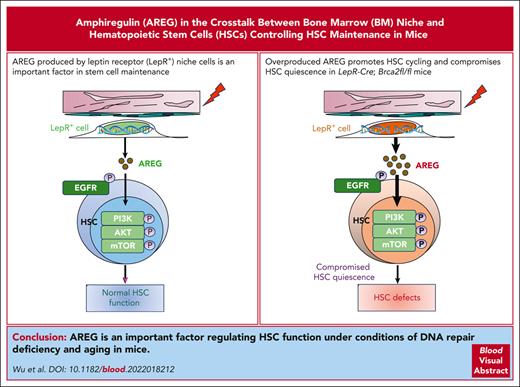
Key Points
Persistent DNA damage induces AREG expression in BM LepR+ cells deficient of the Brca2 gene.
Overproduced AREG activates the PI3K/AKT/mTOR pathway, promotes HSC cycling, and compromises HSC quiescence in LepR-Cre;Brca2fl/fl mice.
Abstract
The cross talk between extrinsic niche-derived and intrinsic hematopoietic stem cell (HSC) factors controlling HSC maintenance remains elusive. Here, we demonstrated that amphiregulin (AREG) from bone marrow (BM) leptin receptor (LepR+) niche cells is an important factor that mediates the cross talk between the BM niche and HSCs in stem cell maintenance. Mice deficient of the DNA repair gene Brca2, specifically in LepR+ cells (LepR-Cre;Brca2fl/fl), exhibited increased frequencies of total and myeloid-biased HSCs. Furthermore, HSCs from LepR-Cre;Brca2fl/fl mice showed compromised repopulation, increased expansion of donor-derived, myeloid-biased HSCs, and increased myeloid output. Brca2-deficient BM LepR+ cells exhibited persistent DNA damage–inducible overproduction of AREG. Ex vivo treatment of wild-type HSCs or systemic treatment of C57BL/6 mice with recombinant AREG impaired repopulation, leading to HSC exhaustion. Conversely, inhibition of AREG by an anti–AREG-neutralizing antibody or deletion of the Areg gene in LepR-Cre;Brca2fl/fl mice rescued HSC defects caused by AREG. Mechanistically, AREG activated the phosphoinositide 3-kinases (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway, promoted HSC cycling, and compromised HSC quiescence. Finally, we demonstrated that BM LepR+ niche cells from other DNA repair–deficient and aged mice also showed persistent DNA damage–associated overexpression of AREG, which exerts similar negative effects on HSC maintenance. Therefore, we identified an important factor that regulates HSCs function under conditions of DNA repair deficiency and aging.
Introduction
Maintenance of the rare hematopoietic stem cell (HSC) population in the bone marrow (BM) and preservation of their functional properties are supported by a highly specialized microenvironment (niche).1-3 The HSC niche comprises different cell populations, including endosteal and sinusoidal endothelial cells, mesenchymal stromal cells (MSCs), and osteoblast-lineage cells,1,2,4,5 which support HSC maintenance through direct interactions or paracrine factors secreted by niche cells, such as C-X-C motif chemokine ligand (CXCL12), thrombopoietin, transforming growth factor (TGF) β1, or stem cell factor (SCF).6,7 Stromal cells in the adult BM that express the leptin receptor (LepR) are heterogenous, including skeletal stem cells and osteogenic and audiogenic progenitors.8 LepR+ cells are known as critical sources of growth factors, including SCF and CXCL12, for the maintenance of HSCs and early restricted progenitors.9,10
The DNA damage response (DDR) machinery orchestrates a complex network of mechanisms that detects and repairs damage to the DNA, thereby maintaining genome integrity.11-13 Dysregulation of the DDR and its repair system can cause human disorders, including hematological malignancies.14 Compelling evidence highlights the occurrence of DDR in HSCs and underscores its role in regulating hematopoiesis.15-17 Persistence of DNA lesions due to DDR deficiency or aging, a generally considered consequence of the unrepaired accumulation of naturally occurring DNA damage,18-20 promotes the functional decline and predisposition of HSCs to leukemic transformation. Cross talk between the BM niche and stem cells is critical for HSC regeneration.1,2,21 Using 2 DDR-deficient mouse models, our recent study identifies a paracrine Wnt5a/Prox1 signaling axis between the BM niche and HSC as a regulator of HSC regeneration under radiation injury.22 However, the interplay between the BM niche and HSCs under conditions of DNA repair deficiency and aging remains elusive.
Breast cancer gene 2 (BRCA2, also known as Fanconi anemia D1 [FANCD1]) is a tumor suppressor gene involved in the DNA double-stranded break (DSB) repair mechanism. Mutant phenotypes of the BRCA2 gene are associated with cancer susceptibility, mostly in breast and ovarian cancer.23 BRCA2 interacting with RAD51 is fundamental for the maintenance of cell division and chromosome structure.24-26 Biallelic mutations in BRCA2 are responsible for ∼3% of all cases of Fanconi anemia (FA), a rare disorder characterized by multiple congenital malformations, progressive BM failure, and susceptibility to malignancies.27 A previous study described hematopoietic dysfunction in a mouse model of Brca2Δ27/Δ27.28 However, the role of BRCA2 in hematopoiesis, especially in the BM microenvironment, remains largely unknown.
The epidermal growth factor (EGF)-like molecule amphiregulin (AREG) is constitutively expressed by a number of epithelial and mesenchymal cell types.29,30 AREG is also produced by multiple immune cells, including Treg cells,31 group 2 innate lymphoid cells,32 mast cells,33 basophils,34 and macrophages.35-38 In addition to being implicated in a variety of physiologic processes,39-41 epithelial-derived AREG promotes tissue repair and integrity.42 AREG has also been implicated in multiple cancer types and potently enhances malignant development in both primary and metastatic lesions.36 A recent study showed that AREG is upregulated in the BM stroma and that coculture of wild-type (WT) Lin–Sca1+c-kit+ (LSK) cells with Areg-knockdown stromal cells leads to apoptosis of LSK cells in vitro.43 However, the function of AREG in hematopoiesis remains to be elucidated. In this study, we showed that LepR+ niche cell–derived AREG under conditions of DNA repair deficiency and aging compromises HSC maintenance, possibly through mechanisms involving activating the PI3K/AKT/mTOR pathways and promoting HSC cycling and exhaustion.
Materials and methods
Mice and treatment
Brca2fl/fl mice44 were crossed with LepR-Cre (The Jackson Laboratory, stock #032457), VE-cadherin-Cre (VE-Cre; stock# 006137), or Osx1-GFP-Cre (Osx1-Cre; stock# 006361) mice. Areg knockout mice (Areg–/–)45 were obtained from Mutant Mouse Resource Research Centers (stock# 011533-UNC) supported by the National Institutes of Health. Fancd2–/– mice were provided by Markus Grompe.46Atm–/– mice (C57BL/6, CD45.2+) were obtained from The Jackson Laboratory (stock# 008536). Young (∼10-12-week-old) or aged (∼20-26-month-old) C57BL/6 mice47 were used to study aging. BoyJ mice (6-8-week-old) were used as the BM transplant recipients. All animals were maintained in specific pathogen-free facilities at the University of Pittsburgh in accordance with the institutional animal care and use committee guidelines.
Results
BRCA2 from LepR+ stromal cells is required for HSC maintenance in the BM
We first compared Brca2 expression in different niche components by quantitative PCR (qPCR) and found that Brca2 was highly expressed in sorted CD45–Osx+, CD45–LepR+, and CD45–VE-cad+ stromal cells1,2,4,5 compared with that in the unfractionated whole BM cells (WBMCs; supplemental Figure 1A, available on the Blood website). By crossing conditional Brca2 knockout mice (Brca2fl/fl)44 with 3 niche-specific deleter strains, namely LepR-Cre, Osx1-Cre, or VE-Cre,48-50 we obtained 3 niche-specific Brca2 knockout strains: LepR-Cre;Brca2fl/fl, Osx1-Cre;Brca2fl/fl, and VE-Cre;Brca2fl/fl. Deletion of Brca2 led to significant DNA damage accumulation in BM niche cells from knockout mice, as detected by flow cytometry–based analysis of γ-H2AX,51 a well-established marker for DNA (DSBs; supplemental Figure 1B-D).52 We also used an enhanced green fluorescent protein (eGFP)-based reporter system,53,54 in which induction of DSB by I-SceI inactivates the eGFP gene, to examine the preferential utilization of the homologous recombination (HR) or nonhomologous end joining (NHEJ) repair pathway in BM niche cells from knockout mice. To exclude potential transfection differences, pDsRed2-N1 plasmid was used as a control. By comparing the ratio of eGFP to DsRed genes, we demonstrated that the efficiency of HR was significantly lower in knockout niche cells than in control cells after irradiation (supplemental Figure 1E). Conversely, NHEJ efficiency in knockout niche cells was much greater than that in the controls (supplemental Figure 1F), indicating that loss of Brca2 in BM niche cells decreased HR but increased error-prone NHEJ, consistent with the fundamental function of BRCA2 in HR previously described in other cell types.55,56
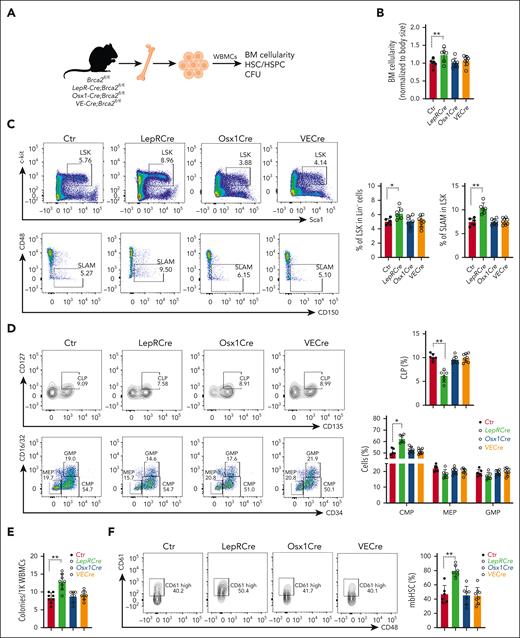
We then examined whether the loss of Brca2 in the BM niche affected hematopoiesis (Figure 1A) and found that the deletion of Brca2 in LepR+ cells (LepR-Cre;Brca2fl/fl) significantly increased BM cellularity (Figure 1B; supplemental Figure 1G). Loss of Brca2 in niche LepR+ cells markedly increased the frequency of progenitor cells (LSK) and phenotypic HSCs (signaling lymphocyte activation molecule [SLAM] and LSKCD48–CD150+)57 in LepR-Cre;Brca2fl/fl mice (Figure 1C; supplemental Figure 1H). LepR-Cre;Brca2fl/fl mice exhibited significantly higher frequencies of common myeloid progenitor (LSKCD34+CD16/32-) but lower common lymphoid progenitor (Lin–Sca1intc-kitintCD135+CD127+) cells than those in control mice (Figure 1D). Additionally, the loss of Brca2 significantly increased the colony-forming units (CFUs) produced by BM cells from LepR-Cre;Brca2fl/fl mice (Figure 1E). Strikingly, the frequencies of myeloid-biased HSCs (mbHSCs: LSKCD150+CD48–CD61+ cells)58 in LepR-Cre;Brca2fl/fl mice were dramatically higher than those in controls (Figure 1F). Together, these data indicate that the loss of Brca2 in niche LepR+ cells affected BM hematopoiesis and suggest that BRCA2 from niche LepR+ cells is required for HSC maintenance in the BM.
BRCA2 from LepR+ stromal cells is required for the maintenance of HSCs in the BM. (A) Schematic presentation of experimental design. (B) BM cellularity in 2 tibias and 2 femurs from mice of the indicated genotypes. Quantification of absolute BM cell numbers normalized to mouse body size are shown; control (Ctr): Brca2fl/fl mice (n = 6-8). (C) Increased HSC frequency in LepR-Cre;Brca2fl/fl mice. Representative flow plots (left) and quantification (right) of LSK (top) or SLAM (signaling lymphocyte activation molecule: LSKCD48–CD150+) (bottom) cells are shown (n = 6-8). (D) Frequencies of hematopoietic progenitor populations in the BM. Representative flow cytometry plots (left) of common lymphoid progenitors, common myeloid progenitors, megakaryocyte/erythroid progenitor (MEPs), and granulocyte/monocyte progenitor (GMPs) in the BM of LepR-Cre;Brca2+/+ and LepR-Cre;Brca2fl/fl mice, along with the frequency (right) of each population (n = 6-8). (E) Increased myeloid colonies generated by WBMCs from LepR-Cre;Brca2fl/fl. Approximately 1000 cells from the indicated mice were plated in a cytokine-supplemented methylcellulose medium. Colonies were enumerated on day 7 (n = 6-8). (F) Increased frequency of mbHSCs in LepR-Cre;Brca2fl/fl mice. Representative flow plots of mbHSCs (LSKCD150+CD48–CD61+) (left); and quantification (right) in the BM of LepR-Cre;Brca2fl/fl and LepR-Cre;Brca2fl/fl mice are shown (n = 6-8). The number of mice analyzed per genotype is shown for each bar in each panel. The results are presented as mean ± standard deviation of 3 independent experiments. One-way analysis of variance (ANOVA) was performed to compare groups (Ctr, LepR-Cre, Osx1-Cre, and VE-Cre). Normality of the data was examined using the Shapiro-Wilk test. If the data were normally distributed, ANOVA was used, followed by t tests. Otherwise, the Kruskal-Wallis test was used, followed by Wilcoxon rank sum tests. ∗P < .05; ∗∗P < .01.
BRCA2 from LepR+ stromal cells is required for the maintenance of HSCs in the BM. (A) Schematic presentation of experimental design. (B) BM cellularity in 2 tibias and 2 femurs from mice of the indicated genotypes. Quantification of absolute BM cell numbers normalized to mouse body size are shown; control (Ctr): Brca2fl/fl mice (n = 6-8). (C) Increased HSC frequency in LepR-Cre;Brca2fl/fl mice. Representative flow plots (left) and quantification (right) of LSK (top) or SLAM (signaling lymphocyte activation molecule: LSKCD48–CD150+) (bottom) cells are shown (n = 6-8). (D) Frequencies of hematopoietic progenitor populations in the BM. Representative flow cytometry plots (left) of common lymphoid progenitors, common myeloid progenitors, megakaryocyte/erythroid progenitor (MEPs), and granulocyte/monocyte progenitor (GMPs) in the BM of LepR-Cre;Brca2+/+ and LepR-Cre;Brca2fl/fl mice, along with the frequency (right) of each population (n = 6-8). (E) Increased myeloid colonies generated by WBMCs from LepR-Cre;Brca2fl/fl. Approximately 1000 cells from the indicated mice were plated in a cytokine-supplemented methylcellulose medium. Colonies were enumerated on day 7 (n = 6-8). (F) Increased frequency of mbHSCs in LepR-Cre;Brca2fl/fl mice. Representative flow plots of mbHSCs (LSKCD150+CD48–CD61+) (left); and quantification (right) in the BM of LepR-Cre;Brca2fl/fl and LepR-Cre;Brca2fl/fl mice are shown (n = 6-8). The number of mice analyzed per genotype is shown for each bar in each panel. The results are presented as mean ± standard deviation of 3 independent experiments. One-way analysis of variance (ANOVA) was performed to compare groups (Ctr, LepR-Cre, Osx1-Cre, and VE-Cre). Normality of the data was examined using the Shapiro-Wilk test. If the data were normally distributed, ANOVA was used, followed by t tests. Otherwise, the Kruskal-Wallis test was used, followed by Wilcoxon rank sum tests. ∗P < .05; ∗∗P < .01.
HSC defects in LepR-Cre;Brca2fl/fl mice
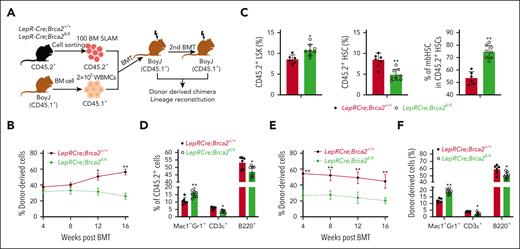
Next, we determined whether the deletion of Brca2 in niche cells affected HSC repopulation. Complete blood count revealed significant increases in white blood cell and neutrophil counts and a decrease in lymphocyte counts in the peripheral blood (PB) of LepR-Cre;Brca2fl/fl mice compared with those in LepR-Cre;Brca2+/+ mice (supplemental Figure 2A). We, then, performed competitive BM transplantation (BMT) by injecting 100 sorted SLAMs from LepR-Cre;Brca2fl/fl mice or their LepR-Cre;Brca2+/+ littermates (CD45.2+), along with 2 × 105 WBMCs isolated from congenic BoyJ mice (CD45.1+), into lethally irradiated BoyJ recipients. Analysis of hematopoietic repopulation at different time points after BMT demonstrated a progressive decline in donor-derived chimera (CD45.2+) in the PB of mice that underwent transplantation with SLAM cells from LepR-Cre;Brca2fl/fl mice compared with those that received transplantation with control cells (Figure 2A-B; supplemental Figure 2B). However, SLAMs from LepR-Cre;Brca2fl/fl mice generated significantly more total HSCs and mbHSCs than those from LepR-Cre;Brca2+/+ mice (Figure 2C; supplemental Figure 2C). The mice that received transplantation with HSCs from LepR-Cre;Brca2fl/fl mice showed a differentiation skew toward myeloid at the expense of lymphoid in multilineage reconstitution (Figure 2D; supplemental Figure 2D). A secondary transplantation assay using BM cells from primary recipient mice showed that HSCs from LepR-Cre;Brca2fl/fl mice gave rise to significantly lower total donor-derived engraftment and higher myeloid output in secondary recipients than those from LepR-Cre;Brca2+/+ mice (Figure 2E-F; supplemental Figure 2E-F). To determine whether increased mbHSCs contributed to the observed compromised HSC functionality, we performed BMT with CD61+ or CD61–SLAM cells and found that although both CD61+ and CD61–HSCs from LepR-Cre;Brca2fl/fl mice gave rise to reduced donor-derived chimera in the recipient mice compared with the respective CD61+ and CD61–HSCs from LepR-Cre;Brca2+/+ mice, only CD61+HSC from LepR-Cre;Brca2fl/fl mice generated significantly lower donor-derived chimera than those from LepR-Cre;Brca2+/+ mice (supplemental Figure 2G). These data indicated that CD61+HSCs from LepR-Cre;Brca2fl/fl mice had compromised hematopoietic repopulation.
HSCs from LepR-Cre;Brca2fl/fl mice show compromised repopulation, increased expansion of donor-derived myeloid-biased HSCs, and myeloid output. (A) Schematic presentation of experimental design. (B) Donor (CD45.2+) cell engraftment over time in CD45.1+ mice that received transplantation with 100 BM SLAM cells from LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice and 2 × 105 competing CD45.1+ BM cells (n = 6-8). (C) Increased expansion of the donor-derived mbHSCs. These mbHSCs from mice that received transplantation were harvested 4 months after BMT for flow cytometry analysis. Shown are the BM frequencies of LSKs (left), total HSCs (LSKCD150+CD48–) (middle), and mbHSCs (LSKCD150+CD48–CD61+) (right) in CD45.2+ cell compartment (n = 6-8). (D) Increased myeloid output in mice (CD45.1+) that received primary transplantation with SLAM cells from LepR-Cre;Brca2fl/fl mice (CD45.2+). Myeloid (Mac1/Gr1), B-cell (B220), and T-cell (CD3ε) engraftment levels at 4 months are shown (n = 6-8). (E-F) Mean levels of donor CD45.2+ cell (E) at 4, 8, 12, and 16 weeks after BMT and lineage engraftment (F) in secondary recipient CD45.1+ mice 16 weeks after transplantation with 3 million WBMCs from the primary mice in panel B (n = 6-8). Results are presented as mean ± standard deviation of 3 independent experiments. Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare LepRCre;Brca2+/+ vs LepRCre;Brca2fl/fl in panel C; LepR-Cre;Brca2+/+ vs LepR-Cre;Brca2fl/fl at time points 4, 8, 12, or 16 weeks after BMT in panels B and E; LepR-Cre;Brca2+/+ vs LepR-Cre;Brca2fl/fl for Mac1+Gr1+, CD3ε+, or B220+ cells in panels D and F. ∗P < .05; ∗∗P < .01.
HSCs from LepR-Cre;Brca2fl/fl mice show compromised repopulation, increased expansion of donor-derived myeloid-biased HSCs, and myeloid output. (A) Schematic presentation of experimental design. (B) Donor (CD45.2+) cell engraftment over time in CD45.1+ mice that received transplantation with 100 BM SLAM cells from LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice and 2 × 105 competing CD45.1+ BM cells (n = 6-8). (C) Increased expansion of the donor-derived mbHSCs. These mbHSCs from mice that received transplantation were harvested 4 months after BMT for flow cytometry analysis. Shown are the BM frequencies of LSKs (left), total HSCs (LSKCD150+CD48–) (middle), and mbHSCs (LSKCD150+CD48–CD61+) (right) in CD45.2+ cell compartment (n = 6-8). (D) Increased myeloid output in mice (CD45.1+) that received primary transplantation with SLAM cells from LepR-Cre;Brca2fl/fl mice (CD45.2+). Myeloid (Mac1/Gr1), B-cell (B220), and T-cell (CD3ε) engraftment levels at 4 months are shown (n = 6-8). (E-F) Mean levels of donor CD45.2+ cell (E) at 4, 8, 12, and 16 weeks after BMT and lineage engraftment (F) in secondary recipient CD45.1+ mice 16 weeks after transplantation with 3 million WBMCs from the primary mice in panel B (n = 6-8). Results are presented as mean ± standard deviation of 3 independent experiments. Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare LepRCre;Brca2+/+ vs LepRCre;Brca2fl/fl in panel C; LepR-Cre;Brca2+/+ vs LepR-Cre;Brca2fl/fl at time points 4, 8, 12, or 16 weeks after BMT in panels B and E; LepR-Cre;Brca2+/+ vs LepR-Cre;Brca2fl/fl for Mac1+Gr1+, CD3ε+, or B220+ cells in panels D and F. ∗P < .05; ∗∗P < .01.
AREG is overproduced and induced by persistent DNA damage in BRCA2-deficient LepR+ stromal cells
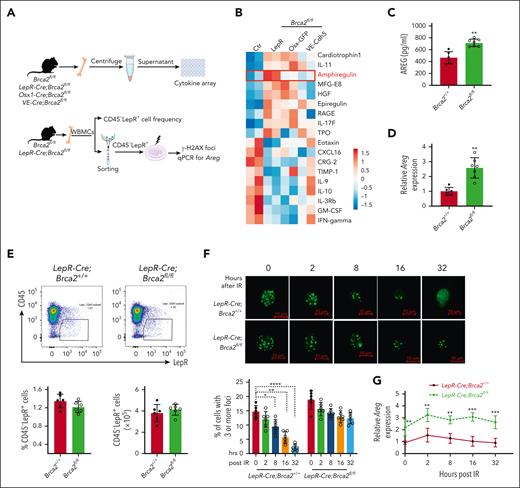
To explore the underlying mechanism, we performed cytokine array59 using the BM supernatant collected from 3 knockout mouse strains. We observed ∼18 significantly dysregulated cytokines in knockout mice compared with those in control mice (Figure 3A,B). Among them, AREG particularly caught our attention because it was specifically upregulated in LepR-Cre;Brca2fl/fl mice but not in Osx1-Cre;Brca2fl/fl or VE-Cre;Brca2fl/fl mice. Because AREG is a key molecule among EGF receptor (EGFR) ligands,38,60 EGF regulates hematopoietic regeneration after radiation injury, and EGFR-dependent DNA repair promotes murine and human hematopoietic regeneration,21,61 we decided to focus on AREG. We, first, confirmed higher levels of AREG in the BM supernatants of mice harboring Brca2 deletion, specifically in LepR+ cells but not in CD45-Osx+ or CD45-VE-cad+ cells, by ELISA (Figure 3C; supplemental Figure 3A). We also measured Areg transcript in 3 niche components by qPCR analysis and found that although Areg messenger RNA levels were comparable in CD45–Osx+ and CD45–VE-cad+ cells (supplemental Figure 3B), Areg transcripts were significantly higher in CD45–LepR+ cells from LepR-Cre;Brca2fl/fl mice than those from the LepR-Cre;Brca2+/+ control mice (Figure 3D; supplemental Figure 3C). Furthermore, deletion of Brca2 in LepR+ cells reduced, albeit not statistically significant, the frequencies of CD45–LepR+ cells (Figure 3E; supplemental Figure 3D), but had no effect on CD45–Osx+ or CD45–VE-Cad+ cells (supplemental Figure 3E).
AREG is overproduced and induced by persistent DNA damage in Brca2-deficient LepR+ stromal cells. (A) Schematic presentation of experimental design. (B) Heatmap of dysregulated cytokines in the knockout mice. Cytokine levels in BM supernatants from the 3 niche-specific Brca2 knockout mouse strains and their WT Ctrs (Brca2fl/fl) were analyzed using a cytokine assay (n = 2 assays per genotype). Heatmap was ordered based on fold change ( 1.5). (C) AREG levels in the BM supernatant of LepR-Cre;Brca2+/+ and LepR-Cre;Brca2fl/fl mice, as detected by ELISA (n = 6-7). (D) Expression of Areg transcript in BM CD45–LepR+ cells of LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice detected by qPCR (n = 6-7). (E) Flow cytometry analysis of BM CD45–LepR+ cell frequencies (top) and absolute numbers (bottom) in LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice. The representative flow (top) and quantification of CD45–LepR+ cells frequencies (middle) and absolute numbers (bottom) are shown (n = 6). (F-G) Persistent DNA damage induces AREG expression. BM CD45–LepR+ cells isolated from LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice after IR (300 cGy) were subjected to immunofluorescence staining for γ-H2AX foci (F) and qPCR analysis for Areg messenger RNA levels (G) at 0, 2, 8, 16, and 32 hours after IR (n = 6). Results are presented as means ± standard deviation of 3 independent experiments. Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare LepR-Cre;Brca2+/+ vs LepR-Cre;Brca2fl/fl in panels C, D, E, and G; and to compare LepR-Cre;Brca2+/+ vs LepR-Cre;Brca2fl/fl at each time point (ie, 0, 2, 8, 16, or 32 hours after IR). For panel F, one-way ANOVA, followed by t tests was performed to compare the indicated time points (2, 8, 16, and 32 vs 0 hours) for each genotype (LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl) separately. ∗P < .05; ∗∗P < .01; ∗∗∗ P < .001. GM-CSF, granulocyte macrophage colony-stimulating factor; HGF, hepatocyte growth factor; IFN, interferon; IL, interleukin; MFG, milk fat globule; TIMP, tissue inhibitor of metalloproteinases; TPO, thrombopoeitin; RAGE, receptor for advanced glycation end products.
AREG is overproduced and induced by persistent DNA damage in Brca2-deficient LepR+ stromal cells. (A) Schematic presentation of experimental design. (B) Heatmap of dysregulated cytokines in the knockout mice. Cytokine levels in BM supernatants from the 3 niche-specific Brca2 knockout mouse strains and their WT Ctrs (Brca2fl/fl) were analyzed using a cytokine assay (n = 2 assays per genotype). Heatmap was ordered based on fold change ( 1.5). (C) AREG levels in the BM supernatant of LepR-Cre;Brca2+/+ and LepR-Cre;Brca2fl/fl mice, as detected by ELISA (n = 6-7). (D) Expression of Areg transcript in BM CD45–LepR+ cells of LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice detected by qPCR (n = 6-7). (E) Flow cytometry analysis of BM CD45–LepR+ cell frequencies (top) and absolute numbers (bottom) in LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice. The representative flow (top) and quantification of CD45–LepR+ cells frequencies (middle) and absolute numbers (bottom) are shown (n = 6). (F-G) Persistent DNA damage induces AREG expression. BM CD45–LepR+ cells isolated from LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice after IR (300 cGy) were subjected to immunofluorescence staining for γ-H2AX foci (F) and qPCR analysis for Areg messenger RNA levels (G) at 0, 2, 8, 16, and 32 hours after IR (n = 6). Results are presented as means ± standard deviation of 3 independent experiments. Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare LepR-Cre;Brca2+/+ vs LepR-Cre;Brca2fl/fl in panels C, D, E, and G; and to compare LepR-Cre;Brca2+/+ vs LepR-Cre;Brca2fl/fl at each time point (ie, 0, 2, 8, 16, or 32 hours after IR). For panel F, one-way ANOVA, followed by t tests was performed to compare the indicated time points (2, 8, 16, and 32 vs 0 hours) for each genotype (LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl) separately. ∗P < .05; ∗∗P < .01; ∗∗∗ P < .001. GM-CSF, granulocyte macrophage colony-stimulating factor; HGF, hepatocyte growth factor; IFN, interferon; IL, interleukin; MFG, milk fat globule; TIMP, tissue inhibitor of metalloproteinases; TPO, thrombopoeitin; RAGE, receptor for advanced glycation end products.
Because BRCA2 is a key factor in HR DNA repair,23-26 we wondered whether high levels of Areg expression in Brca2-deficient LepR+ cells were the result of persistent DNA damage. To this end, we isolated BM CD45–LepR+ cells from mice at different time points after total body irradiation (300 cGy)2,22 and analyzed DNA damage accumulation and Areg transcript. We found that although BM CD45–LepR+ cells from LepR-Cre;Brca2+/+ mice efficiently repaired irradiation (IR)-induced DNA damage over time, IR induced persistent DNA damage in BM CD45–LepR+ cells from LepR-Cre;Brca2fl/fl mice up to 32 hours after IR (Figure 3F). Consistently, persistent high levels of Areg messenger RNA were observed during a 32-hour post-IR period in Brca2-deficient CD45–LepR+ cells (Figure 3G; supplemental Figure 3F) but not in Brca2-deficient CD45–Osx+ or CD45–VE-cad+ cells (supplemental Figure 3G). Taken together, these data indicate that persistent DNA damage may induce AREG overexpression in Brca2-deficient LepR+ stromal cells.
Recombinant AREG treatment impairs repopulation, leading to HSC exhaustion
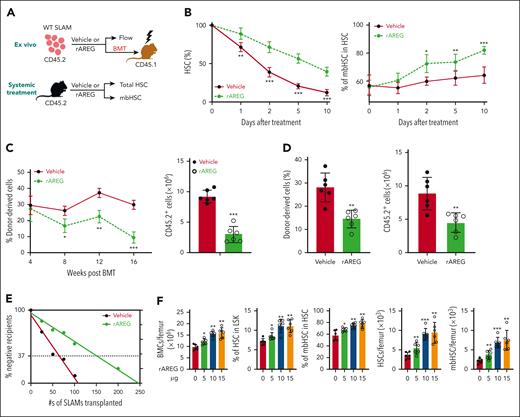
To further understand the effect of AREG on HSCs, we used both in vitro and in vivo approaches (Figure 4A): (1) ex vivo culture of WT SLAM cells with recombinant AREG (rAREG) or vehicle for 10 days and (2) systemic treatment of C57BL/6 mice with increasing doses of rAREG.38 Because 500 ng/mL of rAREG was the optimal dose for liquid (supplemental Figure 4A) and semisolid culture (supplemental Figure 4B), we chose 500 ng/mL for the subsequent experiments. Treatment of WT SLAM cells with 500 ng/mL rAREG in the culture significantly increased the frequencies of total HSCs and mbHSCs (Figure 4B; supplemental Figure 4C). Functionally, rAREG treatment severely compromised the repopulating capacity of cultured HSCs (Figure 4C) and increased myeloid but decreased lymphoid output in recipients (supplemental Figure 4D). Furthermore, rAREG treatment profoundly compromised long-term hematopoiesis in mice that received secondary transplantations (Figure 4D). We also found that rAREG treatment caused more than twofold reduction in the frequency of competitive repopulating units using the limiting dilution assay 54 (Figure 4E; supplemental Figure 4E).
Ex vivo treatment of WT HSCs or systemic treatment of C57BL/6 mice with rAREG impairs repopulation, leading to HSC exhaustion. (A) Schematic presentation of the experimental design. Ex vivo culture of BM SLAM cells from C57BL/6 mice treated with rAREG or vehicle, followed by BMT or flow cytometry analysis of total HSCs and mbHSCs. (B) Time course of the proportion of total HSCs (left) and mbHSCs (right) during 10 days of culture of cells described in panel A (n = 6-7 assays per group). (C) rAREG treatment impairs WT HSC repopulation (CD45.2+) in that mice that received transplantation (CD45.1+). SLAM cells cultured with or without 500 ng/mL rAREG for 5 days, along with 2 × 105 competing CD45.1+ WT BM cells, were transplanted into lethally irradiated BoyJ recipients. Donor CD45.2+ cells engraftment levels (frequencies at different time points after BMT and absolute numbers at 16-week after BMT) in recipient CD45.1+ mice over time after transplantation were determined by flow cytometry (n = 6). (D) rAREG compromises long-term HSC function. WBMCs from the primary recipients described in panel C were transplanted into sublethally irradiated BoyJ recipients. Donor CD45.2+ cells engraftment levels (frequencies and absolute numbers) in recipient CD45.1+ mice were determined using flow cytometry 16 weeks after BMT (n = 6). (E) rAREG treatment causes HSC exhaustion, as determined using a limited dilution transplant assay. Graded numbers of SLAM cells (CD45.2+) from the ex vivo culture plus 2 × 105 radio-protector BM cells were transplanted into lethally irradiated BoyJ recipients (CD45.1+). Plotted are the percentages of recipients containing less than 1% donor (CD45.2+) blood nucleated cells at 16 weeks after transplantation. The frequency of functional HSCs was calculated according to Poisson statistics (competitive repopulating units: vehicle, 1/108.3 and rAREG, 1/246.8). (F) Systemic rAREG treatment alters hematopoiesis in WT mice. C57BL/6 mice were treated with increasing doses (0, 5, 10, and 15 μg) of rAREG every other day for 10 days, followed by analysis of BM cell counts, total HSCs, and mbHSCs 4 weeks later by flow cytometry (n = 6). Frequencies (left) and absolute numbers (right) are shown. Results are presented as mean ± standard deviation of 3 independent experiments. Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare the vehicle vs rAREG at different time points (0, 1, 2, 5, or 10 days after treatment) in panel B; vehicle vs rAREG at different time points (4, 8, 12, or 16 weeks after BMT) in panel C; vehicle vs rAREG in panels D and E. For panel F, one-way ANOVA, followed by t tests was performed to compare different treatments (0, 5, 10, or 15 μg of rAREG). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Ex vivo treatment of WT HSCs or systemic treatment of C57BL/6 mice with rAREG impairs repopulation, leading to HSC exhaustion. (A) Schematic presentation of the experimental design. Ex vivo culture of BM SLAM cells from C57BL/6 mice treated with rAREG or vehicle, followed by BMT or flow cytometry analysis of total HSCs and mbHSCs. (B) Time course of the proportion of total HSCs (left) and mbHSCs (right) during 10 days of culture of cells described in panel A (n = 6-7 assays per group). (C) rAREG treatment impairs WT HSC repopulation (CD45.2+) in that mice that received transplantation (CD45.1+). SLAM cells cultured with or without 500 ng/mL rAREG for 5 days, along with 2 × 105 competing CD45.1+ WT BM cells, were transplanted into lethally irradiated BoyJ recipients. Donor CD45.2+ cells engraftment levels (frequencies at different time points after BMT and absolute numbers at 16-week after BMT) in recipient CD45.1+ mice over time after transplantation were determined by flow cytometry (n = 6). (D) rAREG compromises long-term HSC function. WBMCs from the primary recipients described in panel C were transplanted into sublethally irradiated BoyJ recipients. Donor CD45.2+ cells engraftment levels (frequencies and absolute numbers) in recipient CD45.1+ mice were determined using flow cytometry 16 weeks after BMT (n = 6). (E) rAREG treatment causes HSC exhaustion, as determined using a limited dilution transplant assay. Graded numbers of SLAM cells (CD45.2+) from the ex vivo culture plus 2 × 105 radio-protector BM cells were transplanted into lethally irradiated BoyJ recipients (CD45.1+). Plotted are the percentages of recipients containing less than 1% donor (CD45.2+) blood nucleated cells at 16 weeks after transplantation. The frequency of functional HSCs was calculated according to Poisson statistics (competitive repopulating units: vehicle, 1/108.3 and rAREG, 1/246.8). (F) Systemic rAREG treatment alters hematopoiesis in WT mice. C57BL/6 mice were treated with increasing doses (0, 5, 10, and 15 μg) of rAREG every other day for 10 days, followed by analysis of BM cell counts, total HSCs, and mbHSCs 4 weeks later by flow cytometry (n = 6). Frequencies (left) and absolute numbers (right) are shown. Results are presented as mean ± standard deviation of 3 independent experiments. Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare the vehicle vs rAREG at different time points (0, 1, 2, 5, or 10 days after treatment) in panel B; vehicle vs rAREG at different time points (4, 8, 12, or 16 weeks after BMT) in panel C; vehicle vs rAREG in panels D and E. For panel F, one-way ANOVA, followed by t tests was performed to compare different treatments (0, 5, 10, or 15 μg of rAREG). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Next, we treated WT mice with rAREG (0, 5, 10, and 15 μg)38 and found that AREG level in the BM of treated mice was significantly elevated 1 hour after rAREG administration but returned to near basal level 24 hours later (supplemental Figure 4F). Therefore, we decided to treat the mice every other day for 10 days. We observed a dose-dependent increase in total BM cell counts and frequencies of total HSCs and mbHSCs in rAREG-treated mice compared with those in vehicle controls (Figure 4F). Additionally, rAREG treatment significantly increased myeloid cells but decreased lymphoid cells in the PB of rAREG-treated mice (supplemental Figure 4G). Furthermore, rAREG treatment generated significantly more myeloid colonies in the BM cells from rAREG-treated mice than in those from vehicle-treated mice (supplemental Figure 4H). Mechanistically, rAREG treatment promoted HSC cycling with a negligible effect on apoptosis (supplemental Figure 4I). Together, these results demonstrate a detrimental effect of AREG on HSC maintenance both in vitro and in vivo.
Inhibition of AREG rescues the HSC defects caused by Brca2 deletion
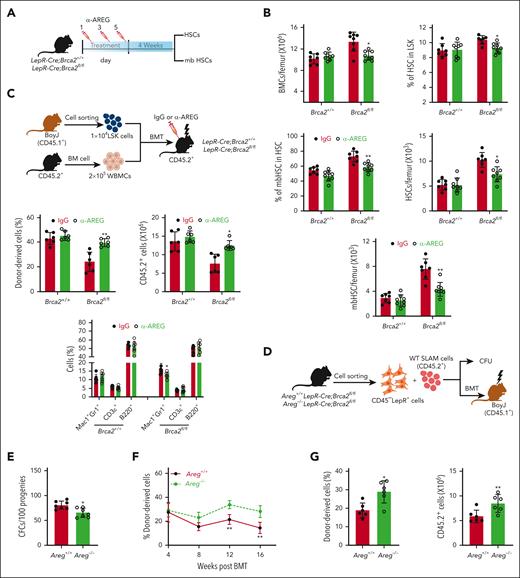
We, then, asked whether inhibition of AREG improved HSC function in LepR-Cre;Brca2fl/fl mice. We, first, treated the mice with increased doses of anti–AREG-neutralizing antibody (α-AREG; intraperitoneally)38 every other day for 5 days and measured AREG levels in the BM supernatants 24 hours after the final injection. Because 50 μg of α-AREG appeared to be the optimal dose for in vivo neutralization (supplemental Figure 5A), we treated the mice with 50 μg of α-AREG every other day for 5 days and examined BM cellularity 4 weeks after treatment (Figure 5A). We found that α-AREG treatment significantly reduced total BM cell counts and the frequencies of total HSCs and mbHSCs in LepRCre;Brca2fl/fl mice compared with immunoglobulin G (IgG)-treated controls (Figure 5B). In addition, α-AREG neutralization restored nearly all PB parameters in LepRCre;Brca2fl/fl mice to normal levels (supplemental Figure 5B).
Inhibition of AREG by anti–AREG-neutralizing antibody or deletion of the Areg gene in LepR-Cre;Brca2fl/fl mice rescues HSC defects caused by AREG. (A) Schematic presentation of the experimental design. Systemic treatment of LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice with anti-AREG or IgG every other day for 5 days. Total BM cells and frequencies of total HSCs and mbHSCs were determined 4 weeks after treatment. (B) Systemic treatment of anti-AREG improves BM parameters of LepR-Cre;Brca2fl/fl mice. LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice were treated with 50 μg anti-AREG or IgG. The numbers of WBMCs/femur (left), HSCs (middle), and mbHSCs (right) cells were determined at 4 weeks after treatment (n = 7-8). Frequencies (left) and absolute numbers (right) are shown. (C) Anti-AREG improves BM niche function in LepR-Cre;Brca2fl/fl mice. A total of 1 × 104 WT CD45.1+ BM LSK cells along with 2 × 105 protector cells (CD45.2+) were transplanted into lethally irradiated LepR-Cre;Brca2+/+ or LepR-Cre;Brca2f/f recipient mice (CD45.2+), followed by 3 doses (every other day for 5 days) of 50 μg anti-AREG or IgG treatment 10 days after BMT (top). Donor (CD45.1+) cell engraftment (left) (frequencies and absolute numbers) and lineage differentiation (right) were determined by flow cytometry 16 weeks after BMT (bottom) (n = 6). (D) Schematic presentation of experimental design. WT SLAM was cocultured with BM CD45–LepR+ cells isolated from Areg+/+LepR-Cre;Brca2fl/fl or Areg–/–LepR-Cre;Brca2fl/fl mice, followed by CFU or BMT assay. (E) Deletion of Areg limits expansion of myeloid progenitors. Progenies of WT SLAM cells cocultured with BM CD45–LepR+ cells from Areg+/+LepR-Cre;Brca2fl/fl or Areg–/–LepR-Cre;Brca2fl/fl mice were subjected to CFU assay (n = 7). (F) Deletion of Areg improves the repopulating capacity of progenies from cocultured SLAM cells. Progenies (CD45.2+) described in panel E, along with 2 × 105 radio-protector cells, were transplanted into lethally irradiated BoyJ recipients (CD45.1+). Donor-derived chimera (CD45.2+) were measured using flow cytometry at different time points after BMT (n = 6). (G) Deletion of Areg improves long-term hematopoietic reconstitution of progenies from cocultured SLAM cells. WBMCs from the primary recipients (CD45.2+) described in panel F were pooled and transplanted into sublethally irradiated BoyJ recipients (CD45.1+). Donor-derived chimera (CD45.2+) were measured using flow cytometry 16 weeks after BMT (n = 6). Frequencies (left) and absolute numbers (right) are shown. Results are presented as mean ± standard deviation of 3 independent experiments. Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare IgG vs α-AREG within Brca2+/+ or Brca2fl/fl groups in panel B and C (left); IgG vs α-AREG within Brca2+/+ or Brca2fl/fl groups for different subpopulations (Mac1+Gr1+, CD3ε+ or B220+ cells) in panel C (right); Areg+/+ vs Areg–/– in panel E and G; Areg+/+ vs Areg–/– at different time points (4, 8, 12, and 16 weeks after BMT) in panel F. ∗P < .05; ∗∗P < .01.
Inhibition of AREG by anti–AREG-neutralizing antibody or deletion of the Areg gene in LepR-Cre;Brca2fl/fl mice rescues HSC defects caused by AREG. (A) Schematic presentation of the experimental design. Systemic treatment of LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice with anti-AREG or IgG every other day for 5 days. Total BM cells and frequencies of total HSCs and mbHSCs were determined 4 weeks after treatment. (B) Systemic treatment of anti-AREG improves BM parameters of LepR-Cre;Brca2fl/fl mice. LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice were treated with 50 μg anti-AREG or IgG. The numbers of WBMCs/femur (left), HSCs (middle), and mbHSCs (right) cells were determined at 4 weeks after treatment (n = 7-8). Frequencies (left) and absolute numbers (right) are shown. (C) Anti-AREG improves BM niche function in LepR-Cre;Brca2fl/fl mice. A total of 1 × 104 WT CD45.1+ BM LSK cells along with 2 × 105 protector cells (CD45.2+) were transplanted into lethally irradiated LepR-Cre;Brca2+/+ or LepR-Cre;Brca2f/f recipient mice (CD45.2+), followed by 3 doses (every other day for 5 days) of 50 μg anti-AREG or IgG treatment 10 days after BMT (top). Donor (CD45.1+) cell engraftment (left) (frequencies and absolute numbers) and lineage differentiation (right) were determined by flow cytometry 16 weeks after BMT (bottom) (n = 6). (D) Schematic presentation of experimental design. WT SLAM was cocultured with BM CD45–LepR+ cells isolated from Areg+/+LepR-Cre;Brca2fl/fl or Areg–/–LepR-Cre;Brca2fl/fl mice, followed by CFU or BMT assay. (E) Deletion of Areg limits expansion of myeloid progenitors. Progenies of WT SLAM cells cocultured with BM CD45–LepR+ cells from Areg+/+LepR-Cre;Brca2fl/fl or Areg–/–LepR-Cre;Brca2fl/fl mice were subjected to CFU assay (n = 7). (F) Deletion of Areg improves the repopulating capacity of progenies from cocultured SLAM cells. Progenies (CD45.2+) described in panel E, along with 2 × 105 radio-protector cells, were transplanted into lethally irradiated BoyJ recipients (CD45.1+). Donor-derived chimera (CD45.2+) were measured using flow cytometry at different time points after BMT (n = 6). (G) Deletion of Areg improves long-term hematopoietic reconstitution of progenies from cocultured SLAM cells. WBMCs from the primary recipients (CD45.2+) described in panel F were pooled and transplanted into sublethally irradiated BoyJ recipients (CD45.1+). Donor-derived chimera (CD45.2+) were measured using flow cytometry 16 weeks after BMT (n = 6). Frequencies (left) and absolute numbers (right) are shown. Results are presented as mean ± standard deviation of 3 independent experiments. Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare IgG vs α-AREG within Brca2+/+ or Brca2fl/fl groups in panel B and C (left); IgG vs α-AREG within Brca2+/+ or Brca2fl/fl groups for different subpopulations (Mac1+Gr1+, CD3ε+ or B220+ cells) in panel C (right); Areg+/+ vs Areg–/– in panel E and G; Areg+/+ vs Areg–/– at different time points (4, 8, 12, and 16 weeks after BMT) in panel F. ∗P < .05; ∗∗P < .01.
Next, we performed reverse BMT, followed by systemic α-AREG or IgG administration 10 days after BMT (Figure 5C, top). α-AREG treatment significantly increased donor-derived chimera in LepR-Cre;Brca2fl/fl recipients compared with that in recipients treated with IgG (Figure 5C, bottom). Additionally, α-AREG treatment rescued myeloid skew, as previously observed in LepR-Cre;Brca2fl/fl mice (supplemental Figure 5C).
Genetic deletion of Areg in LepR-Cre;Brca2fl/fl mice45,62 significantly reduced AREG protein levels in the BM supernatant of Areg–/–LepR-Cre;Brca2fl/fl mice (supplemental Figure 5D). We, then, cocultured BM CD45–LepR+ cells isolated from Areg+/+LepR-Cre;Brca2fl/fl and Areg–/–LepR-Cre;Brca2fl/fl mice with WT SLAM cells for 5 days, followed by CFU and BMT assays (Figure 5D). Myeloid CFU numbers produced by WT SLAM cells cocultured with CD45–LepR+ cells from Areg–/–LepR-Cre;Brca2fl/fl mice were significantly reduced than those cocultured with control cells (Figure 5E). In addition, deletion of Areg in BM CD45–LepR+ cells markedly improved donor engraftment in mice that received transplantation with progenies from coculture with CD45–LepR+ cells of Areg–/–LepR-Cre;Brca2fl/fl mice compared with those cocultured with control cells (Figure 5F). Furthermore, the genetic deletion of Areg in LepR-Cre;Brca2fl/fl CD45–LepR+ cells also improved the long-term repopulating capacity of cocultured progenies in mice that received secondary transplantations (Figure 5G). Collectively, these data indicated that inhibition of AREG in LepR-Cre;Brca2fl/fl mice rescued HSC defects caused by excessive AREG production in the BM niche.
AREG activates PI3K/AKT/mTOR pathway and compromises HSC quiescence
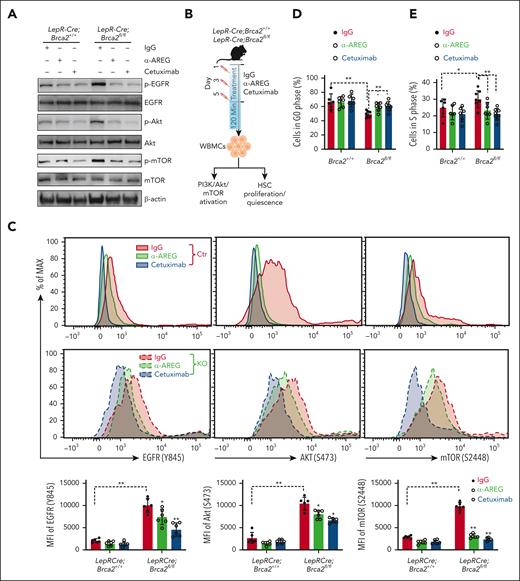
AREG can act in paracrine to active the PI3K/AKT/mTOR pathway in prostate cancer cells.60 We, then, measured AREG-PI3K/AKT/mTOR engagement between the niche cells and the HSCs. We, first, confirmed higher levels of AREG in the culture medium of MSCs from LepR-Cre;Brca2fl/fl mice, but not MSCs from LepR-Cre;Brca2+/+ mice, by ELISA (supplemental Figure 6A). By qPCR, we found that the AREG receptor, EGFR,63 was only expressed on cocultured SLAM cells, but not on MSCs (supplemental Figure 6B). To validate the specific AREG-EGFR engagement, we treated WT BM Lin–c-kit+ cells with 500 ng rAREG in the presence of an EGFR-specific inhibitor, cetuximab or IgG control, and examined PI3K/AKT/mTOR activation. We found that cetuximab significantly reduced the levels of phosphorylated EGFR (Y845), AKT (S473), and mTOR (S2448) in WT Lin–c-kit+ cells (supplemental Figure 6C). Consistently, WT Lin–c-kit+ cells cocultured with CD45–LepR+ cells isolated from LepR-Cre;Brca2fl/fl mice exhibited significantly higher levels of phosphorylated EGFR, AKT, and mTOR than those cocultured with control cells (Figure 6A), indicating the activation of the PI3K/AKT/mTOR pathway in Brca2-deficient CD45–LepR+ cells. Treatment with α-AREG or cetuximab remarkably suppressed the phosphorylation of EGFR, AKT, and mTOR in Lin–c-kit+ cells cocultured with CD45–LepR+ cells from LepR-Cre;Brca2fl/fl mice (Figure 6A), suggesting the direct engagement of AREG-EGFR between niche CD45–LepR+ cells and BM hematopoietic progenitor cells (HPCs).
AREG activates PI3K/AKT/mTOR pathway and compromises HSC quiescence. (A) Stroma-derived AREG activates PI3K/AKT/mTOR pathway in WT Lin–c-kit+ cells. WT Lin–c-kit+ cells were cocultured with BM CD45–LepR+ cells from LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice in the presence of IgG, anti-AREG, or cetuximab for 5 days. Immunoblotting was then performed using 150 000 cells per lane to analyze phosphorylated (p) EGFR and total EGFR; p-AKT and total AKT; p-mTOR and total mTOR; and β-actin. (B) Schematic presentation of the experimental design. LepR-Cre;Brca2+/+ and LepR-Cre;Brca2fl/fl mice were treated with 3 doses of IgG, anti-AREG, or cetuximab (50 μg each; every other day). WBMCs were isolated from mice 120 minutes after the last treatment for flow cytometry analysis of PI3K/AKT/mTOR activation in SLAM cells or HSC quiescence. (C) Anti-AREG or cetuximab suppresses PI3K/AKT/mTOR activation. LepR-Cre;Brca2+/+ and LepR-Cre;Brca2fl/fl mice were treated with IgG, anti-AREG, or cetuximab, as described in panel B, followed by flow cytometry analysis for phosphorylation of EGFR, AKT, and mTOR in SLAM cells 120 minutes later. Representative histogram (top) and quantifications (bottom) are shown (n = 6). (D-E) Anti-AREG or cetuximab treatment improves HSC quiescence and limits HSC proliferation. LepR-Cre;Brca2+/+ and LepR-Cre;Brca2fl/fl mice were treated with IgG, anti-AREG, or cetuximab, as described in panel B, followed by flow cytometry analysis for HSC quiescence by Hoechst/PY (D) or HSC proliferation by 5-bromo-2′-deoxyuridine or 4′,6-diamidino-2-phenylindole (E); n = 6 to 8. Results are presented as mean ± standard deviation of 3 independent experiments. Two-way ANOVA was performed to compare between genotypes (Brca2+/+ vs Brca2fl/fl) and treatments (IgG, α-AREG, or cetuximab), followed by t tests for comparing interested groups in panels C-E. ∗P < .05; ∗∗P < .01. KO, knockout; MFI, mean fluorescence intensity.
AREG activates PI3K/AKT/mTOR pathway and compromises HSC quiescence. (A) Stroma-derived AREG activates PI3K/AKT/mTOR pathway in WT Lin–c-kit+ cells. WT Lin–c-kit+ cells were cocultured with BM CD45–LepR+ cells from LepR-Cre;Brca2+/+ or LepR-Cre;Brca2fl/fl mice in the presence of IgG, anti-AREG, or cetuximab for 5 days. Immunoblotting was then performed using 150 000 cells per lane to analyze phosphorylated (p) EGFR and total EGFR; p-AKT and total AKT; p-mTOR and total mTOR; and β-actin. (B) Schematic presentation of the experimental design. LepR-Cre;Brca2+/+ and LepR-Cre;Brca2fl/fl mice were treated with 3 doses of IgG, anti-AREG, or cetuximab (50 μg each; every other day). WBMCs were isolated from mice 120 minutes after the last treatment for flow cytometry analysis of PI3K/AKT/mTOR activation in SLAM cells or HSC quiescence. (C) Anti-AREG or cetuximab suppresses PI3K/AKT/mTOR activation. LepR-Cre;Brca2+/+ and LepR-Cre;Brca2fl/fl mice were treated with IgG, anti-AREG, or cetuximab, as described in panel B, followed by flow cytometry analysis for phosphorylation of EGFR, AKT, and mTOR in SLAM cells 120 minutes later. Representative histogram (top) and quantifications (bottom) are shown (n = 6). (D-E) Anti-AREG or cetuximab treatment improves HSC quiescence and limits HSC proliferation. LepR-Cre;Brca2+/+ and LepR-Cre;Brca2fl/fl mice were treated with IgG, anti-AREG, or cetuximab, as described in panel B, followed by flow cytometry analysis for HSC quiescence by Hoechst/PY (D) or HSC proliferation by 5-bromo-2′-deoxyuridine or 4′,6-diamidino-2-phenylindole (E); n = 6 to 8. Results are presented as mean ± standard deviation of 3 independent experiments. Two-way ANOVA was performed to compare between genotypes (Brca2+/+ vs Brca2fl/fl) and treatments (IgG, α-AREG, or cetuximab), followed by t tests for comparing interested groups in panels C-E. ∗P < .05; ∗∗P < .01. KO, knockout; MFI, mean fluorescence intensity.
Next, we treated LepR-Cre;Brca2fl/fl mice with α-AREG,38 cetuximab,64 or IgG and determined PI3K/AKT/mTOR activation, cell quiescence, and proliferation (Figure 6B). Flow cytometry showed that 120 minutes was the optimal time point for both α-AREG and cetuximab to significantly reduce PI3K/AKT/mTOR activation in HSCs (supplemental Figure 6D). Indeed, systemic α-AREG or cetuximab treatment markedly reduced the phosphorylation of EGFR, AKT, and mTOR in SLAM cells from LepR-Cre;Brca2fl/fl mice 120 minutes after treatment compared with those treated with the IgG control (Figure 6C). SLAM cells from LepR-Cre;Brca2fl/fl mice were less quiescent and more proliferative than those from LepR-Cre;Brca2+/+ mice (Figure 6D,E; supplemental Figure 6E). The treatment of LepR-Cre;Brca2fl/fl mice with α-AREG or cetuximab significantly improved stem cell quiescence (Figure 6D) and limited HSC cycling (Figure 6E). We also performed a single-cell division assay65,66 and found that HSCs from α-AREG–treated LepR-Cre;Brca2fl/fl mice entered division significantly later and had fewer clones with >2 divisions than those from IgG-treated controls after 48 hours in an ex vivo culture (supplemental Figure 6F). Moreover, mTOR inhibitor (rapamycin) or PI3K inhibitor (LY294002)60 significantly improved HSC quiescence and reduced HSC cycling in LepR-Cre;Brca2fl/fl mice (supplemental Figure 6G-H). Taken together, these data indicate that niche AREG activates the PI3K/AKT/mTOR pathway and compromises HSC quiescence.
Effect of AREG on HSC maintenance in other DNA repair–deficient models and aged mice
Prolonged DNA damage accumulates due to a decreased DNA repair capacity.67 Aging is associated with accumulated DNA damage and compromised stem cell function.18,19,51,68,69 Because persistent DNA damage induces AREG expression in LepR+ cells deficient in Brca2, which in turn compromises HSC maintenance, we investigated the role of niche AREG in regulating HSC function in several other DNA repair–deficient mouse models and aged mice. Indeed, we observed prolonged accumulation of DNA damage in CD45–LepR+ cells of mice deficient of Fancd2 (a key component of the FA pathway46) and Atm (ataxia-telangiectasia mutated; the most upstream DDR kinases70) after IR (300 cGy; Figure 7A; supplemental Figure 7A). IR induced persistently higher levels of γ-H2AX in BM CD45–LepR+ cells from old mice than in those from young mice throughout the 32-hour post-IR period, during which IR-induced DNA damage declined rapidly (Figure 7A; supplemental Figure 7A). Importantly, IR induced high levels of Areg expression in CD45–LepR+ cells from Fancd2–/–, Atm–/–, and aged mice starting at 4 hours and remained elevated up to 32 hours after IR, as compared with those from WT or young control mice (Figure 7A). BM CD45–LepR+ cells from Fancd2–/–, Atm–/–, and aged mice induced significantly higher myeloid-biased progenitor activities of cocultured WT SLAM cells than those from WT or young control mice (Figure 7B). Furthermore, the progenies from WT SLAM cells cocultured with CD45–LepR+ cells from Fancd2–/–, Atm–/–, or aged mice gave rise to significantly lower donor-derived chimera than those cocultured with CD45–LepR+ cells from WT or young control mice in both primary (Figure 7C) and secondary recipients (Figure 7D; supplemental Figure 7B). Additionally, mice that received transplantation with the progenies of WT SLAM cells cocultured with the CD45–LepR+ cells from Fancd2–/–, Atm–/–, or aged mice showed a differentiation skew toward myeloid at the expense of lymphoid in multilineage hematopoietic cell reconstitution (supplemental Figure 7C). Finally, systemic treatment of Fancd2–/–, Atm–/–, and aged mice with α-AREG significantly increased BM total cell counts and HSC frequencies but reduced the frequencies of mbHSCs (Figure 7E; supplemental Figure 7D). AREG neutralization also increased common lymphoid progenitor frequencies, decreased common myeloid progenitor frequencies (supplemental Figure 7E), and reduced myeloid and increased lymphoid outputs (supplemental Figure 7F) in Fancd2–/–, ATM–/–, and aged mice. Finally, systemic treatment of Fancd2–/–, Atm–/–, and aged mice with α-AREG significantly increased donor-derived chimera (Figure 7F; supplemental Figure 7G) and rescued myeloid skew (supplemental Figure 7H) in mice that received transplantation with SLAM cells from α-AREG–treated mice compared with those from IgG-treated controls. Similar results were observed in Areg-KD MSCs from Fancd2–/– and ATM–/– mice (Figure 7G; supplemental Figure 7I-J). Taken together, these data indicate that AREG compromises hematopoiesis under conditions of DNA damage repair deficiency or aging.
Effect of AREG on HSC maintenance in other DNA repair–deficient models and aged mice. (A) IR induces persistent DNA damage and high levels of Areg expression in CD45–LepR+ cells from Fancd2–/–, Atm–/–, and aged mice. BM CD45–LepR+ cells from Fancd2–/–, Atm–/–, and aged mice were subjected to 300 cGy IR, followed by flow cytometry–based γ-H2AX analysis (left) (n = 8-9) and qPCR analysis of Areg transcripts (right) (n= 4) at different time points after IR treatment. One-way ANOVA followed by t tests were performed to compare different genotypes (WT, Fancd2–/–, or Atm–/–) separately by comparing the indicated time points (0, 2, 8, 16, and 32-hours after IR) for γ-H2AX MFI and relative Areg for different genotypes (WT, Fancd2–/–, or Atm–/–). Two-tailed unpaired t test or Wilcoxon rank sum test was performed in the indicated groups: young vs old at time points 0, 2, 4, 8, 16, or 32 hours after IR) for relative Areg expression in young and old mice. (B) Progenies from the coculture of WT SLAM cells with BM CD45–LepR+ cells from Fancd2–/–, Atm–/–, or aged mice produce a high number of myeloid CFUs. WT SLAM cells were cocultured with BM CD45–LepR+ cells from Fancd2–/–, Atm–/–, WT control mice, aged mice, or young control mice for 5 days. One hundred cocultured progenies were subjected to CFU assay (n = 6). One-way ANOVA was performed to compare different genotypes (WT, Fancd2–/–, or Atm–/–) in the left panel. Two-tailed, unpaired t test or Wilcoxon rank sum test was performed in the indicated groups (young vs aged) in the right panel. (C) Progenies from the coculture of WT SLAM cells with BM CD45–LepR+ cells from Fancd2–/–, Atm–/–, or aged mice show compromised hematopoietic repopulation. One thousand cocultured progeny cells (CD45.2+) from the coculture described in panel B, along with 2 × 105 radio-protector cells, were transplanted into lethally irradiated BoyJ recipients (CD45.1+). Donor-derived chimera (CD45.2+) were determined using flow cytometry at different time points after BMT (n = 6). One-way ANOVA, followed by t tests, was performed to compare different genotypes (WT, Fancd2–/–, or Atm–/–), separately comparing them at the indicated time points (4, 8, 12, or 16 weeks after BMT) in the left panel. In the right panel, 2-tailed, unpaired t test or Wilcoxon rank sum test was performed to compare young vs aged at different time points (4, 8, 12, or 16 weeks after BMT). (D) Progenies from coculture with BM CD45–LepR+ from Fancd2–/–, Atm–/–, and aged mice were defective in long-term repopulating. WBMCs from the recipients described in panel C were transplanted into sublethally irradiated BoyJ recipients. Donor-derived chimera (CD45.2+) were determined by flow cytometry 16 weeks after BMT (n = 6-7). One-way ANOVA was performed to compare genotypes (WT, Fancd2–/–, or Atm–/–) in the panel. Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare young vs aged in the right panel. (E) Systemic anti-AREG treatment improves HSC function in Fancd2–/–, Atm–/– (left), and aged (right) mice. Mice of the indicated genotypes were treated with 3 doses of anti-AREG or IgG (50 μg each; every other day for 5 days), followed by the analysis of BM cell counts, total HSCs, and mbHSCs at 4 weeks after treatment (n = 6). Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare IgG vs α-AREG for each genotype (WT, Fancd2–/–, or Atm–/–) (top) or within each group (young or old) (bottom). (F) Systemic anti-AREG treatment improves long-term repopulating of HSCs from Fancd2–/–, Atm–/– (left), and aged mice (right). One hundred SLAM cells from the mice described in panel E (CD45.2+) was transplanted into lethally irradiated BoyJ recipients (CD45.1+). Donor-derived chimera were measured 16 weeks after BMT. Two-tailed, unpaired t test or Wilcoxon rank sum test was performed to compare IgG vs α-AREG within each genotype (WT, Fancd2–/–, or Atm–/–) (left), or within each group (young or old (right). (G) Genetic knockdown of Areg in Fancd2–/– and Atm–/– MSCs improves the repopulating capacity of progenies from cocultured SLAM cells. MSCs from WT, Fancd2–/–, and Atm–/– mice were transduced with lentiviral particle expressing scramble short hairpin RNA or short hairpin RNA targeting Areg. Progenies of WT LSK cells (CD45.2+) cocultured with sorted GFP+ MSCs, along with 2 × 105 radio-protector cells, were transplanted into lethally irradiated BoyJ recipients (CD45.1+). Donor-derived chimera (CD45.2+) were measured by flow cytometry at different time points after BMT (n = 6). Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare IgG vs α-AREG within each genotype (WT, Fancd2–/–, or Atm–/–). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. shAreg, short hairpin Areg.
Effect of AREG on HSC maintenance in other DNA repair–deficient models and aged mice. (A) IR induces persistent DNA damage and high levels of Areg expression in CD45–LepR+ cells from Fancd2–/–, Atm–/–, and aged mice. BM CD45–LepR+ cells from Fancd2–/–, Atm–/–, and aged mice were subjected to 300 cGy IR, followed by flow cytometry–based γ-H2AX analysis (left) (n = 8-9) and qPCR analysis of Areg transcripts (right) (n= 4) at different time points after IR treatment. One-way ANOVA followed by t tests were performed to compare different genotypes (WT, Fancd2–/–, or Atm–/–) separately by comparing the indicated time points (0, 2, 8, 16, and 32-hours after IR) for γ-H2AX MFI and relative Areg for different genotypes (WT, Fancd2–/–, or Atm–/–). Two-tailed unpaired t test or Wilcoxon rank sum test was performed in the indicated groups: young vs old at time points 0, 2, 4, 8, 16, or 32 hours after IR) for relative Areg expression in young and old mice. (B) Progenies from the coculture of WT SLAM cells with BM CD45–LepR+ cells from Fancd2–/–, Atm–/–, or aged mice produce a high number of myeloid CFUs. WT SLAM cells were cocultured with BM CD45–LepR+ cells from Fancd2–/–, Atm–/–, WT control mice, aged mice, or young control mice for 5 days. One hundred cocultured progenies were subjected to CFU assay (n = 6). One-way ANOVA was performed to compare different genotypes (WT, Fancd2–/–, or Atm–/–) in the left panel. Two-tailed, unpaired t test or Wilcoxon rank sum test was performed in the indicated groups (young vs aged) in the right panel. (C) Progenies from the coculture of WT SLAM cells with BM CD45–LepR+ cells from Fancd2–/–, Atm–/–, or aged mice show compromised hematopoietic repopulation. One thousand cocultured progeny cells (CD45.2+) from the coculture described in panel B, along with 2 × 105 radio-protector cells, were transplanted into lethally irradiated BoyJ recipients (CD45.1+). Donor-derived chimera (CD45.2+) were determined using flow cytometry at different time points after BMT (n = 6). One-way ANOVA, followed by t tests, was performed to compare different genotypes (WT, Fancd2–/–, or Atm–/–), separately comparing them at the indicated time points (4, 8, 12, or 16 weeks after BMT) in the left panel. In the right panel, 2-tailed, unpaired t test or Wilcoxon rank sum test was performed to compare young vs aged at different time points (4, 8, 12, or 16 weeks after BMT). (D) Progenies from coculture with BM CD45–LepR+ from Fancd2–/–, Atm–/–, and aged mice were defective in long-term repopulating. WBMCs from the recipients described in panel C were transplanted into sublethally irradiated BoyJ recipients. Donor-derived chimera (CD45.2+) were determined by flow cytometry 16 weeks after BMT (n = 6-7). One-way ANOVA was performed to compare genotypes (WT, Fancd2–/–, or Atm–/–) in the panel. Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare young vs aged in the right panel. (E) Systemic anti-AREG treatment improves HSC function in Fancd2–/–, Atm–/– (left), and aged (right) mice. Mice of the indicated genotypes were treated with 3 doses of anti-AREG or IgG (50 μg each; every other day for 5 days), followed by the analysis of BM cell counts, total HSCs, and mbHSCs at 4 weeks after treatment (n = 6). Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare IgG vs α-AREG for each genotype (WT, Fancd2–/–, or Atm–/–) (top) or within each group (young or old) (bottom). (F) Systemic anti-AREG treatment improves long-term repopulating of HSCs from Fancd2–/–, Atm–/– (left), and aged mice (right). One hundred SLAM cells from the mice described in panel E (CD45.2+) was transplanted into lethally irradiated BoyJ recipients (CD45.1+). Donor-derived chimera were measured 16 weeks after BMT. Two-tailed, unpaired t test or Wilcoxon rank sum test was performed to compare IgG vs α-AREG within each genotype (WT, Fancd2–/–, or Atm–/–) (left), or within each group (young or old (right). (G) Genetic knockdown of Areg in Fancd2–/– and Atm–/– MSCs improves the repopulating capacity of progenies from cocultured SLAM cells. MSCs from WT, Fancd2–/–, and Atm–/– mice were transduced with lentiviral particle expressing scramble short hairpin RNA or short hairpin RNA targeting Areg. Progenies of WT LSK cells (CD45.2+) cocultured with sorted GFP+ MSCs, along with 2 × 105 radio-protector cells, were transplanted into lethally irradiated BoyJ recipients (CD45.1+). Donor-derived chimera (CD45.2+) were measured by flow cytometry at different time points after BMT (n = 6). Two-tailed unpaired t test or Wilcoxon rank sum test was performed to compare IgG vs α-AREG within each genotype (WT, Fancd2–/–, or Atm–/–). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. shAreg, short hairpin Areg.
Discussion
In adult BM, multiple niche cell populations from distinct BM zones regulate HSCs for long-term maintenance, proliferation, and differentiation.1-3,71 In this study, we investigated niche-derived factors in hematopoiesis and identified LepR+ cell–derived AREG as an important factor in regulating HSC function under conditions of DNA damage repair deficiency and aging. Several lines of evidence highlight our findings: (1) mice deficient of Brca2 specifically in BM LepR+ cells exhibit increased frequencies of total and myeloid-biased HSCs; (2) HSCs from LepR-Cre;Brca2fl/fl mice are defective in repopulating and give rise to myeloid-biased lineage differentiation in the recipients; (3) ex vivo culture of WT SLAM cells in the presence of rAREG or systemic rAREG treatment of C57BL/6 mice impairs HSC repopulation capacity, leading to HSC exhaustion; (4) AREG neutralization or genetic deletion of Areg gene in LepR-Cre;Brca2fl/fl mice rescues HSC defects; (5) mechanistically, niche-derived AREG activates the PI3K/AKT/mTOR pathway, promotes HSC cycling, and compromises HSC quiescence; (6) persistent DNA damage in other DNA repair–deficient or aged mice also induces excessive production of AREG in the BM niche LepR+ cells, which compromises HSC maintenance.
BRCA2 is a well-known breast cancer susceptibility gene, also known as FANCD1, which is one of the genes associated with FA, an inherited disorder associated with BM failure and leukemia.27 Compelling studies have shown that FA deficiency leads to severe defects in both the quantity and quality of HSCs,72,73 suggesting a critical role for FA proteins in regulating hematopoiesis. Approximately 3% to 5% of cases in FA are caused by biallelic mutations in the BRCA2 gene.74 Individuals heterozygous for BRCA2 mutations have an increased risk of inherited breast and ovarian cancer. However, the role of BRCA2 in HSC maintenance remains elusive. One study revealed some aspects of hematopoietic dysfunction in a mouse model of a hypomorphic mutation in Brca2/Fancd1 gene.28 Here, we show that mice deficient of Brca2 gene, specifically in niche LepR+ cells, exhibit an altered HSPC pool and compromised HSC function. In this context, our studies identified a novel role for niche BRCA2 in HSC maintenance and added another layer of understanding of FA proteins in regulating hematopoiesis.
Another intriguing finding of this study is that the deletion of Brca2 in CD45–LepR+ cells, but not in CD45–Osx+ or CD45–VE-cad+ cells, affected HSC function in mice (Figures 1 and 2). Using aging and several DNA damage repair–deficient mouse models, we further showed that persistent DNA damage induces AREG overproduction, specifically, in CD45–LepR+ cells but not in the other 2 key niche components in the BM (Figures 3 and 7; supplemental Figures 3 and 7). Recent studies have shown that stromal cells in adult BM that express LepR are a critical source of growth factors, including SCF, CXCL12, and pleiotrophin, for the maintenance of HSCs and early progenitors.8 In line with these studies, our current data highlights the importance of LepR+ niche cells and their secreted factors in HSC maintenance. Moreover, albeit some overlap between OSX-Cre and LepR-Cre expression,6-10 our identification of LepR+ niche–derived AREG as a negative regulator of HSC maintenance using the LepR-Cre–specific Brca2-deficient model suggests that, in the context of paracrine regulation of hematopoiesis, LepR+ niche cells could possess multifaceted functions, including supportive and impeding. Therefore, our findings add another layer to the current understanding of the causal relationship between persistent DNA damage accumulation in HSCs and accelerated failure of hematopoietic system under conditions of DNA repair deficiency and aging.75,76
The PI3K/AKT/mTOR pathway has diverse and important physiological functions.77-79 EGFR differentially modulates the PI3K/AKT/mTOR pathway under cellular stress conditions.80 Previous studies have also suggested that EGFR signaling regulates HSC regeneration after myelosuppressive injury.21 As the most abundant EGFR ligand, AREG is expressed in a variety of cell types during development and homeostasis and can be upregulated in response to diverse stimuli.29,30,38,81 Aberrantly activated AREG-EGFR signaling is required for the growth and survival of CRTC1-MAML2 fusion–positive mucoepidermoid carcinoma cells.82 However, the role of AREG in HSC maintenance remains elusive. Here, we showed that niche-derived AREG compromised HSC function (Figure 4). Inhibition of AREG in LepR-Cre;Brca2fl/fl mice rescues HSC defects (Figure 5). Mechanistically, the overproduction of AREG activates the PI3K/AKT/mTOR pathway, promotes HSC cycling, and compromises HSC quiescence (Figure 6). Although the potential role of hematopoietic AREG remains to be elucidated, this study demonstrated that LepR+ cell–derived AREG regulates HSC function through paracrine AREG-EGFR engagement between the niche and HSCs. In summary, our studyies identify an important niche factor negatively regulating HSC function under conditions of DNA repair deficiency and aging and underscore the therapeutic potential of AREG intervention in improving HSC function.
Acknowledgments
The authors thank S. H. Namekawa (University of California Davis) for Brca2fl/fl mice and Markus Grompe (Oregon Health & Sciences University) for Fancd2+/− mice. The authors also thank Hong Wang from the Biostatistics Facility at the University of Pittsburgh Medical Center (UPMC) Hillman Cancer Center for the statistical support. Schematic presentations were generated using BioRender.
This work is supported by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (grant R01HL151390) (W.D.). This project used the Hillman Animal Facility, which is supported, in part, by NIH, National Cancer Institute (grant P30CA047904). W.D. is a 2021-22 Hillman Senior Fellow for Innovative Cancer Research at the University of Pittsburgh.
Authorship
Contribution: L.W. performed the research and analyzed the data; Q.L., S.C., S.A., A.F.W., N.A., Z.J.G., J.J., and E.V.W. performed some of the research and assisted with data analysis; and W.D. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wei Du, Division of Hematology and Oncology, University of Pittsburgh School of Medicine, UPMC Hillman Cancer Center, 5117 Center Ave, Pittsburgh, PA 15213; e-mail: duw@upmc.edu.
References
Author notes
Information regarding reagents or mouse models generated in this study will be available on request from the corresponding author, Wei Du (duw@upmc.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal