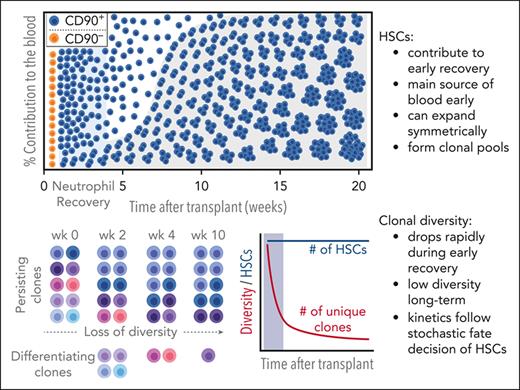
Key Points
HSCs contribute to neutrophil recovery.
HSCs symmetrically divide, forming clonal pools.
Abstract
Hematopoietic stem cells (HSCs) are assumed to be rare, infrequently dividing, long-lived cells not involved in immediate recovery after transplantation. Here, we performed unprecedented high-density clonal tracking in nonhuman primates and found long-term persisting HSC clones to actively contribute during early neutrophil recovery, and to be the main source of blood production as early as 50 days after transplantation. Most surprisingly, we observed a rapid decline in the number of unique HSC clones, while persisting HSCs expanded, undergoing symmetric divisions to create identical siblings and formed clonal pools ex vivo as well as in vivo. In contrast to the currently assumed model of hematopoietic reconstitution, we provide evidence for contribution of HSCs in short-term recovery as well as symmetric expansion of individual clones into pools. These findings provide novel insights into HSC biology, informing the design of HSC transplantation and gene therapy studies.
Introduction
Hematopoietic stem cells (HSCs) have historically been defined as the only long-term persisting cell type capable of self-renewing and of generating all blood lineages. In addition, the general assumption is that adult HSCs are rare, rarely proliferate to prevent exhaustion, do not expand but divide asymmetrically to self-renew and create a progenitor, and do not contribute to short-term hematopoietic recovery after autologous or allogeneic transplantation. Although this definition is currently widely accepted guiding most experimental and clinical research, individual aspects of this definition have been challenged. Longitudinal tracking of murine HSCs in vivo showed robust and continuous contribution during steady-state hematopoiesis, indicating that HSCs are less quiescent than assumed.1-3 Similarly, single murine as well as human HSCs can successfully reconstitute the entire murine host, illustrating extensive proliferative capabilities without immediate exhaustion while maintaining long-term persistence and even engraftment potential in secondary transplants.4-7
In vivo HSC tracing and replication of these findings in humans however is very challenging. Knowledge about the engraftment kinetics and contribution of human HSCs during hematopoietic recovery is currently limited to clinical trials of patients undergoing gene therapy who were simultaneously enrolled in clonal tracking studies using retroviral integration site analysis (ISA) to assess the polyclonality and safety of the graft.8-10 However, the tracking of human HSCs and derivatives remains underinvestigated because the number and volume of blood as well as bone marrow (BM) collections possible to obtain and isolate sufficient genomic material to seamlessly monitor the entire clonal repertoire are limited. Especially during the early phases of reconstitution during which blood cell counts are low, collection of patient material is limited.
In addition, clone tracking is affected by technical limitations. ISA is routinely used to monitor patients undergoing gene therapy and the potential outgrowth of dominant or malignant clones.8,9 Although reliable in detecting and following highly abundant clones, low sensitivity11 and high error rates9 require significant data exclusion and sophisticated statistical tests to ensure data reliability when clonal tracking data are used in an experimental setting to investigate the clonal kinetics of hematopoietic engraftment. Lack of sensitivity and loss of low abundance clones can be overcome by increasing the depth (ie, blood volume), frequency of sampling (high density), as well as repeated sampling,9,11 aspects that are difficult to achieve in patients.
To investigate the clonal dynamics in the early phases of hematopoietic reconstitution and long-term recovery, as well as to determine the involvement of long-term persisting multipotent HSC clones, we applied a new clonal tracking approach. To overcome the known detection limit of ISA, we performed high-density sampling for ISA in nonhuman primates (NHPs) after autologous transplantation of gene-modified cells. Specifically, during neutrophil and platelet recovery, weekly blood samples were taken to enhance data density. After full recovery, animals were followed for up to 4.5 years to identify long-term persisting HSC clones and the contribution of these long-term HSCs to neutrophil recovery was determined in early samples. Finally, the observed loss of unique HSC clones in vivo was used to inform a mathematical model of hematopoietic reconstitution to explore the temporal involvement of HSCs and build a revised timeline of hematopoietic reconstitution.
Materials and methods
Insertion site analysis
Sample processing and sequencing
As described by Adair et al,11 processing of genomic DNA for amplification of integration loci was performed using modified genomic sequencing polymerase chain reaction.12 Next-generation sequencing was performed using paired-end Illumina Miseq. Details for the data processing can be found in supplemental Methods, available on the Blood website.
CITE-seq
Steady-state BM-derived CD34+ cells were stained with TotalSeq-A antibodies and processed using the Chromium Single Cell 3ʹ (version 3) platform from 10× Genomics (Pleasanton, CA). Separation of single cells, library preparation, and RNA extraction were performed in accordance with the 10× Chromium Single Cell Gene Expression Solution protocol. Details on the next-generation sequencing for cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq), messenger RNA alignment and counting, quantification of antibody-derived tags, dimensional reduction and clustering, cell-cycle scoring and regression, cell-type module, SPRING plots, and expressed barcodes can be found in the supplemental Methods.
Statistics
Statistical analysis of data was performed using GraphPad Prism version 5. Details for the statistical analyses used can be found in the figure legends.
Results
Multipotent HSCs contribute to very early neutrophil recovery and persist long term
To reliably identify long-term engrafting HSC clones for our clonal tracking analysis, NHPs undergoing HSC gene therapy were followed-up for up to 4.5 years. Peripheral blood (PB) and BM were analyzed flow cytometrically to confirm stable transgene expression (Figure 1A). Consistent gene marking was seen in PB white blood cells (WBCs) throughout the entire follow-up with nearly identical gene marking efficiency in T cells, B cells, natural killer cells, granulocytes, and monocytes starting at 9 months after transplantation. Gene marking of CD34+ hematopoietic stem and progenitor cells (HSPCs), as well as the HSC-enriched CD34+CD45RA−CD90+ subset in the BM mirrored observed frequencies in the PB.
Longitudinal assessment of gene-modified WBCs and HSPCs in the PB and BM. (A) Two pigtail macaques were transplanted with gene-modified HSPCs after myeloablative conditioning with total body irradiation. Animals were followed-up for up to 50 months through taking PB and BM draws to measure the gene marking in PB WBCs (top), PB lineages (middle), and BM-derived HSPCs (bottom). (B) Bulk PB WBCs were collected and DNA banked for ISA as indicated with gray symbols, whereas PB blood lineages (T cells: CD3+, B cells: CD20+, natural killer [NK] cells: CD16+, monocytes: CD14+, and granulocytes: CD11b+CD14–) were FACS purified as indicated with black symbols. From the BM, CD34+ HSPCs were FACS purified and the DNA banked for ISA. (C) Summary of the animal characteristics, ISA sample collection, and analysis.
Longitudinal assessment of gene-modified WBCs and HSPCs in the PB and BM. (A) Two pigtail macaques were transplanted with gene-modified HSPCs after myeloablative conditioning with total body irradiation. Animals were followed-up for up to 50 months through taking PB and BM draws to measure the gene marking in PB WBCs (top), PB lineages (middle), and BM-derived HSPCs (bottom). (B) Bulk PB WBCs were collected and DNA banked for ISA as indicated with gray symbols, whereas PB blood lineages (T cells: CD3+, B cells: CD20+, natural killer [NK] cells: CD16+, monocytes: CD14+, and granulocytes: CD11b+CD14–) were FACS purified as indicated with black symbols. From the BM, CD34+ HSPCs were FACS purified and the DNA banked for ISA. (C) Summary of the animal characteristics, ISA sample collection, and analysis.
To determine the temporal contribution of multipotent HSCs, PB WBCs, individual PB lineages, as well as BM CD34+ cells were fluorescence-activated cell sorting (FACS) purified for ISA (Figure 1B), and clonal tracking data aggregated (Figure 1C). Highly polyclonal engraftment was seen in both animals without the development of dominant clones with >5% contribution to the PB (supplemental Figure 1).
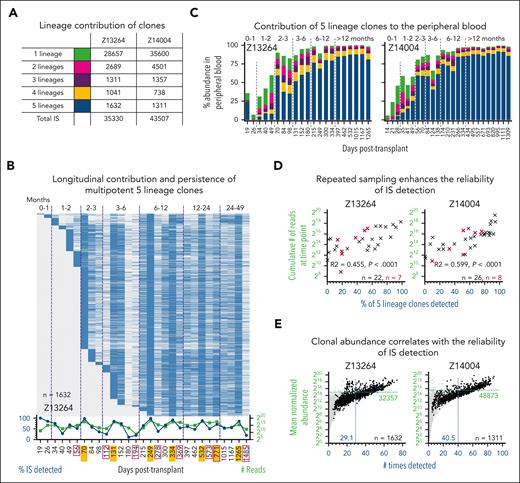
Throughout the follow-up we identified more than a thousand clones contributing to all 5 lineages in Z13264 (1632 clones) and Z14004 (1311 clones) (Figure 2A). HSC-derived cells were detected during neutrophil recovery, persisted long term, and were detected in BM CD34+ cells up to 4 years later (Figure 2B; supplemental Figure 2A). Furthermore, >90% of persisting HSCs contributed to the PB within 131 days in Z13264 and 84 days in Z14004 (Figure 2B; supplemental Figure 2A). Already by day 56, in both animals, >50% of all gene-marked blood cells were derived from long-term persisting HSCs (Figure 2C).
Contribution and persistence of multipotent 5-lineage clones. (A) Summary of retrospectively assigned lineage contribution for unique clones. (B) Longitudinal detection of multipotent 5-lineages clones. Blue and gray indicate the presence and absence of clones, respectively. Graph below heatmap: frequency of 5-lineage clones detected (blue, left y-axis) and number of reads (green, right y-axis) for each time point. BM time points are outlined in red. Time points with multiple FACS-purified lineages are highlighted in yellow. (C) Longitudinal contribution of 5-, 4-, 3-, 2-, and 1-lineage clones to the PB. Color code as defined in panel A. (D) Impact of sampling on the reliability of IS detection. Correlation of the cumulative read count from a single PB (black symbol) or BM (red symbol) time point, with the frequency of multipotent 5-lineage clones detected at the same time point. (E) Impact of clonal abundance on the reliability of IS detection. Correlation of the mean normalized abundance of 5-lineage clones (black dots), with the number of times the clone was detected across all analyzed samples. Gray background indicates clones associated with other groups. The mean normalized abundance and average number of times detected across all clones are indicated with the green horizontal line and the blue vertical line, respectively.
Contribution and persistence of multipotent 5-lineage clones. (A) Summary of retrospectively assigned lineage contribution for unique clones. (B) Longitudinal detection of multipotent 5-lineages clones. Blue and gray indicate the presence and absence of clones, respectively. Graph below heatmap: frequency of 5-lineage clones detected (blue, left y-axis) and number of reads (green, right y-axis) for each time point. BM time points are outlined in red. Time points with multiple FACS-purified lineages are highlighted in yellow. (C) Longitudinal contribution of 5-, 4-, 3-, 2-, and 1-lineage clones to the PB. Color code as defined in panel A. (D) Impact of sampling on the reliability of IS detection. Correlation of the cumulative read count from a single PB (black symbol) or BM (red symbol) time point, with the frequency of multipotent 5-lineage clones detected at the same time point. (E) Impact of clonal abundance on the reliability of IS detection. Correlation of the mean normalized abundance of 5-lineage clones (black dots), with the number of times the clone was detected across all analyzed samples. Gray background indicates clones associated with other groups. The mean normalized abundance and average number of times detected across all clones are indicated with the green horizontal line and the blue vertical line, respectively.
The recapture of multipotent clones in both animals was inconsistent throughout the entire follow-up (Figure 2B; supplemental Figure 2A). Close review of the sequencing data revealed a very uneven distribution of read counts across clones. Although the top 20 to 50 clones account for the vast majority of sequence reads, most clones were only captured <5 times or only had a single read (supplemental Figure 2B). The chance to repeatedly detect clones increased with a greater number of samples taken and cumulative reads recorded at a given time point (Figure 2B,D; supplemental Figure 2A bottom line graph). Furthermore, the reliability of detection increased with the abundance of a clone (Figure 2E).
In these animals, because of enhanced sampling, we observed multipotent long-term persisting HSCs to actively contribute from the very first day of neutrophil recovery to the PB while persisting in the BM stem cell compartment for years. Furthermore, we show that sample density and collection have a significant impact on the sensitivity of ISA and the reliability to detect a clone. ISA data of high abundance clones is highly reliable, whereas the recognition of low abundance clones is inconsistent and requires repeated sampling to detect contribution.
Detection limit of ISA and low clonal abundance cause multipotent clones to appear lineage restricted
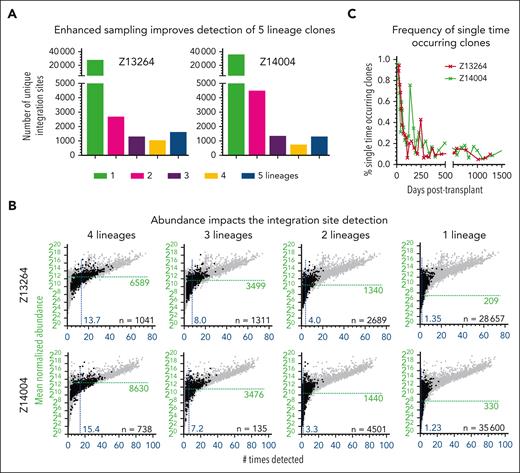
Because of the observed impact of frequent sampling and the read count patterns for clones in ISA data sets described earlier, we closely examined all clones that were found to contribute to only 2, 3, or 4 lineages (Figures 2A and 3A). Many of these clones persisted long term (supplemental Figure 3A). The reliability of integration site (IS) detection (number of times seen across all samples) and the mean abundance across all clones decreased stepwise from multipotent to 1-lineage clones (Figures 2E and 3B). Most importantly, none of the top 100 most highly abundant and confidently detected clones were found to be lineage restricted at any point in the follow-up (supplemental Figure 3B).
Contribution of lineage-restricted and SO clones. (A) Number of clones found contributing to 1, 2, 3, 4, or 5 lineages. (B) Correlation of the mean normalized abundance of 4-, 3-, 2-, and 1-lineage clones with the number of times the clone was detected across all analyzed samples. Gray background indicates clones associated with other groups. The mean normalized abundance and average number of times detected across all clones are indicated by the green horizontal line and the blue vertical line, respectively. (C) Frequency of SO clones over time.
Contribution of lineage-restricted and SO clones. (A) Number of clones found contributing to 1, 2, 3, 4, or 5 lineages. (B) Correlation of the mean normalized abundance of 4-, 3-, 2-, and 1-lineage clones with the number of times the clone was detected across all analyzed samples. Gray background indicates clones associated with other groups. The mean normalized abundance and average number of times detected across all clones are indicated by the green horizontal line and the blue vertical line, respectively. (C) Frequency of SO clones over time.
We further analyzed clones only found to contribute to a single lineage as well as clones that were seen once within a single WBC sample (single-time occurring [SO]). The majority of single-lineage clones were found within the first 6 to 9 months (supplemental Figure 3A). These clones had a very low abundance and strongly overlapped with the group of SO clones (Z13264: 54%; Z14004: 57%). SO clones were the predominant source for WBCs for the first 50 days (Figure 3C). Although we were not able to assign these early SO clones to distinct lineages, granulocytes are the predominant cell type during this phase (Figure 1A). SO clones were continuously detected at low frequencies (5%-10%) in Z13264 as well as Z14004 (Figure 3C).
Based on these data, we show that long-term persisting clones detected in 2, 3, or 4 lineages are low abundance clones inconsistently captured by ISA. Furthermore, early hematopoietic recovery is almost entirely driven by a large number of SO clones.
Rapid loss of unique HSC clones is consistent with stochastic HSC expansion and differentiation
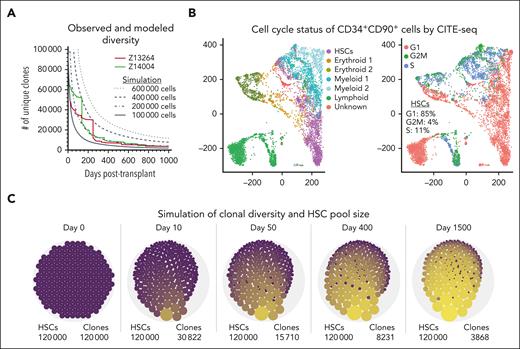
To understand the kinetics of the observed loss of unique clones in vivo, we developed an agent-based, stochastic model of HSCs based on the data observed in these transplant experiments. The goal of the modeling was to determine whether, based on hypothesized mechanisms and the data we observed, we might recapitulate the rapid decrease of unique HSC clones and occurrence of SO clones seen during neutrophil recovery in Z13264 and Z14004 (Figure 4A colored lines).
Mathematical model of clonal loss and stochastic HSC engraftment. (A) Overlay of observed clonal loss in Z13264 (red line) and Z14004 (green line) with the results of our mathematical model of loss of unique clones (black lines, Nt for t = 1 ,….., T = 1000 days). Different starting cell numbers of engrafted HSCs were explored in the simulations as indicated. (B) CITE-seq of a representative human steady-state BM CD34+ population. (Left) Transcriptionally distinct progenitor subsets were color coded as indicated in the color key. (Right) Cell-cycle status of cells. (C) Longitudinal change of clonal loss and HSC clone size. Simulations were performed with 120 000 HSCs at the time of transplant, an initial cell-cycle rate of 100% per day for 20 days and 10% cycle rate per day thereafter. The number of unique clones for each representative time point is shown (bottom right). Dot sizes illustrate the clone sizes.
Mathematical model of clonal loss and stochastic HSC engraftment. (A) Overlay of observed clonal loss in Z13264 (red line) and Z14004 (green line) with the results of our mathematical model of loss of unique clones (black lines, Nt for t = 1 ,….., T = 1000 days). Different starting cell numbers of engrafted HSCs were explored in the simulations as indicated. (B) CITE-seq of a representative human steady-state BM CD34+ population. (Left) Transcriptionally distinct progenitor subsets were color coded as indicated in the color key. (Right) Cell-cycle status of cells. (C) Longitudinal change of clonal loss and HSC clone size. Simulations were performed with 120 000 HSCs at the time of transplant, an initial cell-cycle rate of 100% per day for 20 days and 10% cycle rate per day thereafter. The number of unique clones for each representative time point is shown (bottom right). Dot sizes illustrate the clone sizes.
In the model, represents a clone type during cell cycle (), and the agents are the individual cells within a clone type. Each cell cycle lasts a day and has a unique set of HSC clones (ie, ), and the total number of HSCs per cycle is (supplemental Methods). During a cell cycle, only a fraction of cells from proliferates or differentiates symmetrically and asymmetrically. Each cycling cell in has an equal likelihood of doubling into 2 HSC daughter cells, differentiating into 2 progenitor cells, or asymmetrically dividing into 1 HSC and 1 progenitor, or 1 progenitor and 1 HSC (ie, ) (supplemental Figure 4A).
To design a simplistic and qualitative mathematical model with simulation results consistent with the loss of unique clones seen in Z13264 and Z14004, the following parameters and assumptions were used based on our previous publications, ISA data collected in this study, and additional data generated specifically for this model: (1) because we have seen no evidence for heterogeneity in the CD34+CD90+ population in our previous transplantation13 and single-cell RNA sequencing (scRNA-seq) studies,14 we assume that all CD90+-derived clones have the same engraftment, proliferation, and differentiation potential. In other words, proliferation and/or differentiation are independent events for each cell per cycle. (2) The proliferation rate of HSCs was set to for the simulation shown in Figure 4A based on CITE-seq data from steady-state BM-derived CD34+CD90+ cells showing a range in cell cycling rate of 10% to 20% per day. (Figure 4B; supplemental Figure 4A-C). To avoid a loss or uncontrolled expansion of HSCs, the ratio of self-renewal vs differentiation was defined as population neutral. Furthermore, we included the ability of HSCs to (1) asymmetrically divide, generating an HSC and a progenitor; (2) symmetrically expand, giving rise to 2 HSCs; and (3) symmetrically differentiate into 2 progenitors (supplemental Figure 5A). No limit was set for the total divisions an HSC can undergo.
In our experiments, we transplant a relatively large number of CD34+CD90+ HSCs into each NHP (supplemental Table 1), but we have no data to inform how many of these cells engraft and go on to proliferate and differentiate. Thus, for the model’s initial state, we assumed 1 cell of each clone type and explored the total starting cell and clone number between and (Figure 4A dotted lines; supplemental Figure 5B). Testing a range of starting total cell/clone numbers , we found that the NHP data lay between simulations with initial total cell per clone numbers of 100 000 and 200 000. These simulations yielded long-term ( =1000 days) clone numbers ranging from 3000 to 4000, similar to those observed in vivo, but modeling results underestimated the early number of unique clones (Figure 4A; supplemental Figure 5B).
Next, we simulated the engraftment and blood production of 1.2 × 105 HSCs and compared the simulation with the observed kinetics of vanishing and persisting clones in the PB (supplemental Figure 6A). With a constant cell-cycle rate of 10% per day for HSCs, contribution of long-term persisting clones was significantly delayed (simulation 1, supplemental Figure 6B). An increase of the cycling rate to 20% per day had little impact (simulation 2, supplemental Figure 6B). Next, we tested an alternative 2-step model with a 100% cycling rate per day during a 20-day neutrophil recovery phase and 10% cycling thereafter (simulation 3, supplemental Figure 6B). The higher cycling rate during early recovery did enhance the contribution of persisting HSC clones and more closely aligned with the kinetics observed in the NHPs.
To better understand the clonal dynamics in the BM stem cell niche, we visualized the number and size of HSCs clones over time simulating the engraftment of 1.2 × 105 HSCs with the aforementioned model and using the 2-step dynamics of proliferation (Figure 4C). Although the vast majority of transplanted HSC clones disappear within the first weeks after transplantation, the total number of HSCs did not change. Individual HSC clones probabilistically started to expand and over time formed clonal HSC pools with many identical daughter cells carrying the same viral IS. Despite the growth of individual clones, no dominant clones developed, and loss of unique HSC clones slowed down.
Our mathematical model offers a mechanism by which HSC clones may be expanding, consistent with our experimental results. The loss of unique clones seen in vivo could follow a stochastic pattern in which upon division, each daughter cell may self-renew or differentiate with roughly the same probability. The simulation also supports that transplanted HSCs after ex vivo culture and gene modification could proliferate for an extended time at high rates, rapidly establishing a clonal pool and maintaining an equilibrium number of HSCs similar to the initial number of HSCs that engraft.
Stochastic in vitro expansion and differentiation of CD34+CD45RA−CD90+ HSCs
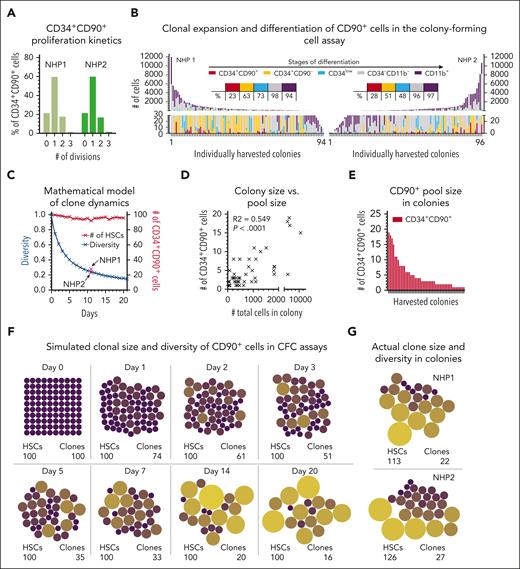
Our mathematical model of clonal dynamics in vivo demonstrates that transplanted HSCs could rapidly cycle at the time of infusion and build clonal pools. To substantiate this hypothesis, we analyzed the proliferation kinetics of phenotypic CD34+CD45RA−CD90+ HSCs, on a clonal level in vitro, of cells taken from control NHPs not involved in the original transplant experiments (NHP1 and NHP2).
Initially, we determined the cell-cycle rate of CD34+CD45RA–CD90+ cells in vitro. Closely mimicking our transplant protocol, bulk CD34+ cells were isolated (day –2), cultured overnight, stained with carboxyfluorescein succinimidyl ester (CFSE) (day –1), rested overnight, and CFSE+CD34+CD45RA–CD90+ cells FACS purified to initiate the culture (day 0 = day of infusion in transplant setting). CFSE staining was analyzed at the day of FACS and the 3 consecutive days. No proliferation of CD34+CD45RA–CD90+ cells was seen before day 1 (within 72 hours of culture). Thereafter, the vast majority of CD34+CD45RA–CD90+ cells underwent 1 division every 24 hours and continued to proliferate at this rate (Figure 5A; supplemental Figure 7A).
Stochastic outgrowth of CD34+CD90+ cells in CFC assays. (A) Number of divisions of CFSE-stained CD34+CD90+ cells after 24-hour culture in CFC medium. CFSE staining was determined flow cytometrically, and the number of divisions as well as the frequency of divided cells were calculated relative to the parental population. (B) Size and composition of CD34+CD90+-derived colonies after 11 days of culture. Phenotypes and cell numbers were determined flow cytometrically. Tables in the center indicate the frequency of colonies containing phenotypically defined stages of differentiation defined above. (C) Simulation of the change in the clonal repertoire (blue line) and number of CD34+CD90+ cells in colonies over time (red line). (D) Correlation of the size of CD34+CD90+-containing colonies with the actual number of CD34+CD90+ cells within each colony. (E) Number of CD34+CD90+ cells within colonies. (F) Visualization of the change in the clonal repertoire and CD34+CD90+ clone size in colonies based on the simulation. (G) Actual size of the clonal repertoire and CD34+CD90+ clone size from the CFC assay in panel C. The dot size is proportional to the number of cells in the colony.
Stochastic outgrowth of CD34+CD90+ cells in CFC assays. (A) Number of divisions of CFSE-stained CD34+CD90+ cells after 24-hour culture in CFC medium. CFSE staining was determined flow cytometrically, and the number of divisions as well as the frequency of divided cells were calculated relative to the parental population. (B) Size and composition of CD34+CD90+-derived colonies after 11 days of culture. Phenotypes and cell numbers were determined flow cytometrically. Tables in the center indicate the frequency of colonies containing phenotypically defined stages of differentiation defined above. (C) Simulation of the change in the clonal repertoire (blue line) and number of CD34+CD90+ cells in colonies over time (red line). (D) Correlation of the size of CD34+CD90+-containing colonies with the actual number of CD34+CD90+ cells within each colony. (E) Number of CD34+CD90+ cells within colonies. (F) Visualization of the change in the clonal repertoire and CD34+CD90+ clone size in colonies based on the simulation. (G) Actual size of the clonal repertoire and CD34+CD90+ clone size from the CFC assay in panel C. The dot size is proportional to the number of cells in the colony.
Next, we tested the clonal outgrowth of CD34+CD45RA–CD90+ HSCs in vitro in colony-forming cell (CFC) assays. Single CFSE+CD34+CD45RA–CD90+ cells at day 0 were FACS sorted into CFC assays, grown for 11 days, and individual colonies harvested for analysis (Figure 5B). Colonies were found to consist of primitive HSPCs (CD34+CD90+), progenitors (CD34+CD90– and CD34low), noncommitted cells (CD34−CD11b−), and mature myeloid cells (CD34–CD11b+) (supplemental Figure 7B). Primitive CD34+CD90+ HSPCs were found in 23% (NHP1) and 28% (NHP2) of colonies, closely resembling the loss of unique clones in our mathematical model (24.4% at day 11) (Figure 5C).
No association was found between the composition of colonies and the presence of CD34+CD90+ cells (supplemental Figure 7C). Greater quantities of CD34+CD90+ cells correlated with the total size of colonies (Figure 5D-E) (R2 = 0.507), and the cumulative number of CD34+CD90+ cells across all colonies indicated a slight overall expansion (NHP1: 119%; NHP2: 131%). Consistent with our mathematical model, HSCs formed clonal pools with a great range of sizes and a significant loss of unique clones (Figure 5F-G).
Formation of clonal HSC pools in the NHP BM
To test our hypothesis that HSCs symmetrically expand and form clonal pools, we analyzed the BM HSC compartment of Z14004 at a clonal level, quantifying the number of CD90+ HSCs with identical integrations. Five years after transplantation, green fluorescent protein (GFP)–positive CD34+CD90+ HSCs from Z14004 were FACS purified, introduced into CFC assays, and 500 single-cell–derived colonies picked after 10 to 12 days (supplemental Figure 8A). Half of the genetic material from each colony was pooled for ISA to create a reference for all ISs present (supplemental Figure 8B), and primers generated for the top 10 ISs detected (supplemental Figure 8B-C). Bulk ISA data as well as the polymerase chain reaction on 98 individual colonies confirmed formation of clonal pools consistent with our mathematical model (supplemental Figure 8B,D).
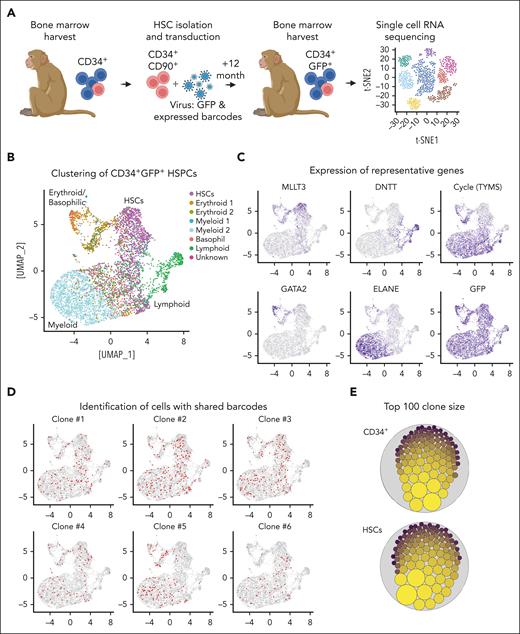
To further validate HSC pool formation suggested by our ISA data, models, and CFC assays, we transplanted another NHP with CD34+CD90+ cells transduced with lentiviral vectors encoding for GFP and expressed barcodes (Figure 6A).15 After 12 months, GFP+CD34+ cells from the BM were harvested and analyzed with 10× scRNA-seq. After clustering (Figure 6B; supplemental Figure 8E), cell types were identified based on the gene expression of lineage-specific genes (Figure 6C). Finally, cells sharing the same expressed barcodes were identified (1015 unique clones total) and overlaid on the reference map (Figure 6D), and the size of each clone quantified within bulk CD34+ cells and the HSC cluster (Figure 6E). Consistent with our mathematical model, formation of clonal pools was evident in bulk CD34+ cells as well as the more refined HSC cluster (Figure 6E) with a similar clonal distribution as seen in Z14004 (supplemental Figure 8D). Quantification of cells sharing the same barcode across all transcriptionally distinct clusters further showed no enrichment or amplification of cells within distinct progenitor subsets (supplemental Figure 8F).
Development of clonal HSC pools in vivo. (A) Experimental design. NHP CD34+CD90+ cells were transduced with lentivirus encoding for GFP and expressed barcodes. After 12 months, GFP+CD34+ cells were isolated from the BM and analyzed by scRNA-seq. (B) scRNA-seq of GFP+CD34+ cells. Transcriptionally distinct progenitor subsets were color coded as indicated in the color key. (C) Expression of GFP, representative genes associated with primitive HSCs (MLLT3), lymphoid (DNTT), erythroid (GATA2), and myeloid (ELANE) progenitors, as well as proliferating cells (TYMS). (D) Identification of cells sharing the same expressed barcode for the 6 most abundant clones. (E) Graphical representation of the clone size in CD34+ cells (all clusters combined) and the transcriptionally primitive HSC subset. The dot size is proportional to the number of cells sharing the same expressed barcode identified by scRNA-seq.
Development of clonal HSC pools in vivo. (A) Experimental design. NHP CD34+CD90+ cells were transduced with lentivirus encoding for GFP and expressed barcodes. After 12 months, GFP+CD34+ cells were isolated from the BM and analyzed by scRNA-seq. (B) scRNA-seq of GFP+CD34+ cells. Transcriptionally distinct progenitor subsets were color coded as indicated in the color key. (C) Expression of GFP, representative genes associated with primitive HSCs (MLLT3), lymphoid (DNTT), erythroid (GATA2), and myeloid (ELANE) progenitors, as well as proliferating cells (TYMS). (D) Identification of cells sharing the same expressed barcode for the 6 most abundant clones. (E) Graphical representation of the clone size in CD34+ cells (all clusters combined) and the transcriptionally primitive HSC subset. The dot size is proportional to the number of cells sharing the same expressed barcode identified by scRNA-seq.
In summary, formation of clonal HSC pools in vivo was confirmed using expressed barcodes. Clonal pools vary in size with very few large and many smaller clonal pools equally distributed across all transcriptionally defined stem and progenitor clusters.
Discussion
Here, we show that long-term engrafting multilineage HSCs actively contribute during neutrophil recovery and are the predominant source of blood cell production as early as 50 to 60 days after myeloablative conditioning and transplantation. Most importantly, observed changes in the clonal repertoire over time suggest a stochastic HSC fate decision process with the formation of long-term persisting clonal pools. Our data imply that HSCs are involved much earlier in hematopoietic reconstitution than previously reported and symmetrically expand after transplantation, suggesting a revised model of hematopoietic reconstitution.
Hematopoietic recovery within the first 6 to 9 months after conditioning and HSC transplantation is thought to be exclusively driven by short-living progenitor cells, whereas persisting multipotent HSCs do not contribute before 6 to 9 month after transplantation.16 This model has been presumed for several decades, with more recent clonal tracking data from autologous human gene therapy trials and NHP transplantation studies seeming to confirm this concept.8,17,18 Our high-density ISA data challenges the currently assumed timing and delayed contribution of HSC after transplantation. We provide evidence that persisting multipotent HSCs actively contribute to neutrophil recovery within the first weeks after transplantation. The abundance of blood cells derived from these persisting multipotent HSC clones during neutrophil recovery is very low and, because of the poor sensitivity of ISA, can easily be missed when samples are taken too late or several months apart. In addition, the very rapid loss of unique clones during neutrophil recovery, with thousands of clones contributing only for a very short period of time, requires a very high sampling density to reliably detect these persisting HSC clones. Such comprehensive sampling would be prohibitive in patients and is only possible in large-animal models like the NHP models. Although samples from patients who received gene therapy were consistently taken throughout the long-term follow-up, early sampling was limited to monthly draws, with the first samples available for analysis 4 to 6 weeks after transplantation.8 Previous NHP studies similarly focused on the long-term tracing of clones with limited data density, specifically in the first 3 months after transplantation, likely missing the vast majority of HSC-derived cells contributing during early recovery.17,18
Various models of HSC contribution during steady-state hematopoiesis and recovery after transplantation have been proposed.19 The common assumption has long been that HSCs are quiescent during recovery, rarely contribute to everyday blood production, and have a very limited number of cell cycles before they imminently differentiate.20,21 This led to the assumption that the initial recovery is driven by short-living progenitors, and that steady-state hematopoiesis is maintained by multipotent progenitors to prevent exhaustion and consumption of HSCs. However, a variety of recent studies in mice determined robust and continuous everyday contribution of HSCs to the blood.1-3,22 Contribution of HSCs during steady-state hematopoiesis was further found in baboons, with a reported estimation of between 56% and 84% of HSCs to cycle at least once a year.23 Similarly, involvement of HSCs during hematopoietic recovery after transplantation in cats had been proposed >2 decades ago by Abkowitz et al.24 These models further predict that the HSC fate to self-renew or differentiate is likely a stochastic event in mice,25,26 cats,24 primates,27 as well as humans.28,29
Our HSC engraftment model further suggests, and in vivo barcode tracking confirms, that multipotent long-term persisting HSC clones form clonal pools over time in vivo. These findings suggest that adult HSCs can symmetrically expand. To understand the underlying kinetics resulting in this HSC pool formation, we evaluated various models to understand the initial rapid decline in the clonal repertoire and the long-term persistence of only a couple-thousand clonal HSC pools seen in our ISA data. To our surprise, the simulations of a stochastic fate decision of HSCs using an agent-based modeling approach did demonstrate the best representation of the observed long-term loss of unique HSC clones in vivo. Our data as well as the simulation therefore imply that the decision of a dividing HSC to either differentiate and contribute to the blood or to expand and persist building up a pool is likely a stochastic event. Our model further suggests that early blood production may be potentially driven by HSC clones stochastically undergoing symmetric differentiation and only contribute to a limited number of lineages before they disappear, a population of cells currently described as short-term engrafting progenitors in humans8 or as short-term HSCs in mice.30 This hypothesis is supported by the current inability to demonstrate the coexistence of ≥2 functionally distinct subsets with short- and long-term engraftment features within the human and NHP CD34+CD45RA−CD90+ phenotype using scRNA-seq.31,32 However, to this end it remains speculative whether SO clones driving the blood production during the first 50 to 60 days of recovery are stochastically differentiating HSCs, predetermined short-term HSCs, or short-term progenitors. More sensitive methods and new tracking approaches will be needed to closely monitor this currently underinvestigated time window of reconstitution.
Our stochastic model focuses on a limited number of mostly constant parameters with the cycling rate as the only factor of variability. No aspects of positive/negative feedback mechanisms, loss of HSCs during homing, divisional limits, variations in the symmetric/asymmetric division rate, or the capacity limit of the BM niche (and others) were included.29 Because changes in the ratio of symmetric vs asymmetric division are frequently associated with a loss or gain of stem cells, either leading to exhaustion or an excessive and potentially cancerogenic outgrowth over years or even decades,33-37 we kept this parameter constant but expect a slight expansion to counteract HSC loss and apoptosis over time. Introduction of environmental regulators, feedback loops, as well as the BM capacity into our simulation are expected to modify the cell-cycle rate and, presumably, accelerate the loss of unique clones specifically in the early phase of engraftment. We currently assume in our model that all transplanted HSCs cycle at very high rates for 20 days, but in vivo HSCs may gradually slow down until steady-state hematopoiesis is reached. Little is known about the actual cycling rate of CD34+CD45RA–CD90+ HSCs in vivo during very early hematopoietic recovery, how it may change over time, and what external factors are responsible for the changes (eg, niche feedback, cytokines, or lack of blood cells). Lastly, we would expect a certain degree of competition with remaining endogenous HSCs that survive the conditioning. To this end, a multitude of additional factors currently not included in our model may help to explain the faster decline of unique clones in the early phases of recovery, including the potential existence of a predetermined short-term engrafting subset within the CD34+CD45RA–CD90+ phenotype.
Despite enhanced resolution of our ISA data set, we are still experiencing sample-to-sample variability. Individual time points with poor sample quality or low numbers of sequencing reads only detected highly abundant clones and introduced a seemingly oscillating contribution of multipotent HSCs. Although other groups have reported the presence of lymphoid- and myeloid-biased HSC clones,8,9 we show that lineage skewing may be a combined result of ISA sensitivity, sample quality, and clone abundance. None of the most highly abundant persisting HSC clones showed signs of lineage bias and all lineage-restricted clones were low in abundance, closer to the detection limit of ISA and therefore less consistently detected. Although Six et al9 addressed this issue by rerunning samples from the same time point multiple times, the limited material from patients only contains a fraction of contributing clones and does not represent the entire repertoire of clones present at a given time point. More sensitive longitudinal approaches will be required to reliably address the question of lineage bias in low abundance clones, account for the difference in lifetime of different blood cell types, and overcome current technical limitations.
In summary, high-density clonal tracking in NHPs revealed an unexpected early contribution of HSCs and formation of long-term persisting clonal pools, thereby challenging the currently proposed involvement of HSCs during hematopoietic recovery after myeloablative conditioning and transplantation. Our findings, however, should have major implications for HSC transplantation and particularly for ex vivo as well as in vivo HSC gene therapy. The ability to directly modify CD34+CD90+ cells should substantially reduce the amount of reagents and thus the cost needed for the genetic engineering of HSCs, and pave the path for in vivo HSC gene therapy applications.
Acknowledgments
The authors thank Helen Crawford for help in preparing the manuscript and figures.
This work was supported, in part, by National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) grant R01 AI135953-01, NIH, National Heart, Lung, and Blood Institute grant R01 HL136135 (H.-P.K.), NIH, NIAID grant R01 AI150500 (E.F.C.-O. and J.T.S.), Markey Molecular Medicine Investigator (H.-P.K.), the José Carreras/E. Donnall Thomas Endowed Chair for Cancer Research (H.-P.K., inaugural recipient), and the Stephanus Family Endowed Chair for Cell and Gene Therapy (H.-P.K.).
Authorship
Contribution: S.R., M.E., and H.-P.K. designed and supervised the study; S.R., M.E., D.P., R.M., S.O., G.K., M.C., A.M.P., E.R.D., E.F.C.-O., and J.T.S. developed the methodology; M.E. and D.P. performed bioinformatic analysis; M.E., E.R.D., E.F.C.-O., and J.T.S. performed mathematical modeling; S.R., M.E., and D.P. were responsible for data visualization; H.-P.K. acquired funding; and S.R., M.E., D.P., H.-P.K., E.R.D., E.F.C.-O., and J.T.S. wrote the manuscript.
Conflict-of-interest disclosure: S.R. is a consultant to Forty-Seven Inc (Gilead Sciences) and Ensoma Inc. H.-P.K. is, or was, a consultant to and has or had ownership interests with Rocket Pharmaceuticals, Homology Medicines, VOR Biopharma and Ensoma Inc, and has also been a consultant to CSL Behring and Magenta Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Stefan Radtke, Fred Hutchinson Cancer Center, PO Box 19024, 1100 Fairview Ave, Seattle, WA 98109-1024; e-mail: sradtke@fredhutch.org; and Hans-Peter Kiem, Fred Hutchinson Cancer Center, PO Box 19024, 1100 Fairview Ave, Seattle, WA 98109-1024; e-mail: hkiem@fredhutch.org.
References
Author notes
∗S.R. and M.E. contributed equally to this study.
Custom scripts are available on GitHub (https://github.com/KiemLab-RIS).
Integration site analysis and cellular indexing of transcriptomes and epitopes by sequencing data reported in this article have been deposited in the National Center for Biotechnology BioProject ID PRJNA756001 and CellTag barcoding data in National Center for Biotechnology BioProject PRJNA906058.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Longitudinal assessment of gene-modified WBCs and HSPCs in the PB and BM. (A) Two pigtail macaques were transplanted with gene-modified HSPCs after myeloablative conditioning with total body irradiation. Animals were followed-up for up to 50 months through taking PB and BM draws to measure the gene marking in PB WBCs (top), PB lineages (middle), and BM-derived HSPCs (bottom). (B) Bulk PB WBCs were collected and DNA banked for ISA as indicated with gray symbols, whereas PB blood lineages (T cells: CD3+, B cells: CD20+, natural killer [NK] cells: CD16+, monocytes: CD14+, and granulocytes: CD11b+CD14–) were FACS purified as indicated with black symbols. From the BM, CD34+ HSPCs were FACS purified and the DNA banked for ISA. (C) Summary of the animal characteristics, ISA sample collection, and analysis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/1/10.1182_blood.2022018564/2/m_blood_bld-2022-018564-gr1.jpeg?Expires=1769106512&Signature=Wgyp2CHYjhcoQruoyU68T08ftfMZ-JDBfAuWMo-rUqqN9ksWySenYfIaha8cZDkIZY9AlRIOjAtO4soG0E25VZY5w0aelgN-JYZTmQqUmz-5mVCcWIGSidjroj8YPXJl63adIfIRQ1pdmOrirfomguTMmWhpbm1HQE9f~tqDZcQilI1hwDFyfW1a6LG2t3HouMabl53FvpLjoJBf-BJ~FQ7cObYX3aURMNrDdazUAcG-SQ1kEXdakwGpEbZdq7mA3FNx9j6MDx6HR8aRrnjuL-fPK5PHuD5L-DyUROwhXowiTnX-fut1mXNmn-f9QHTj8lVVP1BrOpHBcWZOMgGyQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal