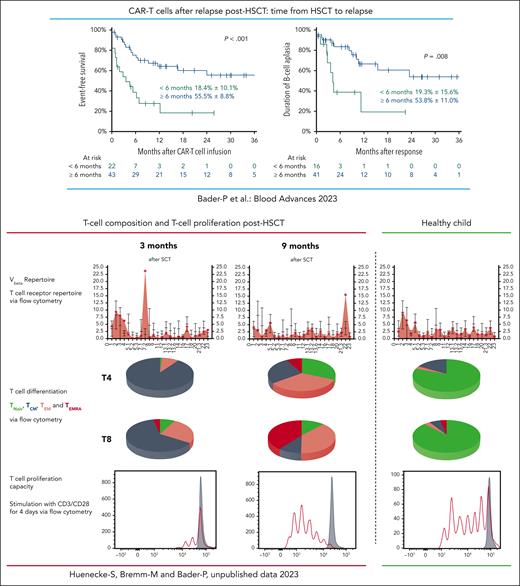
Several studies and analyses of real-world data have convincingly shown that approximately 50% of theses highest-risk patients with R/R BCP-ALL could be rescued with second-generation 4.1BB CAR-T cells as stand-alone therapy. Most recently, real-world data from Germany revealed that 53% of patients with BCP-ALL who relapsed after HSCT were successfully treated with CAR-T cells. Probability of overall survival at 2 years was 74% in patients who relapsed ≥6 months post-HSCT but only 16% for patients who relapsed <6 months post-HSCT.8 As can be seen in the figure, persistence of CAR-T cells was far shorter in those patients who relapsed early after allogeneic HSCT. In general, the composition of the T-cell repertoire in patients is skewed immediately after allogeneic HSCT. The ability of these T cells to proliferate is reduced compared with a later time after HSCT and compared with T cells of healthy individuals. It is of great importance to understand whether patients who relapse early after HSCT can benefit from autologous CAR T-cell treatment at all or whether these patients might need CAR-T cells from alternative sources with different compositions.9
Treatment with autologous CAR-T cells in patients who relapsed >6 months from HSCT led to superior survival compared with patients who relapsed <6 months from transplant. In patients with early relapse, B-cell persistence was shorter than in patients who relapsed >6 months post-HSCT.8 Early after HSCT the composition of the T-cell repertoire in patients is skewed and normalizes with time. The ability of these T cells to proliferate is reduced compared with a later time after HSCT and compared with T cells of healthy individuals. This might cause the diminished persistence of autologous CAR-T cells when given early after HSCT and demonstrates the importance of the report from del Bufalo et al in which CAR-T cells were generated from healthy donors. TCM, central memory T cell; TEM, effector memory T cell; TEMRA, effector memory T cell reexpressing CD45RA; TNaiv, naive T cell; Vbeta, V beta T cell repertoire.
Treatment with autologous CAR-T cells in patients who relapsed >6 months from HSCT led to superior survival compared with patients who relapsed <6 months from transplant. In patients with early relapse, B-cell persistence was shorter than in patients who relapsed >6 months post-HSCT.8 Early after HSCT the composition of the T-cell repertoire in patients is skewed and normalizes with time. The ability of these T cells to proliferate is reduced compared with a later time after HSCT and compared with T cells of healthy individuals. This might cause the diminished persistence of autologous CAR-T cells when given early after HSCT and demonstrates the importance of the report from del Bufalo et al in which CAR-T cells were generated from healthy donors. TCM, central memory T cell; TEM, effector memory T cell; TEMRA, effector memory T cell reexpressing CD45RA; TNaiv, naive T cell; Vbeta, V beta T cell repertoire.
The strength of the approach reported by del Bufalo and colleagues is to offer an alternative route to cure for patients who relapse early after allogeneic HSCT or for those with hypoplastic hematopoiesis lacking sufficient T-cell numbers for an autologous approach. In these patients, use of T cells from the original stem cell donor offers theoretical benefits for the successful generation of CAR-T cells. This might contribute to successful transduction, in vivo proliferation, expansion, and functional effectiveness of the T cells. Although no severe cases were reported, the high proportion of naive T cells and the high plasticity of healthy donor T cells theoretically might increase the risk of severe cytokine release syndrome and severe immune-cell associated neurotoxicity syndromes postallogeneic CAR T-cell therapy. In addition, as potential alloreactive T cells remain present in the CAR-T product, a risk for severe and life-threatening GVHD remains. GVHD was only reported in 1 patient in the study, and this was controllable with immunosuppressive treatment; however, this risk remains an important concern. As a consequence, the important and compelling findings reported here need to be investigated in a prospective, controlled, multicenter trial.
Conflict-of-interest disclosure: P.B. declares research grants from Neovii, Riemser, Medac, and Bristol Myers Squibb (to institution); is a member of advisory boards for Novartis, Celgene, Amgen, Medac, Servier (personal and institutional); has received speaker fees from Miltenyi, Jazz, and Riem.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal