Key Points
Genome-wide CRISPR screens identify the IL-1R pathway as a novel oncogenic driver in pC ALK− ALCL, which is activated by IL-1α autocrine.
IL-1R pathway cooperates with JAK signaling, and the JAK2/IRAK1 dual inhibitor pacritinib exhibits strong activities against pC ALK− ALCL.
Abstract
Anaplastic large cell lymphoma (ALCL), a subgroup of mature T-cell neoplasms with an aggressive clinical course, is characterized by elevated expression of CD30 and anaplastic cytology. To achieve a comprehensive understanding of the molecular characteristics of ALCL pathology and to identify therapeutic vulnerabilities, we applied genome-wide CRISPR library screenings to both anaplastic lymphoma kinase positive (ALK+) and primary cutaneous (pC) ALK− ALCLs and identified an unexpected role of the interleukin-1R (IL-1R) inflammatory pathway in supporting the viability of pC ALK− ALCL. Importantly, this pathway is activated by IL-1α in an autocrine manner, which is essential for the induction and maintenance of protumorigenic inflammatory responses in pC-ALCL cell lines and primary cases. Hyperactivation of the IL-1R pathway is promoted by the A20 loss-of-function mutation in the pC-ALCL lines we analyze and is regulated by the nonproteolytic protein ubiquitination network. Furthermore, the IL-1R pathway promotes JAK-STAT3 signaling activation in ALCLs lacking STAT3 gain-of-function mutation or ALK translocation and enhances the sensitivity of JAK inhibitors in these tumors in vitro and in vivo. Finally, the JAK2/IRAK1 dual inhibitor, pacritinib, exhibited strong activities against pC ALK− ALCL, where the IL-1R pathway is hyperactivated in the cell line and xenograft mouse model. Thus, our studies revealed critical insights into the essential roles of the IL-1R pathway in pC-ALCL and provided opportunities for developing novel therapeutic strategies.
Introduction
Anaplastic large cell lymphoma (ALCL), characterized by elevated expression of CD30 and anaplastic cytology,1 is a subgroup of peripheral T-cell lymphomas, a diverse group of mature T-cell and natural killer cell neoplasms, accounting for 10% to 15% of non-Hodgkin lymphoma diagnoses in the United States. Two variants have been described in ALCL: anaplastic lymphoma kinase (ALK)–positive (ALK+) and ALK-negative (ALK−).2,3 ALK+ ALCL is characterized by recurrent chromosomal translocations affecting the ALK gene and several different partners, most frequently the nucleophosmin (NPM) gene.4,5 ALK− ALCLs, including systemic ALK−, primary cutaneous ALCL (pC-ALCL), and recently noticed breast implant–associated (BIA) ALCL that arises in the seroma cavity surrounding breast implants,6 are a heterogeneous group of disorders defined as tumors similar to ALK+ ALCL but lacking ALK expression. Systemic ALK− ALCLs often have an aggressive clinical course and poor outcome,7 and pC-ALCLs usually have a good prognosis and low mortality, although partial or complete regression may still occur in these patients. Thus, there is an urgent need to develop targeted therapies based on the oncogenic pathways aberrantly activated in these T-cell malignancies.
Several advances have been made in understanding the etiology of ALCL. Using ALCL cell line models and primary samples, we and others have discovered the involvement of several critical factors in ALCL, particularly in the ALK+ subtype, including AKT/mammalian target of rapamycin (MTOR),8-10 AP-1 family members cJUN, JUNB, and BATF/BATF3,11-14 and IRF4.11,15-17 Of particular interest is the JAK/STAT pathway, which was recently revealed to play a central role in the pathogenesis of several subtypes of ALK− ALCL.18-24 Activating mutations of JAK1 and/or STAT3 genes are frequent in primary ALK− ALCL cases, which lead to the constitutive activation of the JAK/STAT3 pathway, representing a critical driver in these tumors. Although these findings have provided critical insights into ALK− ALCL pathogenesis, some key gaps in knowledge remain, as addressed herein.
Although STAT3 is activated in nearly all ALK− ALCLs,18 the basis for its activation remains unclear. JAK/STAT3 mutations are evident in only ∼30% to 40% ALK− ALCL tumors,19,21-24 suggesting that an activated upstream signal(s) is required to promote the JAK/STAT3 pathway in these tumors. Moreover, observed gain-of-function JAK/STAT3 mutations only facilitate and augment signals from upstream but alone are insufficient to initiate ALCL cell proliferation.18 The identity of the upstream pathway(s) to activate JAK/STAT and support ALK− ALCL is critical, but completely unknown. Likewise, the epistatic relationships between this pathway(s) and the recurrent genetic lesions in ALCL are unclear, which are needed to determine the clinical application of targeting JAK/STAT for a broader patient population.
Addressing these gaps will enable a complete understanding of the molecular pathogenesis of ALCL to identify and exploit critical therapeutic vulnerabilities. In this study, we decided to use genome-wide CRISPR library screenings to address these unresolved questions and gain a complete understanding of the unique factors in ALK− ALCL pathogenesis.
Methods
Refer to supplemental Experimental Procedures, available on the Blood website.
Genome-wide depletion (loss-of-function) CRISPR library screen
The genome-wide human CRISPR-Cas9 library was described in.25 In brief, 200 million cells were infected with the pooled lentiviral lymphoma signaling sgRNA library at a multiplicity of infection of 0.3 and selected with 2 μg/mL puromycin. The blue fluorescence protein (BFP) percentage was examined to ensure the completion of selection. Doxycycline was then added to induce sgRNAs expression. After 21 days of in vitro culture, doxycycline-induced cells and uninduced cells were collected for genomic DNA extraction (Qiagen) (refer to supplemental Experimental Procedures for details).
Tumor model and therapy study
For the xenograft tumor model, the MAC1 cells were subcutaneously injected into the flanks of female nonobese diabetic/severe combined IL2Rgammanull immunodeficient (NOD scid gamma) mice. Tumor growth was monitored by measuring tumor size in 2 orthogonal dimensions. All animals were maintained in the Laboratory Animal Health Facility at Fox Chase Cancer Center, and all experiments were performed in accordance with procedures approved by the Fox Chase Cancer Center Animal Care and Use Committee (refer to supplemental Experimental Procedures for details).
Tumor biopsy specimens were obtained from patients with ALK ALCL. All human samples were anonymously coded as stipulated by the Declaration of Helsinki. All samples were studied according to a protocol approved by the institutional review board of Fox Chase Cancer Center and Rhode Island Hospital.
Results
Genome-wide CRISPR library screens identify the interleukin-1R (IL-1R) pathway as a novel oncogenic driver in pC ALK− ALCL
To fill the gaps in knowledge and identify unique factors involved in ALCL pathogenesis, we performed genome-wide CRISPR functional screenings using an optimized minimal genome-wide human CRISPR-Cas9 library,25 which targets 18 761 genes with 2 optimal single-guide-RNAs (sgRNAs) per gene. A depletion (loss-of-function) screen was performed in the ALK− ALCL line MAC1 and the ALK+ ALCL line Karpas299 (Figure 1A). The genes required explicitly for each line were identified in the loss-of-function screens (Figure 1A; supplemental Figure 1; supplemental Table 1). The essential genes supporting both MAC1 and Karpas299 survival included the most well-established molecules, including STAT3, AKT/MTOR pathway components (MTOR, RPTOR, and PDPK1) (Figure 1A). AP-1 family members JUNB and BATF/BATF3 were identified as strong oncogenic hits in the ALK+ ALCL line Karpas299, as expected (Figure 1A, right). These results demonstrated the validity of the screen setup.
Genome-wide CRISPR library screens identify the IL-1R pathway as a novel oncogenic driver in pC-ALCL. (A) Outline of the workflow of the depletion CRISPR library screens in lymphoma cell lines (upper). Overview of the genome-wide CRISPR screen results (lower). Shown are the volcano plots of all genes in 2 lines. y-axis indicates the significance (−log10p); x-axis indicates the log2 fold change (sgRNA on/off). The dashed lines indicate P = .05 and log2(FC) = ±4. (B) Top significantly enriched cellular pathways identified among the oncogenic hits identified from MAC1 or Karpas299 genome-wide CRISPR screens [log2(fold change) < −4 and P < .05] in panel A. (C) Left, diagrammatic representation of the workflow for the sgRNA toxicity assay. Right, ALCL lines were transduced with IRAK1, MYD88, IL1R1, IL1RAP, or control sgRNAs along with green fluorescent protein (GFP). The fraction of viable GFP+/sgRNA+ cells relative to the live cell fraction is plotted at the indicated times after sgRNA induction, normalized to day 0 values. Error bars denote standard deviation (SD) of triplicates. P was calculated comparing day 0 to each time point of indicated sgRNA induction in MAC1 and MAC2A lines. ∗∗P < .01.
Genome-wide CRISPR library screens identify the IL-1R pathway as a novel oncogenic driver in pC-ALCL. (A) Outline of the workflow of the depletion CRISPR library screens in lymphoma cell lines (upper). Overview of the genome-wide CRISPR screen results (lower). Shown are the volcano plots of all genes in 2 lines. y-axis indicates the significance (−log10p); x-axis indicates the log2 fold change (sgRNA on/off). The dashed lines indicate P = .05 and log2(FC) = ±4. (B) Top significantly enriched cellular pathways identified among the oncogenic hits identified from MAC1 or Karpas299 genome-wide CRISPR screens [log2(fold change) < −4 and P < .05] in panel A. (C) Left, diagrammatic representation of the workflow for the sgRNA toxicity assay. Right, ALCL lines were transduced with IRAK1, MYD88, IL1R1, IL1RAP, or control sgRNAs along with green fluorescent protein (GFP). The fraction of viable GFP+/sgRNA+ cells relative to the live cell fraction is plotted at the indicated times after sgRNA induction, normalized to day 0 values. Error bars denote standard deviation (SD) of triplicates. P was calculated comparing day 0 to each time point of indicated sgRNA induction in MAC1 and MAC2A lines. ∗∗P < .01.
To better understand the cellular pathways that are essential for the growth of these cells, we carried out Biocarta pathway enrichment analysis for the oncogenic hits identified from MAC1 or Karpas299 genome-wide CRISPR screens [log2(fold change) < −4 and P < .05], and the top 10 most significantly enriched cellular pathways (P < .05) in each line were shown in Figure 1B. Notably, MAC1 and Karpas299 shared some common essential pathways, including DNA replication, chromatin remodeling, spliceosomal assembly, protein translation, and the AKT/mTOR pathway (Figure 1B; supplemental Figure 1). Surprisingly, in addition to those shared pathways, the IL-1R pathway and NF-κB signaling were highly enriched among oncogenic hits only in MAC1, but not in Karpas299 (Figure 1B; supplemental Figure 1). Indeed, a complete set of components in the IL-1R and canonical NF-κB signaling pathways, including IRAK1, MYD88, IL1R1, IL1RAP(IL1R3), ECSIT, TRAF6, MAP3K7(TAK1), IKBKB, IKBKG, and RELA, were identified as strong oncogenic hits only in MAC1 (Figure 1A).
To confirm the CRISPR screen results, we depleted IL-1R pathway components IRAK1, MYD88, IL1R1, and IL1RAP by 2 individual sgRNAs (supplemental Figure 2A) in a large panel of ALCL cell lines covering ALK+ and ALK− subgroups and measured the sgRNAs toxicities in these cells (Figure 1C). Depletions of the IL-1 pathway components’ expression were selectively toxic to pC ALK− ALCL cell lines MAC1 and MAC2A but not to ALK+ ALCL controls (Figure 1C). In line with these observations, IL-1R pathway inhibition promoted apoptosis in these 2 lines (supplemental Figure 2B). Interestingly, 2 ALK− ALCL cell lines, TLBR1 and TLBR2, belonging to the BIA subtype of ALK− were only slightly sensitive to IL-1R pathway inhibition compared with the pC ALK− ALCL lines (Figure 1C). To further investigate the role of the IL-1R pathway in all subtypes of ALK− ALCL, we use another ALK− ALCL cell line, FEPD, belonging to the systemic ALK− subtype. FEPD was unable to be transduced with an inducible Cas9; we therefore silenced MYD88 expression in a panel of ALCL cell lines using a highly specific MYD88 short hairpin RNA (shRNA)26 (supplemental Figure 2C). Consistent with the sgRNA results, although silencing MYD88 expression showed some general toxicity in all ALK− ALCL cell lines, including FEPD, the effects were much more potent in pC ALK− ALCL ones (supplemental Figure 2D). Therefore, our screen unexpectedly identified the IL-1R pathway as a novel pathway that plays a critical role in supporting the viability of pC ALK− ALCL.
IL-1α expression and secretion maintain protumorigenic signaling in pC ALK− ALCL cell lines and primary cases
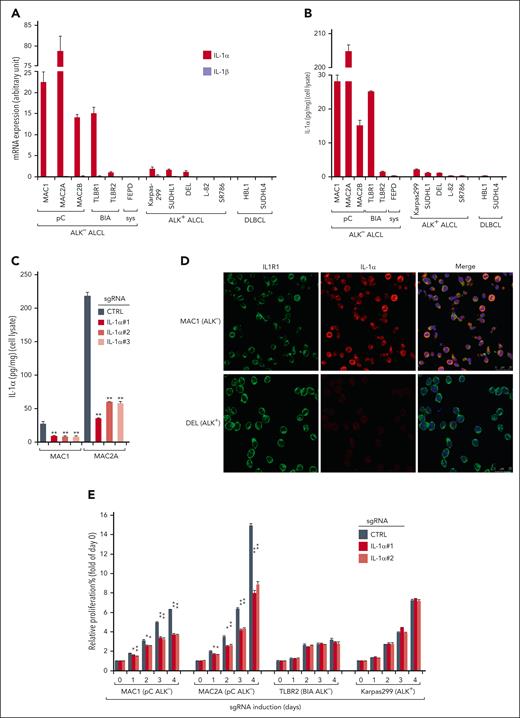
The novel oncogenic role of IL-1R signaling in pC ALK− ALCL identified by a genome-wide CRISPR screen (Figure 1) led us to evaluate the expression of IL-1R ligands IL-1α and IL-1β in a completed panel of lymphoma cell lines. Remarkably, although IL-1β messenger RNA (mRNA) expression was absent in all the lines, relatively elevated levels of IL-1α mRNA expression were observed in all pC ALK− ALCL lines and in BIA ALCL line TLBR1, but not in FEPD, ALK+ lines L-82 and SR786, or diffuse large B-cell lymphoma controls (Figure 2A). Of note, certain levels of IL-1α expression were also found in TLBR2 and 3 ALK+ ALCL lines, Karpas299, SUDHL1, and DEL, although at a much lower level (Figure 2A). These results were further confirmed by IL-1α in-cell enzyme-linked immunosorbent assays (ELISA) (Figure 2B). Interestingly, most of the IL-1α molecule in these cells appeared to be likely membrane-bound and was not released into the media, as it was mostly detectable by in-cell ELISA and released to the supernatant upon the inflammasome activator nigericin treatment (supplemental Figure 3A), in line with the unique ability of this cytokine.27,28 The specificity of the in-cell ELISA was confirmed by sgRNAs targeting IL-1α (Figure 2C). In agreement, a clear colocalization of IL-1α with IL1R1 was detected in pC ALK− ALCL line MAC1 (Figure 2D). Accordingly, depletion of IL-1α using sgRNAs significantly impaired growth of pC ALK− ALCL lines with higher IL-1α production, but only had limited effects in TLBR2 and Karpas299 (Figure 2E). Notably, adding IL-1α neutralizing antibody to the supernatant in pC-ALCL only has limited effects (supplemental Figure 3B) compared with IL-1α depletion, supporting the idea that most of the IL-1α molecule was not released into the media. Together, all these data indicate that IL-1α expression is common in ALCL, although the levels among subtypes are variable, and that it has important implications for the induction and maintenance of prosurvival responses in pC-ALCL.
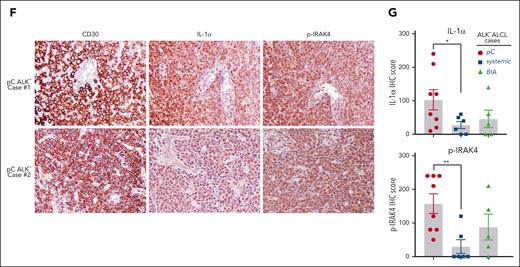
IL-1α production in ALK− ALCL cell lines and primary cases. (A) Relative IL-1α and IL-1β mRNA expressions, measured by real-time polymerase chain reaction (PCR) and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) values, in indicated lymphoma cell lines. Error bars denote standard error of the mean (SEM) of triplicates. (B) IL-1α production, measured by in-cell ELISA (cell lysate) and normalized to cell numbers, in indicated lymphoma cell lines. Error bars denote SEM of triplicates. (C) MAC1 and MAC2A lines were transduced with IL-1α or ctrl sgRNAs, selected, and expression induced. IL-1α production was measured by in-cell ELISA as in panel B. P was calculated comparing the ctrl sgRNA (sgCTRL) and sgIL-1α groups; ∗∗P < .01. Error bars denote SEM of triplicates. (D) Immunofluorescence confocal microscopy analysis of the distribution of endogenous IL1R1 and IL-1α in the indicated lines. Antibodies used are indicated on the top. (E) Indicated ALCL lines were transduced with Ctrl or IL-1α sgRNAs and selected. Relative proliferations upon sgRNA induction were measured by the Promega CellTiter Cell Proliferation Assay (MTS) and normalized to the Ctrl sgRNA groups. P was calculated comparing sgCTRL and sgIL-1α groups; ∗P < .05; ∗∗P < .01. Error bars denote SEM of triplicates. (F) Immunohistochemistry (IHC) of IL-1α, p-IRAK4, and CD30 expression of 2 pC ALK− ALCL cases. Sections were examined microscopically using 100× original magnification. The depicted images are representative of the 19 ALK− ALCL cases examined. (G) IL-1α and p-IRAK4 IHC scores (detailed at supplemental Table 2) in 3 subgroups of primary ALK− ALCL cases. ∗P < .05; ∗∗P < .01.
IL-1α production in ALK− ALCL cell lines and primary cases. (A) Relative IL-1α and IL-1β mRNA expressions, measured by real-time polymerase chain reaction (PCR) and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) values, in indicated lymphoma cell lines. Error bars denote standard error of the mean (SEM) of triplicates. (B) IL-1α production, measured by in-cell ELISA (cell lysate) and normalized to cell numbers, in indicated lymphoma cell lines. Error bars denote SEM of triplicates. (C) MAC1 and MAC2A lines were transduced with IL-1α or ctrl sgRNAs, selected, and expression induced. IL-1α production was measured by in-cell ELISA as in panel B. P was calculated comparing the ctrl sgRNA (sgCTRL) and sgIL-1α groups; ∗∗P < .01. Error bars denote SEM of triplicates. (D) Immunofluorescence confocal microscopy analysis of the distribution of endogenous IL1R1 and IL-1α in the indicated lines. Antibodies used are indicated on the top. (E) Indicated ALCL lines were transduced with Ctrl or IL-1α sgRNAs and selected. Relative proliferations upon sgRNA induction were measured by the Promega CellTiter Cell Proliferation Assay (MTS) and normalized to the Ctrl sgRNA groups. P was calculated comparing sgCTRL and sgIL-1α groups; ∗P < .05; ∗∗P < .01. Error bars denote SEM of triplicates. (F) Immunohistochemistry (IHC) of IL-1α, p-IRAK4, and CD30 expression of 2 pC ALK− ALCL cases. Sections were examined microscopically using 100× original magnification. The depicted images are representative of the 19 ALK− ALCL cases examined. (G) IL-1α and p-IRAK4 IHC scores (detailed at supplemental Table 2) in 3 subgroups of primary ALK− ALCL cases. ∗P < .05; ∗∗P < .01.
We then investigated primary patient samples for evidence of IL-1α protein expression and IL-1R signaling activation by evaluating IL-1α and p-IRAK4 (IRAK4 kinase activation) through immunohistochemical staining in 19 primary cases diagnosed with ALK− ALCL (supplemental Table 2; Figure 2F, 2 representative cases are shown), including 8 in pC, 6 in systemic, and 5 in BIA subtypes. Notably, medium-to-high levels of IL-1α expression were detected in 8 out of 19 primary cases (Figure 2G). Moreover, elevated IL-1α expression appeared to be directly correlated with the activation of IL-1R signaling (p-IRAK4 level), which was detected in 12 out of 19 ALK− ALCL cases. Importantly, elevated IL-1α expression and IRAK4 phosphorylation were more prevalent in pC cases compared with those in systemic or BIA cases (Figure 2G), in line with the cell line results. Furthermore, elevated expression of IL1RAP was observed in pC cases (3 out of 8), but rarely in systemic or BIA cases (supplemental Figure 3C-D). Indeed, in a previously published data set in which survival data was available in ALK− ALCL,29 patients with ALK− ALCL with higher expressions of IL1RAP showed significant survival disadvantages compared with those with lower expressions (P = .032) (supplemental Figure 3E). Thus, we conclude that IL-1α signaling is frequently upregulated in primary pC ALK− ALCL and likely represents a novel driver with adverse prognoses in these tumors.
IL-1R pathway maintains prosurvival NF-κB activation in pC ALK− ALCL with abnormal IL-1α expression
To determine the cellular pathways regulated by the IL-1R pathway in pC-ALCL, we profiled gene expression changes upon sgRNA depletion of IL-1α in the IL-1α high-expression pC ALK− lines MAC1 and MAC2A and, for comparison, in the IL-1α low-expression ALK− line TLBR2 (Figure 3A, upper). Gene expression signatures enriched among IL-1α targets in both MAC1 and MAC2A lines were highly overlapped, and the shared signatures were shown in Figure 3B. Among those signatures, a signature of NF-κB activation was the most enriched (P < .0001), indicating that NF-κB is the primary pathway downstream of IL-1R activation in these cells (Figure 3A-B; supplemental Figure 4). Interestingly, signatures that reflect autocrine IL-6 and/or IL-2 signaling were enriched among genes that were activated by IL-1α (P < .0001; Figure 3A-B; supplemental Figure 4). Finally, a signature of the inflammatory-response pathway was also significantly represented among IL-1α–activated genes. In contrast, none of these pathways were enriched among genes that were activated by IL-1α in BIA ALK− line TLBR2 (supplemental Figure 5A-C). Thus, the IL-1R pathway likely promotes NF-κB signaling and JAK-STAT activation in pC ALCLs that have elevated IL-1α expression.
IL-1R pathway mediates NF-κB activation in ALK− ALCL with higher IL-1α. (A) The workflow of using RNA-seq analysis to profile gene expression changes upon sgRNAs depletion of IL-1α in ALCL lines (upper). IL-1α–upregulated genes grouped according to gene expression signatures, in both MAC1 and MAC2A cell lines (lower). (B) Gene set enrichment analysis (GSEA) of gene expression signatures that were enriched among IL-1α upregulated genes in both MAC1 (upper) and MAC2A (lower) lines. Only the overlapped and shared signatures among all sgRNA transduced samples in both cell lines were shown. (C) NF-κB-driven luciferase reporter–engineered MAC1 (upper) or Karpas299 (lower) lines were transduced with indicated sgRNAs. Relative NF-κB reporter activities were measured after 4 days of induction. P was calculated comparing sgCTRL and the indicated sgRNA groups; ∗P < .05; ∗∗P < .01. (D) Indicated ALCL lines were transduced with MYD88, IL-1α, or Ctrl sgRNAs, selected, and expression induced. Lysates were analyzed by immunoblotting for the indicated proteins. (E) Indicated ALCL lines were transduced with MYD88 or Ctrl shRNAs, selected, and expression induced. Lysates were analyzed by immunoblotting for the indicated proteins. (F) MAC1 line was stable engineered with IKKβ WT, IKKβ S176/180E, or empty control, then transduced with MYD88 or Ctrl shRNAs along with GFP. The fraction of viable, GFP+/shRNA+ cells relative to the live cell fraction is plotted at the indicated times after shRNA induction, normalized to day 0 values. Error bars denote SD of triplicates. P was calculated by comparing the IKKβ S176/180E and IKKβ WT engineered lines with MYD88 shRNA transduction. ∗∗P < .01.
IL-1R pathway mediates NF-κB activation in ALK− ALCL with higher IL-1α. (A) The workflow of using RNA-seq analysis to profile gene expression changes upon sgRNAs depletion of IL-1α in ALCL lines (upper). IL-1α–upregulated genes grouped according to gene expression signatures, in both MAC1 and MAC2A cell lines (lower). (B) Gene set enrichment analysis (GSEA) of gene expression signatures that were enriched among IL-1α upregulated genes in both MAC1 (upper) and MAC2A (lower) lines. Only the overlapped and shared signatures among all sgRNA transduced samples in both cell lines were shown. (C) NF-κB-driven luciferase reporter–engineered MAC1 (upper) or Karpas299 (lower) lines were transduced with indicated sgRNAs. Relative NF-κB reporter activities were measured after 4 days of induction. P was calculated comparing sgCTRL and the indicated sgRNA groups; ∗P < .05; ∗∗P < .01. (D) Indicated ALCL lines were transduced with MYD88, IL-1α, or Ctrl sgRNAs, selected, and expression induced. Lysates were analyzed by immunoblotting for the indicated proteins. (E) Indicated ALCL lines were transduced with MYD88 or Ctrl shRNAs, selected, and expression induced. Lysates were analyzed by immunoblotting for the indicated proteins. (F) MAC1 line was stable engineered with IKKβ WT, IKKβ S176/180E, or empty control, then transduced with MYD88 or Ctrl shRNAs along with GFP. The fraction of viable, GFP+/shRNA+ cells relative to the live cell fraction is plotted at the indicated times after shRNA induction, normalized to day 0 values. Error bars denote SD of triplicates. P was calculated by comparing the IKKβ S176/180E and IKKβ WT engineered lines with MYD88 shRNA transduction. ∗∗P < .01.
Confirming the RNA-seq results, CRISPR depletion of IL-1α, MYD88, IRAK1, IL1R1, or IL1RAP almost completely inhibited NF-κB reporter activities in MAC1 (Figure 3C, upper). By contrast, in Karpas299, the depletion of IL-1R pathway components affected NF-κB activity to a much lower degree (Figure 3C, lower). Adding human IL-1α significantly reverted the effect of IL-1α depletion on NF-kB inhibition (supplemental Figure 5D), attesting to the specificity. In line with these results, IL-1α or MYD88 CRISPR depletion significantly diminished the phosphorylation of IκBα (NF-κB activation) and NF-κB p65 subunit DNA binding activities in pC ALK− lines and, to a lesser extent, also in ALK+ lines (Figure 3D; supplemental Figure 5E). Silencing of MYD88 expression using shRNA also impaired NF-κB activation in MAC1, but not in the systemic ALK− ALCL cell line FEPD (Figure 3E). Finally, ectopic expression of the constitutively active IKKβ mutant S177/181E largely mitigated the effects of MYD88 shRNA on MAC1 cell viability, whereas expression of the IKKβ wild type (WT) failed to do so (Figure 3F).
IL-1R pathway is regulated by the nonproteolytic protein ubiquitination network in pC ALK− ALCL
Unlike activated B-cell-like diffuse large B-cell lymphoma (ABC DLBCL),26 genetic aberrations of MYD88 have not been found in ALCL, indicating that IL-1R signaling is activated in an intracrine manner that maintains local inflammatory responses. However, it is still possible that the IL-1R pathway cooperates with recurrent genetic lesions in ALCL, especially those that regulate NF-κB and inflammatory-response pathways. We therefore reanalyzed the RNA sequencing results of all available ALK− ALCL cell lines18 (supplemental Table 3), and the mutation patterns of genes related to the inflammatory pathways are displayed in Figure 4A. Our analyzed sequence results are consistent with previous studies, where genes in the JAK-STAT pathway were frequently mutated in ALK− ALCL lines (Figure 4A). However, A20 (TNFAIP3), a potent anti-inflammatory protein that inhibits NF-κB, was found to be inactivated by frameshift mutations in the 3 clonally related pC ALK− MAC lines with abnormal IL-1α expression (Figure 4A). The A20 frameshift mutations were confirmed by Sanger sequencing (supplemental Figure 6A), and loss of A20 protein expression was confirmed by immunoblotting (Figure 4B). To evaluate the expression pattern of A20 in primary T-cell lymphoma cases, we analyzed an integrated whole-genome mRNA expression microarray data set comprising a large cohort of 417 T-cell lymphoma samples and T cells from 61 healthy donors.29 Our analysis revealed that A20 expression is significantly silenced in T cells from patients with ALK− (P = .000113) and ALK+ ALCL (P = .00000009) than in those from healthy donors (Figure 4C). Thus, the frequent deletion or downregulation of A20 in cell lines and primary cases indicates its tumor-suppressor role for ALCL. Importantly, using the same data set, patients with ALK− ALCL with higher expression of A20 showed significant survival advantages as compared with those with lower expressions (P = .016) (Figure 4D).
The nonproteolytic protein ubiquitination network in ALCL. (A) Distribution of mutations in genes related to the inflammatory pathways of ALK−ALCL cell lines, obtained from RNA-seq analysis. (B) A20 protein expression in indicated ALCL lines. (C) TNFAIP3 (A20) mRNA expression in 417 T-cell lymphoma samples (144 peripheral T-cell lymphomas [PTCL] NOS, 127 AITL, 69 ALK− ALCL, 56 ALK+ ALCL, 21 ATLL) and 61 T cells from healthy donors from publicly available microarray data set. t tests were performed between the control normal T-cell group and the other PTCL subtypes, and a P < .05 is considered statistically significant. (D) Overall survival in ALK− ALCL with higher or lower expression values of A20. The threshold (cut-point value for high or low-expression group) was set using maximally selected rank statistics with the “survminer” R package. The cut-point value has been determined as 8.29134 (log2 expression), and the number of patients in the high-expression and low-expression groups is 29 and 8, respectively. (E) NF-κB–driven luciferase reporter–engineered MAC1 line was transduced with inducible lentiviral constructs encoding A20 WT, C103A, and C779/782A complementary DNAs or empty control. Relative NF-κB reporter activities were measured after 1 day of induction. (F) NF-κB–driven luciferase reporter–engineered MAC1 line was transduced with indicated sgRNAs. Relative NF-κB reporter activities were measured after 4 days of induction. (G) MAC1 cells were transduced with RNF31 or ctrl sgRNAs, selected, and expression induced. Cell lysates were subjected to biotin-labeled M1-specific TUBE binding and streptavidin purification and analyzed by immunoblotting. (H) MAC1 cells were transduced with RNF31 or ctrl sgRNAs, selected, and expression induced. IRAK1 immunoprecipitations or total lysates from these lines were immunoblotted for the indicated proteins. In panels E-F, error bars denote SEM of triplicates. P was calculated comparing sgCTRL and the indicated sgRNA groups or comparing empty vector and A20 expression groups; ∗∗P < .01.
The nonproteolytic protein ubiquitination network in ALCL. (A) Distribution of mutations in genes related to the inflammatory pathways of ALK−ALCL cell lines, obtained from RNA-seq analysis. (B) A20 protein expression in indicated ALCL lines. (C) TNFAIP3 (A20) mRNA expression in 417 T-cell lymphoma samples (144 peripheral T-cell lymphomas [PTCL] NOS, 127 AITL, 69 ALK− ALCL, 56 ALK+ ALCL, 21 ATLL) and 61 T cells from healthy donors from publicly available microarray data set. t tests were performed between the control normal T-cell group and the other PTCL subtypes, and a P < .05 is considered statistically significant. (D) Overall survival in ALK− ALCL with higher or lower expression values of A20. The threshold (cut-point value for high or low-expression group) was set using maximally selected rank statistics with the “survminer” R package. The cut-point value has been determined as 8.29134 (log2 expression), and the number of patients in the high-expression and low-expression groups is 29 and 8, respectively. (E) NF-κB–driven luciferase reporter–engineered MAC1 line was transduced with inducible lentiviral constructs encoding A20 WT, C103A, and C779/782A complementary DNAs or empty control. Relative NF-κB reporter activities were measured after 1 day of induction. (F) NF-κB–driven luciferase reporter–engineered MAC1 line was transduced with indicated sgRNAs. Relative NF-κB reporter activities were measured after 4 days of induction. (G) MAC1 cells were transduced with RNF31 or ctrl sgRNAs, selected, and expression induced. Cell lysates were subjected to biotin-labeled M1-specific TUBE binding and streptavidin purification and analyzed by immunoblotting. (H) MAC1 cells were transduced with RNF31 or ctrl sgRNAs, selected, and expression induced. IRAK1 immunoprecipitations or total lysates from these lines were immunoblotted for the indicated proteins. In panels E-F, error bars denote SEM of triplicates. P was calculated comparing sgCTRL and the indicated sgRNA groups or comparing empty vector and A20 expression groups; ∗∗P < .01.
A20 is a ubiquitin-modifying enzyme that suppresses NF-κB activation.30 Therefore, A20 loss is likely to support IL-1α–induced NF-κB activation in pC-ALCL. To test this, A20 null ALCL line MAC1 was reconstituted with A20 WT as well as A20 mutants that disable its deubiquitinating or linear-ubiquitin–binding ability (A20 C103A and C779/782A, respectively).31 Re-expression of A20 WT, compared with the C103A or C779/782A forms, resulted in significant downregulation of NF-κB activity in MAC1 (Figure 4E; supplemental Figure 6B).
The A20 C779/782A linear-ubiquitin–binding mutant failed to inhibit MAC1 NF-κB activation, indicating the role of the linear-ubiquitin chain assembly complex (LUBAC) in these cells.32-35 In line with this result, depletion of RNF31 (the main E3 subunit of LUBAC) largely impaired NF-κB activities in MAC1 (Figure 4F; supplemental Figure 6C). We then assessed the linear ubiquitination status of the IKKγ subunit (NEMO), the primary substrate of RNF31, by using linear-ubiquitin (M1)–specific tandem ubiquitin-binding entities (M1-TUBE). Our analysis revealed that NEMO is constitutively hypermodified by linear ubiquitin chains, and RNF31 depletion substantially reduced the level of ubiquitinated NEMO bound by the M1-TUBE (Figure 4G; supplemental Figure 6D). In the IL-1R pathway, nonproteolytic ubiquitination mediates the binding of NEMO-IKK to IRAK1, which is essential for downstream NF-κB activation.36-38 In our studies, IRAK1 was found to bind to NEMO in MAC1 cells constitutively, but depletion of RNF31 or the specific LUBAC inhibitor HOIPIN-839,40 inhibited this binding (Figure 4H; supplemental Figure 6E).
The epistatic relationships between JAK- STAT3 mutation and IL-1R pathway in ALCL
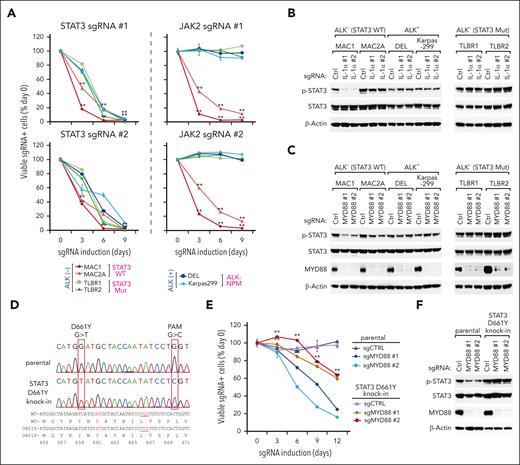
Interestingly, the 3 clonally related pC ALK− ALCL MAC lines with the A20 frameshift mutation and higher IL-1α expression carry WT STAT3 (Figure 4A). Given the fact that only the IL-1α high-expression pC-ALCL lines depend on IL-1R signaling to survive, it is likely that the IL-1R signaling induces autocrine secretion of proinflammatory cytokines that drive JAK kinase activation and eventually turn on STAT3, whereas ALCL lines carrying STAT3 gain-of-function mutations or the NPM-ALK oncoprotein that strongly activates STAT341 are less addicted to this autocrine signaling. Of note, the MAC lines harbor a PCM1-JAK2-fusion (Figure 4A), which may increase their sensitivity to IL-1α induced inflammatory cytokines.
To test these, we designed sgRNAs targeting STAT3 or JAK2 (supplemental Figure 7A) and assessed their toxicities in a panel of ALCL lines. STAT3 depletion was highly toxic to all lines tested, including those carrying WT STAT3 (Figure 5A, left). Similarly, C188-9, a new and effective STAT3 inhibitor targeting the SH2 domain,42 was highly toxic in all ALCL lines we tested (supplemental Figure 7B). However, depletion of JAK2 was only toxic to the STAT3 WT lines but had no effects on STAT3 mutant lines or ALK+ ALCL (Figure 5A, right). In contrast, CRISPR depletion of IL-1α or MYD88 impaired STAT3 activation in 2 STAT3 WT lines but not in lines carrying STAT3 mutations or NPM-ALK (Figure 5B-C; supplemental Figure 7C-D). Importantly, using CRISPR site-specific knockin technology, we created a reformed MAC1 cell line that carries the TLBR2 STAT3 D661Y mutation (Figure 5D). Compared with the parental control, the STAT3 D661Y knockin cell line was much more resistant to the effects of MYD88 or IL-1α sgRNAs on cell viability (Figure 5E; supplemental Figure 7E-F) and STAT3 activation (Figure 5F; supplemental Figure 7G).
Cooperation of the JAK- STAT3 mutation and the IL-1R pathway in ALCL. (A) Indicated ALCL lines were transduced with STAT3 or JAK2 sgRNAs along with GFP. The fraction of viable GFP+/sgRNA+ cells relative to the live cell fraction is plotted at the indicated times after sgRNA induction, normalized to day 0 values. Error bars denote SD of triplicates. P was calculated comparing day 0 to each time point of indicated sgRNA induction in the MAC1 and MAC2A lines. ∗∗P < .01. (B-C) Indicated ALCL lines were transduced with MYD88, IL-1α, or ctrl sgRNAs, selected, and expression induced. Lysates were analyzed by immunoblotting for the indicated proteins. (D) Using CRISPR site-specific knockin to generate a MAC1 line carrying the STAT3 D661Y mutation. The 661st amino acid in WT and mutated STAT3 sequences and their coding sequences are marked red. To prevent the continuous DNA cutting by the Cas9 protein, the guiding RNA targeting protospacer adjacent motif (PAM) was also switched from “TGG” to “TCG” to produce a silent mutation. (E) MAC1 parental or STAT3 D661Y knockin lines were transduced with MYD88 or ctrl sgRNAs along with GFP. The fraction of viable GFP+/sgRNA+ cells relative to the live cell fraction is plotted at the indicated times after sgRNA induction, normalized to day 0 values. Error bars denote SD of triplicates. P was calculated by comparing the parental and STAT3 D661Y knockin lines with the indicated MYD88 sgRNA transduction. ∗∗P < .01. (F) MAC1 parental or STAT3 D661Y knockin lines were transduced with MYD88 or ctrl sgRNAs, selected, and expression induced. Lysates were analyzed by immunoblotting for the indicated proteins.
Cooperation of the JAK- STAT3 mutation and the IL-1R pathway in ALCL. (A) Indicated ALCL lines were transduced with STAT3 or JAK2 sgRNAs along with GFP. The fraction of viable GFP+/sgRNA+ cells relative to the live cell fraction is plotted at the indicated times after sgRNA induction, normalized to day 0 values. Error bars denote SD of triplicates. P was calculated comparing day 0 to each time point of indicated sgRNA induction in the MAC1 and MAC2A lines. ∗∗P < .01. (B-C) Indicated ALCL lines were transduced with MYD88, IL-1α, or ctrl sgRNAs, selected, and expression induced. Lysates were analyzed by immunoblotting for the indicated proteins. (D) Using CRISPR site-specific knockin to generate a MAC1 line carrying the STAT3 D661Y mutation. The 661st amino acid in WT and mutated STAT3 sequences and their coding sequences are marked red. To prevent the continuous DNA cutting by the Cas9 protein, the guiding RNA targeting protospacer adjacent motif (PAM) was also switched from “TGG” to “TCG” to produce a silent mutation. (E) MAC1 parental or STAT3 D661Y knockin lines were transduced with MYD88 or ctrl sgRNAs along with GFP. The fraction of viable GFP+/sgRNA+ cells relative to the live cell fraction is plotted at the indicated times after sgRNA induction, normalized to day 0 values. Error bars denote SD of triplicates. P was calculated by comparing the parental and STAT3 D661Y knockin lines with the indicated MYD88 sgRNA transduction. ∗∗P < .01. (F) MAC1 parental or STAT3 D661Y knockin lines were transduced with MYD88 or ctrl sgRNAs, selected, and expression induced. Lysates were analyzed by immunoblotting for the indicated proteins.
IL-1R pathway is modulated by JAK-STAT3 signaling and contributes to the JAK inhibitor treatment sensitivity in pC-ALCL
IL1R1 and IL1RAP, the receptors of IL-1α, are among the top upregulated genes in primary ALCL,43 which is likely to be regulated by STAT3.17 Analyses of the IL1R1 and IL1RAP genes revealed a series of highly conserved STAT3-binding sites (supplemental Figure 8A). Consistent with these observations, our chromatin immunoprecipitation-coupled real-time polymerase chain reaction experiments demonstrated that STAT3 bound avidly to DNA fragments (∼300 bp) containing the IL1R1 transcription start site (S-1245 and S-965) and the IL1RAP enhancer region (S+812) in ALCL lines (Figure 6A; supplemental Figure 8B), supporting that STAT3 directly mediates IL1R1 and IL1RAP gene expression in ALCL. Indeed, STAT3 deletion diminished IL1R1 and IL1RAP mRNA transcription in all ALCL lines we tested (Figure 6B; supplemental Figure 8C). In contrast, JAK2 only regulated the expression of IL1R1 and IL1RAP in pC ALK− ALCL lines in which IL-1R pathways were hyperactivated but not in ALK+ and BIA ALCL (Figure 6C; supplemental Figure 8D), in line with our previous results. Similar results can be achieved using a highly specific JAK inhibitor, ruxolitinib (Figure 6D; supplemental Figure 8E). Therefore, JAK-STAT3 also contributes to the constitutive activation of the IL-1R pathway through a feed-forward loop.
IL-1R pathway contributes to the JAK inhibitor treatment sensitivity in pC-ALCL. (A) Chromatin IPs from the indicated antibodies were subjected to real-time PCR analysis for candidate STAT3-binding regions in the IL1R1 and IL1RAP loci in the indicated ALCL lines. (B-C) Indicated ALCL lines were transduced with STAT3, JAK2, or Ctrl sgRNAs, selected, and expression induced, IL1R1 and IL1RAP expressions were measured by real-time PCR. (D) Indicated ALCL lines were treated with the JAK inhibitor ruxolitinib at the indicated concentrations for 24 hours, and IL1R1 and IL1RAP expressions were measured. (E) Viability of indicated cell lines after treatment with AS2444697, ruxolitinib, or both (left). Formal calculation of synergism between AS2444697 and ruxolitinib (right). Positive highest-single-agent (HSA) synergy score values indicate synergy. (F) NSG mice bearing MAC1 xenografts were treated with AS2444697, ruxolitinib, the combination of AS2444697 and ruxolitinib, as well as vehicle controls. Tumor growth was measured as a function of tumor volume. Error bars denote SEM. (G-H) Tumor weight (G) and size (H) at the treatment end point. In panels A-D, error bars denote SEM of triplicates. P was calculated comparing sgCTRL and the indicated sgRNA groups or comparing untreated and treated groups; ∗P < .05; ∗∗P < .01. In panels F-G, error bars denote SEM. ∗P < .05; ∗∗P < .01.
IL-1R pathway contributes to the JAK inhibitor treatment sensitivity in pC-ALCL. (A) Chromatin IPs from the indicated antibodies were subjected to real-time PCR analysis for candidate STAT3-binding regions in the IL1R1 and IL1RAP loci in the indicated ALCL lines. (B-C) Indicated ALCL lines were transduced with STAT3, JAK2, or Ctrl sgRNAs, selected, and expression induced, IL1R1 and IL1RAP expressions were measured by real-time PCR. (D) Indicated ALCL lines were treated with the JAK inhibitor ruxolitinib at the indicated concentrations for 24 hours, and IL1R1 and IL1RAP expressions were measured. (E) Viability of indicated cell lines after treatment with AS2444697, ruxolitinib, or both (left). Formal calculation of synergism between AS2444697 and ruxolitinib (right). Positive highest-single-agent (HSA) synergy score values indicate synergy. (F) NSG mice bearing MAC1 xenografts were treated with AS2444697, ruxolitinib, the combination of AS2444697 and ruxolitinib, as well as vehicle controls. Tumor growth was measured as a function of tumor volume. Error bars denote SEM. (G-H) Tumor weight (G) and size (H) at the treatment end point. In panels A-D, error bars denote SEM of triplicates. P was calculated comparing sgCTRL and the indicated sgRNA groups or comparing untreated and treated groups; ∗P < .05; ∗∗P < .01. In panels F-G, error bars denote SEM. ∗P < .05; ∗∗P < .01.
JAK-STAT3 signaling was found downstream of IL-1R activation, and importantly, its activity can also promote the expression of IL-1 receptors in ALCL. Thus, blocking the IL-1R pathway represents an effective way to promote JAK inhibitor sensitivity in pC-ALCL. To test this concept, we applied a highly selective IRAK4 inhibitor, AS2444697, which has demonstrated encouraging clinical potential.44 In MAC1 and MAC2A lines, AS2444697 treatment significantly diminishes IRAK4 phosphorylation, demonstrating on-target suppression (supplemental Figure 9A). We then treated MAC1 and MAC2A lines with AS2444697 in combination with the FDA-approved JAK inhibitor ruxolitinib (Figure 6E, left). In both lines, tumor cells were killed more efficiently with the combination of AS2444697 and ruxolitinib than with either drug alone. Significantly, these combinational effects appeared to be synergistic when assessed using the mathematical algorithm described previously45 (Figure 6E, right). Importantly, in the MAC1 xenograft mouse model, oral treatment with ruxolitinib alone slowed the growth of established tumors compared with vehicle treatment (Figure 6F). However, the combination of AS2444697 and ruxolitinib treatment further arrested the tumor growth (combination vs ruxolitinib only, P < .01 at days 6, 8, and 10 of treatment) (Figure 6F). Furthermore, the effectiveness of this combination for the established tumors was confirmed by tumor weight and size at the treatment end point (Figure 6G-H). At the doses used, this combination was also well tolerated by mice, with no change in body weight observed (supplemental Figure 9B).
The JAK2/IRAK1 dual inhibitor pacritinib is selectively toxic for IL-1R pathway–dependent pC-ALCL cell lines and xenograft models
Our results strongly support the notion that simultaneously targeting the IL-1R pathway and JAK2 will be ideal for pC ALCLs in which the IL-1R pathway is hyperactivated. Pacritinib, a recently FDA-approved drug for patients with myelofibrosis, is a novel oral kinase inhibitor with high specificity for both JAK2 and IRAK1.46-52 In MAC1 cells, pacritinib treatment was able to reduce both IRAK1 and STAT3 phosphorylation, demonstrating the on-target suppression by this drug (Figure 7A; supplemental Figure 9C). To investigate the therapeutic potential, we determined the viability of ALCL lines after treatment with pacritinib (Figure 7B). In all 3 pC ALK− ALCL lines, pacritinib was toxic in a dose-dependent manner. By contrast, pacritinib had little toxicity in 2 systemic ALK− ALCL lines or 5 ALK+ ALCL lines. Of note, pacritinib also had modest toxicity in 2 BIA ALCL lines at the highest dosage, consistent with the sgRNA results (Figure 1C). To quantitatively assess the blocking potency of pacritinib in ALCL lines, we measured the half-maximal inhibitory concentration accordingly (Figure 7C). In line with our previous results, pacritinib was about 10 times more potent in pC ALK− ALCL cells in which the IL-1R pathway is hyperactivated (Figure 7C right), supporting its evaluation in vivo.
Targeting IRAK1/JAK2 using pacritinib in pC ALK− ALCL cell lines and xenograft mouse models. (A) Indicated ALCL lines were treated with pacritinib at the indicated concentrations for 24 hours. Lysates were analyzed by immunoblotting for the indicated proteins. (B) Indicated ALCL lines were treated with pacritinib at the indicated concentrations for 3 days. Viability was measured by an MTS assay and normalized to DMSO-treated cells. Error bars denote SEM of triplicates. (C) The half-maximal inhibitory concentration (IC50) of pacritinib in ALCL lines. The IC50 was calculated using GraphPad prism (right). (D) NSG mice bearing MAC1 xenografts were treated with pacritinib or vehicle control. Tumor growth was measured as a function of tumor volume. Error bars denote SEM. ∗P < .05; ∗∗P < .01. (E-F) Tumor weight (E) and size (F) in pacritinib and vehicle treatment groups at the treatment end point. ∗∗P < .01. (G) Model of the action of the IL-1R pathway in pC ALK− ALCL presented in this study.
Targeting IRAK1/JAK2 using pacritinib in pC ALK− ALCL cell lines and xenograft mouse models. (A) Indicated ALCL lines were treated with pacritinib at the indicated concentrations for 24 hours. Lysates were analyzed by immunoblotting for the indicated proteins. (B) Indicated ALCL lines were treated with pacritinib at the indicated concentrations for 3 days. Viability was measured by an MTS assay and normalized to DMSO-treated cells. Error bars denote SEM of triplicates. (C) The half-maximal inhibitory concentration (IC50) of pacritinib in ALCL lines. The IC50 was calculated using GraphPad prism (right). (D) NSG mice bearing MAC1 xenografts were treated with pacritinib or vehicle control. Tumor growth was measured as a function of tumor volume. Error bars denote SEM. ∗P < .05; ∗∗P < .01. (E-F) Tumor weight (E) and size (F) in pacritinib and vehicle treatment groups at the treatment end point. ∗∗P < .01. (G) Model of the action of the IL-1R pathway in pC ALK− ALCL presented in this study.
In the pC-ALCL xenograft mouse model, oral treatment with pacritinib, as a single agent, significantly slowed the growth of established tumors compared with vehicle treatment (P < .01 at days 4, 6, 8, and 10 of treatment) (Figure 7D), which is comparable with the effects of the AS2444697 and ruxolitinib combinational treatment (Figure 6F), and well tolerated (supplemental Figure 9D). Accordingly, the effectiveness of pacritinib was confirmed by tumor size and weight in both pacritinib and vehicle treatment groups at the treatment end point (Figure 7E-F). Hence, simultaneously targeting the IL-1R pathway and JAK2 with pacritinib represents great therapeutic potential in these tumors.
Discussion
Here, using high-throughput genome-wide CRISPR screening technologies, we revealed an unexpected role of the IL-1R pathway in supporting the viability of pC ALK− ALCL and provided a strategy to exploit this knowledge therapeutically using the JAK2/IRAK1 dual inhibitor pacritinib. Interestingly, this pathway is activated by IL-1α in an autocrine manner, which is essential for the induction and maintenance of protumorigenic inflammatory responses in pC-ALCL cell lines and primary cases. In ALK− ALCL cell lines, the A20 loss-of-function mutation, and the nonproteolytic protein ubiquitination network promote IL-1R pathway signaling and downstream NF-κB activation. In contrast, a STAT3 gain-of-function mutation or ALK translocation likely reduces this pathway’s prerequisite for supporting tumor survival. Because of these several observations, pacritinib has favorable and specific activities to prevent tumor growth of pC ALCLs in which the IL-1R pathway is hyperactivated, both in vitro and in mice xenograft models (Figure 7G).
Accumulating data indicate that tumor development depends not only on genetic alternations within malignant or premalignant cells but also on the inflammatory microenvironment.53,54 As a critical mediator of immunity and inflammation, the IL-1R signaling pathway has been shown to play an essential role in many solid tumors in response to increased IL-1 in the tumor microenvironment.55 However, its role in lymphoid malignancies has not been evaluated. In solid tumors, chronic inflammation could increase the tendency to develop IL-1–related tumors.56 However, such an inflammatory environment is usually rare in ALCL, though it would occur in some BIA-ALCL through plasticity.57 Our analysis demonstrated that IL-1R signaling is activated in an IL-1α autocrine and intracrine manner, which is unique among all published studies, to our knowledge, for the first time in hematologic malignancies. Interestingly, pC-ALCL, which primarily affects the skin, exhibits the highest IL-1α production in cell line models and primary cases. This observation raises the possible link between inflammatory skin conditions and the development of pC-ALCL, as dysregulation of the IL-1 system and the nonproteolytic ubiquitination pathway have been demonstrated to result in inflammatory skin diseases.58,59
Compared with pC-ALCL, other ALCL subtypes are generally less dependent on IL-1R signaling to survive. At this point, it remains unclear whether this is due to the lower IL-1α expression or the oncogenic STAT3/ALK genetic alterations in these cells. The frequency of JAK/STAT3 mutation in pC-ALCL is only 5%,19 much lower than that in other ALCL subtypes, supporting the role of IL-1α signaling in pC-ALCL independent of STAT3 mutation. In our analysis, CRISPR knockin of the STAT3 gain-of-function mutant D661Y in STAT3 WT ALCL cells partially mitigates the effect of IL-1R pathway inhibition on cell viability, indicating the oncogenic STAT3 mutation can at least compensate for the hyper-reactivated IL-1R signaling in ALCL. In ALCL, we have previously demonstrated that the STAT3 D661Y mutation promotes alternative NF-κB activation by stabilizing NIK, the central kinase in the alternative NF-κB pathway, in a CD30-dependent manner.60 Likewise, the NPM-ALK oncoprotein induces STAT3 activity, leading to minimal alternative NF-κB activation.60 Because the major consequence of IL-1R signaling activation is to trigger NF-κB, it is most likely that the NIK stabilization in STAT3 mutant ALCLs counteracts the survival advantage of IL-1R pathway hyperactivation.
Finally, our findings provide a comprehensive mechanistic basis for clinical trials in pC ALK− ALCL using the JAK2/IRAK1 dual inhibitor, pacritinib. Our studies indicated that not only the IL-1R pathway promotes JAK-STAT3 signaling, but also JAK signaling contributes to the IL-1R pathway hyperactivation through a feed-forward loop. As a single agent, we observed the striking activity of pacritinib in blocking the IL-1R pathway and STAT3 activation and killing STAT3 WT pC ALK− ALCL cells in vitro and in mice xenograft models, supporting clinical evaluation of this treatment regimen. Because more than 90% of pC-ALCL harbor WT STAT3, this therapeutic approach might benefit many patients diagnosed with this subtype of ALCL.
Acknowledgments
The authors thank Alan L. Epstein (University of Southern California Keck School of Medicine) for the TLBR1 and TLBR2 cell lines, and Annarosa Del Mistro (The Veneto Institute of Oncology) for the FEPD cell line. The authors thank Andrey Efimov (Fox Chase Cancer Center) for his assistance with the confocal microscopy.
This research was supported by National Institutes of Health/National Cancer Institute R01 CA259188 and R01 CA251674 (Y.Y.), Scholar Award from the Leukemia & Lymphoma Society (Y.Y.), and Cancer Research Conventional Grant from Gabrielle's Angel Foundation and the Mark Foundation for Cancer Research (Y.Y.). Z.S. was partially supported by the Greenwald Postdoctoral Fellowship for Research, Fox Chase Cancer Center.
Authorship
Contribution: Y.Y. designed and oversaw the project; Z.S., W. Wu, and W. Wei performed experiments and collected data; Y.Y., Z.S., W. Wu, and W. Wei analyzed and interpreted the data; W.X. analyzed library sequencing data; M.L. performed the differential gene expression analysis and the survival risk analysis; K.Q.C. performed the IHC analysis; D.W.H. analyzed the RNA-seq data for ALK− ALCL cell line mutations; S.J., J.-P.Z., H.W., M.E.K., T.A.W., L.M.S., and M.N., provided technical support and critical materials; and Y.Y. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Thomas A. Waldmann died on 25 September 2021.
Correspondence: Yibin Yang, Blood Cell Development and Function Program, Fox Chase Cancer Center, 333 Cottman Ave, Philadelphia, PA 19111; e-mail: yibin.yang@fccc.edu.
References
Author notes
∗Z.S., W. Wu, and W. Wei contributed equally to this work.
The high-throughput RNA sequencing data from this study have been submitted to the NCBI Sequence Read Archive under accession number SUB12286764.
All data generated or analyzed during this study are included in this published article and its supplementary information files.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Genome-wide CRISPR library screens identify the IL-1R pathway as a novel oncogenic driver in pC-ALCL. (A) Outline of the workflow of the depletion CRISPR library screens in lymphoma cell lines (upper). Overview of the genome-wide CRISPR screen results (lower). Shown are the volcano plots of all genes in 2 lines. y-axis indicates the significance (−log10p); x-axis indicates the log2 fold change (sgRNA on/off). The dashed lines indicate P = .05 and log2(FC) = ±4. (B) Top significantly enriched cellular pathways identified among the oncogenic hits identified from MAC1 or Karpas299 genome-wide CRISPR screens [log2(fold change) < −4 and P < .05] in panel A. (C) Left, diagrammatic representation of the workflow for the sgRNA toxicity assay. Right, ALCL lines were transduced with IRAK1, MYD88, IL1R1, IL1RAP, or control sgRNAs along with green fluorescent protein (GFP). The fraction of viable GFP+/sgRNA+ cells relative to the live cell fraction is plotted at the indicated times after sgRNA induction, normalized to day 0 values. Error bars denote standard deviation (SD) of triplicates. P was calculated comparing day 0 to each time point of indicated sgRNA induction in MAC1 and MAC2A lines. ∗∗P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/15/10.1182_blood.2022019166/3/m_blood_bld-2022-019166-gr1.jpeg?Expires=1769096040&Signature=4ZoJfTCy5lxmB4eN5L52iiVSHtcw~HqbdCELN~KjgxaKIWBJFT9ldGqpfCqrlW-0vuUsMynOuxu5VMLmgR76fKiXElq3oS~npeNaB2ODlyS5NSm0XMlUyDMOUxm85TZ~IW6D2B93dhTiH7POQOOarAn-ZKI8TJFqUgg9g6FhUujKd9K1aVOvUi~qtyTyUwLWfMd8Y2uLKeD4qQ9PqljdxI3pZqP-Eek6JSJX357ycV9SX7x9NkNrhyYDGXLX~W4uHeITDDfMhUDI6yqDa0uBP-lPRlQD~Eh1-OwaAUgvyj5oNGsf~VZUQA-I4nFZ7Yu0IFtM34mtcbTh1Sb9iZ5tCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![The nonproteolytic protein ubiquitination network in ALCL. (A) Distribution of mutations in genes related to the inflammatory pathways of ALK−ALCL cell lines, obtained from RNA-seq analysis. (B) A20 protein expression in indicated ALCL lines. (C) TNFAIP3 (A20) mRNA expression in 417 T-cell lymphoma samples (144 peripheral T-cell lymphomas [PTCL] NOS, 127 AITL, 69 ALK− ALCL, 56 ALK+ ALCL, 21 ATLL) and 61 T cells from healthy donors from publicly available microarray data set. t tests were performed between the control normal T-cell group and the other PTCL subtypes, and a P < .05 is considered statistically significant. (D) Overall survival in ALK− ALCL with higher or lower expression values of A20. The threshold (cut-point value for high or low-expression group) was set using maximally selected rank statistics with the “survminer” R package. The cut-point value has been determined as 8.29134 (log2 expression), and the number of patients in the high-expression and low-expression groups is 29 and 8, respectively. (E) NF-κB–driven luciferase reporter–engineered MAC1 line was transduced with inducible lentiviral constructs encoding A20 WT, C103A, and C779/782A complementary DNAs or empty control. Relative NF-κB reporter activities were measured after 1 day of induction. (F) NF-κB–driven luciferase reporter–engineered MAC1 line was transduced with indicated sgRNAs. Relative NF-κB reporter activities were measured after 4 days of induction. (G) MAC1 cells were transduced with RNF31 or ctrl sgRNAs, selected, and expression induced. Cell lysates were subjected to biotin-labeled M1-specific TUBE binding and streptavidin purification and analyzed by immunoblotting. (H) MAC1 cells were transduced with RNF31 or ctrl sgRNAs, selected, and expression induced. IRAK1 immunoprecipitations or total lysates from these lines were immunoblotted for the indicated proteins. In panels E-F, error bars denote SEM of triplicates. P was calculated comparing sgCTRL and the indicated sgRNA groups or comparing empty vector and A20 expression groups; ∗∗P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/15/10.1182_blood.2022019166/3/m_blood_bld-2022-019166-gr4.jpeg?Expires=1769096040&Signature=b6Gor7j5M-djKSWK9aIXxr~VPhBueCa17E5V9Jh5rwcPVu8~dRGWhXOfcI7We8SlOY~DmIyJf0KAiH4Z7xjnz1TY5KTWzfPaXLXIuFw9y-e00PioGDGZ-bzM7WNCDAHb5Li3lHttgErvKeExqS7JvZ2ZafIbcLOisqvUAAbwlflwtQ0oDQXRzhxYy-rA0jwIRLCUsQrfUsTUnMS83EQBgOrN3eoHWoEZM~vcZyrJfxysTBazk4IR9~vkrc7KKaNixugKdf28NRMc8p5FQGYlJ3eUohZJqblPq5MVmUxGCILmmpLGQC8U~0I4o-WUgw6NfvKKxXdH0sYNXy63W~74xw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal