In this issue of Blood, Wu et al1 have demonstrated that leptin receptor-expressing (LepR+) bone marrow (BM) stromal cells upregulate and secrete amphiregulin (AREG) in mice during aging and in mice deficient in the DNA repair gene Brca2. This increase in AREG promotes a decline in hematopoietic stem cell (HSC) repopulating capacity. In the current paradigm of hematopoiesis, niche-derived paracrine growth factors, such as stem cell factor, CXCL12, and pleiotrophin,2-4 provide essential support for HSC maintenance. The study by Wu et al adds importantly to this paradigm by demonstrating the contribution of a niche-derived paracrine factor in pathologic conditions, such as genotoxic injury, or during aging.
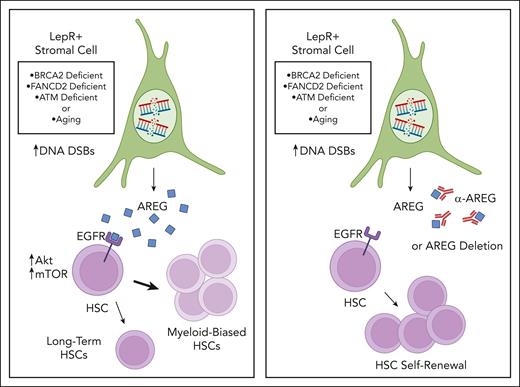
The authors found that deletion of the DNA repair gene Brca2 in BM osteoblasts, endothelial cells, or LepR+ stromal cells caused an increase in DNA double-stranded breaks in all cell types; however, derangements in hematopoiesis were only observed when Brca2 was deleted in LepR+ stromal cells. Specifically, mice with Brca2 deficiency in LepR+ stromal cells displayed increased phenotypic hematopoietic progenitors, HSCs, and myeloid-biased HSCs, coincident with a loss of HSC self-renewal capacity as measured in competitive transplantation assays.
Cytokine array analysis revealed increased levels of the epidermal growth factor receptor (EGFR) ligand, AREG, in the BM supernatant of mice with Brca2-deficient LepR stromal cells compared with controls. Additionally, following total body irradiation, Brca2-deficient stromal cells exhibited persistent DNA damage and increased Areg expression compared with Brca2+ stromal cells in irradiated, control mice. The authors then tested the direct effects of AREG on murine HSCs and showed that 10-day culture with AREG increased the percentages of phenotypic HSCs and myeloid-biased HSCs but caused a significant decline in long-term HSCs as measured by secondary transplantation assays and limiting dilution analysis. Systemic administration of AREG to wild-type mice caused similar increases in myeloid-biased HSCs and myeloid skewing at the expense of lymphoid production. Importantly, treatment of mice bearing Brca2-deficient stromal cells with a neutralizing anti-AREG antibody corrected the increased percentages of phenotypic HSCs and myeloid-biased HSCs compared with controls, and administration of anti-AREG to mice with Brca2-deficient stromal cells significantly increased donor cell engraftment following competitive transplantation of wild-type BM cells. In keeping with these findings, deletion of Areg in Brca2-deficient LepR+ stromal cells restored the capacity of these cells to support HSC maintenance in short-term culture, resulting in increased engraftment of progeny in transplanted mice compared with mice transplanted with HSCs cultured with Areg-expressing LepR+ stromal cells (see figure).
Schematic overview of AREG regulation of hematopoiesis in response to DNA repair defects or aging. DSB = double strand breaks. Illustration by Lisa Valicente, Cedars Sinai Medical Center. Images and shapes utilized from Biorender.com.
Schematic overview of AREG regulation of hematopoiesis in response to DNA repair defects or aging. DSB = double strand breaks. Illustration by Lisa Valicente, Cedars Sinai Medical Center. Images and shapes utilized from Biorender.com.
BM hematopoietic cells from mice bearing Brca2-deficient stromal cells displayed increased activation of EGFR and downstream effectors Akt and mammalian target of rapamycin (mTOR), whereas neutralization of AREG via systemic administration of anti-AREG suppressed AREG-mediated activation of EGFR/Akt/mTOR signaling. AREG-mediated induction of Akt/mTOR activation was also associated with decreased HSC quiescence, which was corrected by anti-AREG antibody or cetuximab, an anti-EGFR antibody, suggesting that AREG mediated these effects through EGFR activation.
The authors also showed that LepR+ stromal cells increased Areg expression when other DNA repair genes, specifically Fancd2 and Atm, were deleted, as well as in aged mice that harbored persistent DNA damage following irradiation. Further, wild-type BM HSCs cultured with Fancd2- or Atm-deficient stromal cells demonstrated decreased HSC repopulating capacity compared with HSCs cultured with control stromal cells; similarly, BM HSCs cultured with stromal cells from aged mice displayed decreased in vivo repopulating capacity compared with HSCs cultured with young stromal cells. Treatment of mice bearing Fancd2- or Atm-deficient stromal cells, as well as aged mice, with anti-AREG antibody partially corrected the HSC repopulating capacity defect in these animals.
The current study highlights the important role of DNA repair proteins, particularly BRCA2 (also known as Fanconi anemia D1 [FANCD1]), FANCD2, and ATM in maintaining the genomic and functional integrity of BM stromal cells in the HSC niche. Although up to 5% of Fanconi anemia cases are caused by mutations in the Brca2 gene, the current study provides new insight into the niche-specific function of these DNA repair proteins and their essential role in maintaining the overall integrity of the hematopoietic system.
This study raises an important mechanistic question as to how deficiencies in Brca2, Fancd2, or Atm, or the onset of aging, promote the increased transcription of Areg in BM stromal cells. BRCA2 is a tumor suppressor protein that maintains cellular genome integrity via function in homologous recombination, G2 checkpoint control, and protection of replication forks.5 BRCA1 is also a tumor suppressor protein that functions in DNA damage repair and transcriptional regulation.6 Interestingly, BRCA1 was shown to repress Areg gene expression via binding to the Areg promoter, and Brca1 deletion caused an increase in Areg transcription.7 It will be important to determine whether a common transcriptional regulation links the deficiencies of DNA repair genes and aging to Areg transcription or whether Areg expression can be induced by multiple different processes.
The results from Wu et al build importantly upon prior studies that have suggested a role for EGFR signaling in regulating HSC regeneration.8 Interestingly, treatment with AREG, an EGFR ligand, caused a decline in HSC repopulating capacity, whereas EGF has been shown to promote HSC regeneration in irradiated mice.8 These opposing results may reflect fundamental differences in the binding and signaling capacity of AREG and EGF through EGFR,9 an EGFR “dosage” effect similar to that described for Wnt signaling in HSCs,10 or the probability that HSCs respond uniquely to cytokines in the context of homeostasis versus injury.
Conflict-of-interest: J.P.C. declares no competing financial interest.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal