Clinical and biological heterogeneity challenges the development of curative treatment for mantle cell lymphoma (MCL). In their timely article in this issue of Blood, Decombis et al describe a targetable mechanism of acquired resistance to combined inhibition of Bruton tyrosine kinase (BTK), BCL2, and CD20—a clinical strategy currently under intensive clinical investigation—and animate the development of biologically informed therapy to overcome this resistance.1
There exists no unified frontline therapy for MCL. Although multiple chemoimmunotherapy regimens can benefit younger, fit patients, none is curative. In addition, the older age of most patients, the adverse predictive implications of TP53 mutations with chemotherapy, and the dependence of MCL on B-cell receptor (BCR) and NF-κB signaling have motivated the development of less intensive induction regimens incorporating BTK and other targeted inhibitors.2 Approximately 20% of patients achieve complete remission (CR) on Ibrutinib or venetoclax monotherapy in the relapsed and refractory (R/R) setting,3,4 but progression invariably occurs. Preclinical synergy motivated ibrutinib/venetoclax combination therapy in the ABT-199 and Ibrutinib in MCL (AIM) trial,5 in which CRs by positron emission tomography (PET) occurred in 62% of patients with R/R MCL. Poor responses remained, however, with inactivating alterations in genes of the switch/sucrose nonfermentable (SWI-SNF) complex skewing survival signals through upregulation of BCL2L1 (Bcl-xL).6 In addition, Chiron et al identified microenvironmental cues upregulating NF-κB and BCL2 family peptides, including Bcl-xL, reducing ex vivo apoptotic priming and venetoclax sensitivity.7 This resistance was reverted ex vivo by targeting CD20 with obinutuzumab. These findings motivated multiple groups, including these authors, to initiate studies combining BTK, BCL2, and CD20 inhibition in the R/R and frontline settings, including the OAsIs (obinutuzumab, ABT-199, ibrutinib; NCT02558816),8 BOVen (zanubrutinib, obinutuzumab, venetoclax; NCT03824483),9 and AVO trials (acalabrutinib, venetoclax, obinutuzumab; NCT04855695). Although such targeted combination regimens induce high rates of PET CR (>80%) among treatment-naïve patients, the frequency of primary refractory disease and early progression mandates the search for biological mechanisms of resistance to inform the development of next-generation therapies.
This is precisely what Decombis et al sought to do. They identified 3 of 5 patients who progressed on OAsIs and underwent targeted panel sequencing who harbored treatment-emergent or treatment-expanded CARD11 gain-of-function (GOF) mutations, supporting the selective advantage of CARD11 activation. To elucidate downstream effects, single-cell RNA sequencing was applied to serial specimens from a hallmark patient in whom a preexisting CARD11G123S subclone expanded on OAsIs treatment to form the dominant clone at progression. Transcriptomic analysis revealed enrichment of transcriptional and translational activity as well as RelA and NF-κB1 expression at progression, with evidence of clonal expansion of an NF-κB1 transcriptional cluster, corroborating the advantage of CARD11 GOF mutations signaling through NF-κB1. When assessed within 5 additional OAsIs participants, expression of the NF-κB1 cluster was increased in MCL cells from refractory patients vs responders and in a subset of cells from a patient who rapidly relapsed after an initial response. The top 16 genes in this cluster were then treated as a focused resistance signature called the MCL_R16.
MCL_R16 clinical associations mirrored those of the full cluster. In addition, its expression was magnified in MCL cells from lymph nodes vs peripheral blood, reflecting the authors’ previous findings regarding lymphoid microenvironment-mediated resistance. Strikingly, the MCL_R16 correlated with inferior survival when retrospectively applied to 2 independent cohorts of patients treated with chemoimmunotherapy, suggesting possible generalized resistance biology.
The Bcl-2 family member BCL2A1 emerged as one of the genes most strongly associated with progression on OAsIs. Functional experiments in cell line models demonstrated dependence of BCL2A1 activity on BCR and CARD11-BCL10-MALT1 (CBM) complex signaling. Critically, mutationally activated but not wild-type (WT) CARD11 could bypass BCR dependence through direct induction of BCL2A1, implicating the CBM-BCL2A1 axis in ibrutinib resistance. Additional in vitro experiments revealed BCL2A1 upregulation after in vitro treatment with venetoclax or other BH3 peptides, correlating with the acquisition of apoptotic priming to BCL2A1. Taken together, these data support a model in which BCL2A1 upregulation promotes resistance to inhibition of BTK, BCL2, or the combination and highlight the potential for targeting MALT1 to overcome resistance mediated by CARD11 GOF mutations (see figure).
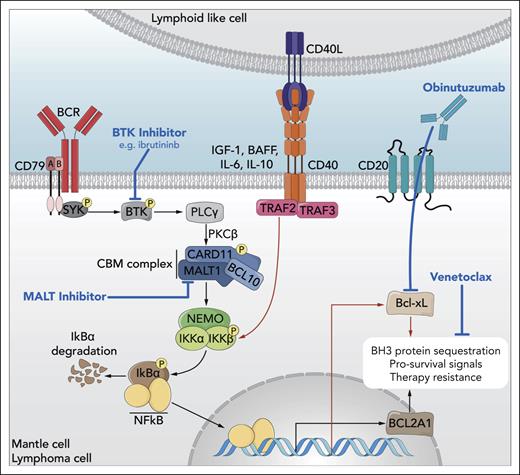
Elucidation of oncogenic signaling in mantle cell lymphoma highlights opportunities for rational therapeutic combinations. B-cell receptor (BCR) signaling via Bruton tyrosine kinase (BTK) activates the CARD11-BCL10-MALT1 (CBM) complex, which induces NF-κB nuclear translocation and transcriptional activation of target genes, including antiapoptotic BCL2A1. Cell-extrinsic input from lymphoid-like cells in the microenvironment also signals through NF-κB to upregulate antiapoptotic genes, like BCL2L1 (Bcl-xL). These and other biological programs converge to promote targeted therapy resistance in mantle cell lymphoma but are subject to targeted inhibition by ibrutinib (BTK), venetoclax (BCL2 family peptides), and obinutuzumab (CD20, indirectly downregulating Bcl-xL). Here, Decombis et al have identified gain-of-function mutations in CARD11 that confer autonomous activation of the CBM complex, thereby circumventing pharmacologic inhibition of BTK with ibrutinib and potentially mediating resistance to combined BTK-BCL2-CD20 triplet therapy, as in the OAsIs trial. This illuminated the potential for therapeutic targeting of MALT1 to suppress the CBM–NF-κB–BCL2A1 resistance axis in mantle cell lymphoma as part of future rational combination regimens. BAFF, B-cell activating factor; IGF, insulin-like growth factor; IL, interleukin; PKC, protein kinase C; PLC, phospholipase C; SYK, spleen associated tyrosine kinase. Professional illustration by Somersault18:24.
Elucidation of oncogenic signaling in mantle cell lymphoma highlights opportunities for rational therapeutic combinations. B-cell receptor (BCR) signaling via Bruton tyrosine kinase (BTK) activates the CARD11-BCL10-MALT1 (CBM) complex, which induces NF-κB nuclear translocation and transcriptional activation of target genes, including antiapoptotic BCL2A1. Cell-extrinsic input from lymphoid-like cells in the microenvironment also signals through NF-κB to upregulate antiapoptotic genes, like BCL2L1 (Bcl-xL). These and other biological programs converge to promote targeted therapy resistance in mantle cell lymphoma but are subject to targeted inhibition by ibrutinib (BTK), venetoclax (BCL2 family peptides), and obinutuzumab (CD20, indirectly downregulating Bcl-xL). Here, Decombis et al have identified gain-of-function mutations in CARD11 that confer autonomous activation of the CBM complex, thereby circumventing pharmacologic inhibition of BTK with ibrutinib and potentially mediating resistance to combined BTK-BCL2-CD20 triplet therapy, as in the OAsIs trial. This illuminated the potential for therapeutic targeting of MALT1 to suppress the CBM–NF-κB–BCL2A1 resistance axis in mantle cell lymphoma as part of future rational combination regimens. BAFF, B-cell activating factor; IGF, insulin-like growth factor; IL, interleukin; PKC, protein kinase C; PLC, phospholipase C; SYK, spleen associated tyrosine kinase. Professional illustration by Somersault18:24.
In vitro experiments demonstrated that MALT1 protease inhibition abrogated BCL2A1 promoter and NF-κB activity as well as expression of the MCL_R16 signature in cell lines. Although BTK inhibitors downregulated the MCL_R16 signature only in CARD11 WT models, MALT1 inhibitors exerted these effects independent of CARD11 mutation status. These data pointed to the potential for MALT1/BCL2 combination therapy. Indeed, therapeutic synergy was observed in cell lines treated with venetoclax and MALT1 protease inhibitors independent of CARD11 mutation status but dependent on BCL2A1. These findings were validated within patient-derived in vivo models of CARD11-mutated, BTK inhibitor-resistant MCL, in which venetoclax and the MALT1 inhibitor JNJ-67856633 inhibited tumor growth with synergistic effect.
In summary, this work provides important new insights into the mechanism by which CARD11 mutations mediate resistance to BTK inhibitor-inclusive therapies, such as in OAsIs. Their identification of BCL2A1 as a BCR/CBM-dependent mediator of this resistance, which could be overcome by MALT1 inhibition, informed the rational combination of MALT1 and BCL2 inhibitors, which exert synergistic activity within models of BTK inhibitor-resistant MCL. These efforts will certainly continue and, in fact, complement recent findings on the preclinical activity of combined MALT1 and BTK inhibition for ibrutinib-resistant MCL.10 Looking ahead, one can envision a wider spectrum of MALT1 inhibitor combinations, although this will require immune-competent model systems to understand the consequences of MALT1 modulation on regulatory T cells and other key immune subsets.
Additional impact from this work arises from data regarding the mutational (CARD11 GOF) and transcriptional (MCL_R16) correlates of adverse clinical outcomes, which may nucleate novel predictive biomarkers for patients treated with OAsIs-like and other regimens. Although these resistance features were identified in a small number of patients and require considerable optimization and prospective validation, the biological and clinical heterogeneity of MCL underscores the importance of this endeavor. Most important, this work by Decombis et al further illuminates the complex and dynamic biology of MCL in the context of a novel therapeutic regimen, enabling the development of biologically informed, tolerable treatment strategies to circumvent resistance in this recalcitrant disease.
Conflict-of-interest disclosure: M.A.M. has received research support from Genentech/Roche and Generate Biomedicines and has served on scientific advisory boards for Novartis and CancerModels.org. None relate directly to this commentary.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal