Key Points
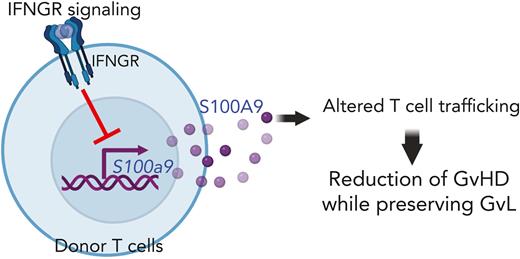
S100A9 upregulated by IFNGR signaling blockade functions as a novel GVHD suppressor without compromising GVL in mice.
Administration of recombinant S100A9 proteins or upregulation of S100A9 by anti-IFNGRα antibodies reduces GVHD in mice.
Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative treatment for both malignant and nonmalignant hematologic disorders. However, graft-versus-host disease (GVHD) and malignant relapse limit its therapeutic success. We previously demonstrated that the blockade of interferon-gamma receptor (IFNGR) signaling in donor T cells resulted in a reduction in GVHD while preserving graft-versus-leukemia (GVL) effects. However, the underlying molecular mechanisms remain inconclusive. In this study, we found that S100A9 is a novel GVHD suppressor upregulated when IFNGR is blocked in T cells. Both Ifngr1−/− and S100a9-overexpressing T cells significantly reduced GVHD without compromising GVL, altering donor T-cell trafficking to GVHD target organs in our mouse model of allo-HSCT. In addition, in vivo administration of recombinant murine S100A9 proteins prolongs the overall survival of recipient mice. Furthermore, in vivo administration of anti-human IFNGRα neutralizing antibody (αhGR-Nab) significantly upregulates the expression of S100A9 in human T cells and improved GVHD in our mouse model of xenogeneic human peripheral blood mononuclear cell transplantation. Consistent with S100a9-overexpressing T cells in our allo-HSCT model, αhGR-Nab reduced human T-cell trafficking to the GVHD target organs. Taken together, S100A9, a downstream molecule suppressed by IFNGR signaling, functions as a novel GVHD suppressor without compromising GVL.
Introduction
We previously reported that Ifngr1−/− T cells significantly improved graft-versus-host disease (GVHD) severity and overall survival without reducing graft-versus-leukemia (GVL) in our mouse models of allogeneic hematopoietic stem cell transplantation (allo-HSCT).1-3 Several mechanisms underlying the reduction of GVHD by Ifngr1−/− T cells have been proposed by several groups including ours: (1) alteration of allogeneic T-cell trafficking to GVHD target organs by down-regulating CXCR3 expression1,3; (2) an increase in Treg cells by reducing IRF1 that inhibits Foxp3 gene expression4; and (3) preferential T-cell differentiation to Th2 (GATA3+) over Th1 (T-bet+) cells.3,5 Although each of these proposed mechanisms plays a role in GVHD reduction by Ifngr1−/− T cells, none of these are definitive: (1) Cxcr3−/− T cells only partially reduced GVHD,1 (2) Treg-depleted Ifngr1−/− T cells could partially but significantly reduce GVHD (J. Ritchey and J. Choi, unpublished data, February 4, 2014), and (3) GATA3-overexpressing T cells failed to reduce GVHD.3 All of these suggest that Ifngr1−/− T cells use multiple pathways to reduce GVHD. To the best of our knowledge, this is the first study to demonstrate that targeting interferon-gamma receptor (IFNGR) signaling in T cells results in a reduction of GVHD while maintaining GVL through the overexpression of S100A9, a novel GVHD suppressor molecule.
Study design
Mouse models of GVHD/GVL
Allo-HSCT (B6 to Balb/c with/without A20 cells) was performed as previously described.1-3,6-8 For the xenograft model, 5 × 106 human peripheral blood mononuclear cells (PBMCs) were transplanted on day 0 into sublethally irradiated (250 cGy at day −1) NOD/SCID/γcnull (NSG) mice. Detailed information on the mouse model of GVHD/GVL and all other methods are described in the supplemental Data (available on the Blood website).
Results and discussion
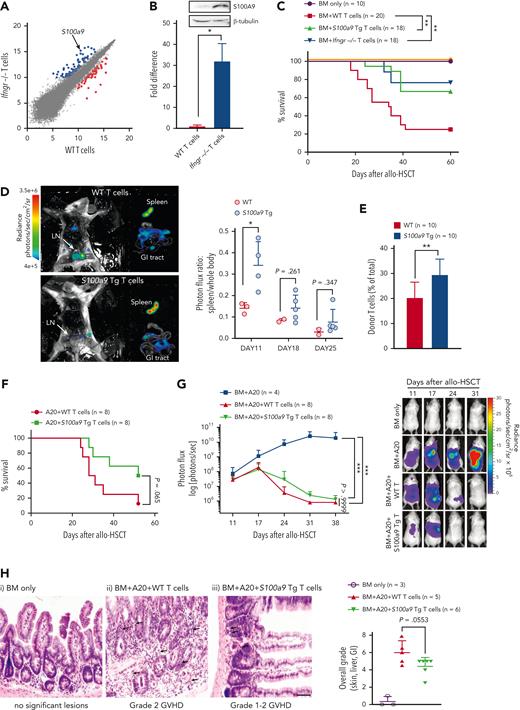
To identify the GVHD suppressor genes expressed in alloreactive Ifngr1−/− donor T cells, we performed RNA profiling analyses on T cells (wild-type [WT] or Ifngr1−/−) stimulated with allogeneic antigen-presenting cells (supplemental Table 1). The expression of S100a9 was upregulated 26-fold in Ifngr1−/− T cells compared with that in WT T cells (Figure 1A). Further validation by quantitative polymerase chain reaction and western blot analysis also showed a significant increase in S100a9 messenger RNA and protein expression in Ifngr1−/− T cells compared with that in WT T cells (Figure 1B). We then determined whether S100A9 acts as a suppressor molecule that Ifngr1−/− T cells use to reduce GVHD. We found that both Ifngr1−/− and S100a9-overexpressing T cells significantly increased the overall survival of recipients compared with WT T cells after allo-HSCT (Figure 1C).
S100a9-overexpressing T cells separate GVHD from GVL, increasing T-cell retention in the spleen. (A) WT or Ifngr1−/− CD4+CD25− T cells obtained from C57BL/6 were cocultured with irradiated (2000 rad) whole splenocytes obtained from Balb/c as alloantigen stimulators. After 6 days of coculture, total RNA was purified from CD4+CD25+ cells sorted by flow cytometry. RNA profiling analyses were performed using the Mouse Genome 430 2.0 array. Shown are up- (blue dots) or down-(red dots) regulated genes in Ifngr1−/− T cells. (B) Expression of S100a9 mRNA and S100A9 protein was determined using real-time PCR and western blotting, respectively. (C) Allo-HSCT was performed as follows; 5 × 106 TCD-BM (CD45.1+ WT) and 5 × 105 T cells (CD45.2+ WT, Ifngr1−/−, or S100a9-Tg) obtained from B6 mice were transplanted on day 0 into lethally irradiated (900 cGy on day −1) Balb/c allogeneic recipient mice. The TCD-BM only group serves as no GVHD control. The mice were monitored for survival. A pool of 3 independent experiments is shown. (D) In vivo BLI was performed to track T cells after allo-HSCT. The representative BLI images are obtained from dissected mice transplanted with WT (upper) and S100a9-overexpressing T cells (lower) on day 11 after allo-HSCT. Photon flux (photons/s) was measured from the spleen, GI tract, and the rest of the whole body. The ratio of signal intensities (photons/s per cm2 per sr) from the spleen and the rest of the body were compared (right panel). (E) The percent donor T cells in the spleens of recipient mice transplanted with WT or S100a9-overexpressing T cells was measured on day 21 after allo-HSCT. The donor T cells were determined by H2-Kd− and CD45.2+. (F-H) Allo-HSCT was performed as follows; luciferase/RFP-expressing A20 leukemia cells (1 × 105) were injected on the day of TCD-BM cell infusion (day 0), followed by delayed donor lymphocyte infusion (DLI; 2 × 106 T cells) on day 11. The leukemia burden was measured weekly using BLI. A pool of 2 independent experiments. (F) Survival rate and (G) leukemia burden. Representative images of each group from days 11 to 31 are shown. (H) Representative images of the small intestine of each group on day 21 after allo-HSCT. (i, TCD-BM only; ii, TCD-BM+A20+WT T cells; and iii, TCD-BM+A20+S100a9-overexpressing T cells). TCD-BM only group has no significant lesions. The arrows indicate crypt apoptosis, luminal debris, and crypt dropout. The scale bar represents 100 μm. Overall histological grades on day 21 after allo-HSCT (right panel). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. All error bars are presented as mean ± standard deviation. BLI, bioluminescence imaging; GI, gastrointestinal; mRNA, messenger RNA; PCR, polymerase chain reaction; TCD, T-cell depleted.
S100a9-overexpressing T cells separate GVHD from GVL, increasing T-cell retention in the spleen. (A) WT or Ifngr1−/− CD4+CD25− T cells obtained from C57BL/6 were cocultured with irradiated (2000 rad) whole splenocytes obtained from Balb/c as alloantigen stimulators. After 6 days of coculture, total RNA was purified from CD4+CD25+ cells sorted by flow cytometry. RNA profiling analyses were performed using the Mouse Genome 430 2.0 array. Shown are up- (blue dots) or down-(red dots) regulated genes in Ifngr1−/− T cells. (B) Expression of S100a9 mRNA and S100A9 protein was determined using real-time PCR and western blotting, respectively. (C) Allo-HSCT was performed as follows; 5 × 106 TCD-BM (CD45.1+ WT) and 5 × 105 T cells (CD45.2+ WT, Ifngr1−/−, or S100a9-Tg) obtained from B6 mice were transplanted on day 0 into lethally irradiated (900 cGy on day −1) Balb/c allogeneic recipient mice. The TCD-BM only group serves as no GVHD control. The mice were monitored for survival. A pool of 3 independent experiments is shown. (D) In vivo BLI was performed to track T cells after allo-HSCT. The representative BLI images are obtained from dissected mice transplanted with WT (upper) and S100a9-overexpressing T cells (lower) on day 11 after allo-HSCT. Photon flux (photons/s) was measured from the spleen, GI tract, and the rest of the whole body. The ratio of signal intensities (photons/s per cm2 per sr) from the spleen and the rest of the body were compared (right panel). (E) The percent donor T cells in the spleens of recipient mice transplanted with WT or S100a9-overexpressing T cells was measured on day 21 after allo-HSCT. The donor T cells were determined by H2-Kd− and CD45.2+. (F-H) Allo-HSCT was performed as follows; luciferase/RFP-expressing A20 leukemia cells (1 × 105) were injected on the day of TCD-BM cell infusion (day 0), followed by delayed donor lymphocyte infusion (DLI; 2 × 106 T cells) on day 11. The leukemia burden was measured weekly using BLI. A pool of 2 independent experiments. (F) Survival rate and (G) leukemia burden. Representative images of each group from days 11 to 31 are shown. (H) Representative images of the small intestine of each group on day 21 after allo-HSCT. (i, TCD-BM only; ii, TCD-BM+A20+WT T cells; and iii, TCD-BM+A20+S100a9-overexpressing T cells). TCD-BM only group has no significant lesions. The arrows indicate crypt apoptosis, luminal debris, and crypt dropout. The scale bar represents 100 μm. Overall histological grades on day 21 after allo-HSCT (right panel). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. All error bars are presented as mean ± standard deviation. BLI, bioluminescence imaging; GI, gastrointestinal; mRNA, messenger RNA; PCR, polymerase chain reaction; TCD, T-cell depleted.
To define the mechanisms by which S100A9 reduces GVHD, we tested whether overexpressed S100a9 in T cells alters T-cell trafficking to GVHD target organs, as we previously reported in Ifngr1−/− T cells.1 The S100a9-overexpressing T cells displayed a significant increase in donor T-cell retention in the spleen compared with WT T cells after allo-HSCT (Figure 1D-E). Notably, S100a9-overexpressing T cells have no defects in proliferation, differentiation (Th1, Th2, Th17, and Tregs), and expression of CXCR3 and S1PR1, which are critical for T-cell trafficking9,10 (supplemental Figures 1-3). In addition, S100a9-overexpressing T cells did not increase the frequency of myeloid–derived suppressor cells (supplemental Figure 4).11 These results indicate that the GVHD suppressor function of S100a9-overexpressing T cells is independent of these factors.
Next, we examined whether S100a9-overexpressing donor bone marrow (BM) cells also reduced GVHD. The overexpression of S100a9 in donor T cells, but not in donor BM cells, reduced GVHD, as S100a9-overexpressing donor BM cells did not change overall survival (supplemental Figure 5). In addition, the frequencies of donor BM-derived B cells, an indicator of healthy immune reconstitution and less GVHD, were higher in the peripheral blood of mice transplanted with S100a9-overexpressing T cells than in those of WT T cells along with either WT or S100a9-overexpressing BM cells.1 These results suggest that the expression of S100a9 in donor T cells, but not in donor BM cells, attenuates GVHD.
Based on our previous report that Ifngr1−/− donor T cells prevent GVHD without compromising GVL, we examined whether S100a9-overexpressing T cells preserve the beneficial GVL effect after allo-HSCT. The mice transplanted with S100a9-overexpressing T cells demonstrated improved overall survival with equivalent tumor clearance compared with those transplanted with WT T cells (Figure 1F-G; supplemental Figure 6A). In addition, we found significantly reduced overall histological GVHD grades, clinical GVHD scores, and weight loss in mice transplanted with S100a9-overexpressing T cells compared with those transplanted with WT T cells (Figure 1H; supplemental Figure 6B-C). These observations suggest that the overexpression of S100a9 in donor T cells resulted in the reduction of GVHD while preserving the beneficial GVL effect.
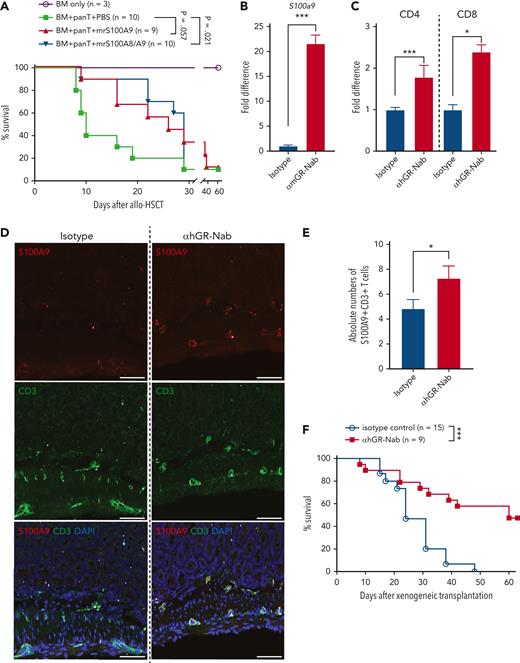
As we found that S100A9 was overexpressed in Ifngr1−/− T cells, we tested whether in vivo administration of recombinant murine S100A9 (mrS100A9) could suppress GVHD in mice. We orally administered mrS100A9 and mrS100A8 proteins to maximize the bioavailability of the proteins in the gut, where the proteins exert antimicrobial functions by forming S100A9 homodimers and S100A8/A9 heterodimers.12-14 Although the optimal timing and dose of mrS100A9 proteins remain undetermined, mrS100A9 proteins with or without mrS100A8 prolonged survival and reduced GVHD compared with the vehicle control (Figure 2A; supplemental Figure 7). As gastrointestinal GVHD is associated with significant changes in the intestinal microbiota and antimicrobial functions of immune cells against pathogenic microorganisms,15 mrS100A9 might have contributed to the modulation of the intestinal microbiota to reduce GVHD. Nonetheless, its effects on microbiota in our study are not clear. Interestingly, fecal S100A8/A9 are biomarkers of gastrointestinal GVHD in patients.16 It is possible that S100A8/A9 are increased to serve as counter-regulatory molecules to maintain immune homeostasis in the development of GVHD after allo-HSCT.
Recombinant S100A9 proteins or upregulation of S100A9 by treatment of anti-IFNGRα antibodies reduce GVHD by altering donor cell trafficking. (A) Survival rate of mice treated with recombinant murine S100A9 with or without S100A8 proteins (1 μg/injection starting on day 0, 5 times per week for 2 weeks), after allo-HSCT. (B) T cells obtained from C57BL/6 were cocultured with irradiated (2000 rad) whole splenocytes obtained from Balb/c in the presence of αmGR-Nab (10 μg/mL) or isotype control (10 μg/mL). After 6 days of coculture, mRNA was isolated and the expression of S100a9 was determined by real-time PCR. (C) Human PBMCs were stimulated with a CD3/CD28 activator and LPS (100 ng/mL) in the presence of αhGR-Nab (10 μg/mL) or isotype control (10 μg/mL). After 4 days, CD4 and CD8 T cells were sorted by flow cytometry and mRNA was extracted from them. The expression of S100A9 was determined by real-time PCR. (D-F) Xenogeneic cell transplantation was performed as follows. 5 × 106 human PBMCs were transplanted on day 0 into sublethally irradiated (250 cGy at day −1) NSG recipient mice. αhGR-Nab (200 μg/injection) was administered on days 0, 3, 7, and 10. (D) On day 14 after xenogeneic cell transplantation, intestines were harvested, and immunofluorescence staining was performed with anti-human S100A9 (red) and CD3 (green). DAPI was used for counterstaining (blue). (E) The absolute numbers of S100A9+CD3+ T cells in mm2 of the small intestines are shown. (F) Survival rate after xenogeneic cell transplantation. Shown is a pool of 2 independent experiments. The scale bar represents 50 μm. ∗P < .05 and ∗∗∗P < .001. The error bars for panels B-C and F are represent the mean ± standard deviation and mean ± SEM, respectively. mRNA, messenger RNA; PCR, polymerase chain reaction; SEM, standard error of the mean.
Recombinant S100A9 proteins or upregulation of S100A9 by treatment of anti-IFNGRα antibodies reduce GVHD by altering donor cell trafficking. (A) Survival rate of mice treated with recombinant murine S100A9 with or without S100A8 proteins (1 μg/injection starting on day 0, 5 times per week for 2 weeks), after allo-HSCT. (B) T cells obtained from C57BL/6 were cocultured with irradiated (2000 rad) whole splenocytes obtained from Balb/c in the presence of αmGR-Nab (10 μg/mL) or isotype control (10 μg/mL). After 6 days of coculture, mRNA was isolated and the expression of S100a9 was determined by real-time PCR. (C) Human PBMCs were stimulated with a CD3/CD28 activator and LPS (100 ng/mL) in the presence of αhGR-Nab (10 μg/mL) or isotype control (10 μg/mL). After 4 days, CD4 and CD8 T cells were sorted by flow cytometry and mRNA was extracted from them. The expression of S100A9 was determined by real-time PCR. (D-F) Xenogeneic cell transplantation was performed as follows. 5 × 106 human PBMCs were transplanted on day 0 into sublethally irradiated (250 cGy at day −1) NSG recipient mice. αhGR-Nab (200 μg/injection) was administered on days 0, 3, 7, and 10. (D) On day 14 after xenogeneic cell transplantation, intestines were harvested, and immunofluorescence staining was performed with anti-human S100A9 (red) and CD3 (green). DAPI was used for counterstaining (blue). (E) The absolute numbers of S100A9+CD3+ T cells in mm2 of the small intestines are shown. (F) Survival rate after xenogeneic cell transplantation. Shown is a pool of 2 independent experiments. The scale bar represents 50 μm. ∗P < .05 and ∗∗∗P < .001. The error bars for panels B-C and F are represent the mean ± standard deviation and mean ± SEM, respectively. mRNA, messenger RNA; PCR, polymerase chain reaction; SEM, standard error of the mean.
Lastly, we examined whether anti-murine/human IFNGRα neutralizing antibodies (αmGR-Nab and αhGR-Nab) also reduced GVHD through upregulation of S100A9. Treatment with αmGR-Nab and αhGR-Nab upregulated the expression of S100a9 in mouse and human T cells, respectively (Figure 2B-C). Likewise, in vivo administration of αhGR-Nab significantly increased the number of S100A9-expressing human T cells in the intestine (Figure 2D-E). In addition, αhGR-Nab significantly improved overall survival, clinical GVHD scores, and body weight loss compared with the isotype control in our mouse model of human PBMC–mediated xenogeneic GVHD17,18 (Figure 2F; supplemental Figure 8). Because we found that both Ifngr1−/− (refer to Choi et al1) and S100a9-overexpressing donor T cells display altered donor T-cell trafficking, we examined whether αhGR-Nab also alters human T-cell trafficking to GVHD target organs in our xenogeneic transplantation model. Strikingly, human donor cells were observed significantly less in the liver and lungs (2 of the major GVHD target organs of NSG mice after human PBMC–mediated xenogeneic transplantation)19 of the mice treated with αhGR-Nab compared with the control mice, even though there was no difference in the number of T cells that infiltrated the intestines and skin, the other 2 GVHD target organs19 (supplemental Figure 9).
To date, separating GVHD from the beneficial GVL is a major goal of allo-HSCT because current immune suppressants reduce both GVHD and GVL, thereby increasing malignancy relapse.20 Although several therapeutic strategies to reduce GVHD without abrogating GVL in animal models and human patients have been proposed, the mechanisms by which allogeneic donor T cells differentially modulate GVHD and GVL remain largely unknown. Our studies demonstrated that blockade of the IFNGR signaling in T cells upregulates S100A9, a novel effector molecule that selectively suppresses GVHD over GVL. Thus, our findings provide new insights into the mechanism by which Ifngr1−/− T cells reduce GVHD while maintaining GVL levels.
Acknowledgment
J.C. is a Gabrielle’s Angel Foundation for Cancer Research (GAFCR) Research Fellow and supported by Amy Strelzer Manasevit Research Program which is funded through Be The Match Foundation and the National Marrow Donor Program, the Alvin J. Siteman Cancer Center through The Foundation for Barnes-Jewish Hospital and the National Institutes of Health (NIH), National Cancer Institute (NCI) (P30 CA091842; the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH). St. Baldrick’s Foundation, and Washington University SPORE in Leukemia, SPORE Diversity Program (NIH, NCI grant P50 CA171963), NIH, National Institute of Allergy and Infectious Diseases (R21 AI155990), Emerson Collective, and the Alvin J. Siteman Cancer Center through The Foundation for Barnes-Jewish Hospital. J.F.D. is supported by NIH, NCI grant R35 CA210084-01. M.L.C. is supported by Washington University Molecular Imaging Center pilot Research Project. The Washington University Molecular Imaging Center provided bioluminescence imaging and is supported by the NIH, Office of the Director grant S10 OD027042 and NIH, NCI grant P30 CA091842 (Siteman Cancer Center Small Animal Cancer Imaging shared resource).
Authorship
Contribution: S.K., M.L.C., J.F.D., and J.C. conceptualized the study; S.K., M.L.C., J.F.D., and J.C. designed the methodology; S.K., S.L., B.K., J.R., K.V., J.P., L.M., A.S., F.G., S.A., M.L.C., and J.C. conducted the study and data validation; S.K., S.L., M.L.C., and J.C. performed the formal analysis; S.K., M.L.C., J.F.D., and J.C. wrote the first version of the manuscript:; S.K., M.L.C., and J.C. visualized the data; M.L.C., J.F.D., and J.C. acquired the funding; and J.C. supervised the study and is a senior author.
Conflict-of-interest disclosure: J.C. received an honorarium from Incyte Corporation, received research funding from Mallinckrodt Pharmaceuticals, and served as a consultant for Daewoong Pharmaceutical. J.F.D. served on advisory boards for Incyte Corporation, Macrogenics, Rivervest, and Bioline and is a cofounder of Magenta Therapeutics and Wugen. M.L.C. is a cofounder and Chief Scientific Officer of Wugen. The remaining authors declare no competing financial interests.
Correspondence: Matthew L. Cooper, Division of Oncology, Department of Medicine, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8007, St. Louis, MO 63110; e-mail: matthewcooper@wustl.edu; John F. DiPersio, Division of Oncology, Department of Medicine, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8007, St. Louis, MO 63110; e-mail: jdipersi@wustl.edu; and Jaebok Choi, Division of Oncology, Department of Medicine, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8007, St. Louis, MO 63110; e-mail: jchoi25@wustl.edu.
References
Author notes
Following the peer-reviewed publication of our mouse models described in this study, mice will be distributed to investigators at academic institutions wanting them for noncommercial research at no cost except for standard maintenance and transportation expenses. The recipient investigators must provide written assurance and evidence that the animals will be used solely in accord with their local Institutional Animal Care and Use Committee review, that animals will not be further distributed by the recipient without our consent, and that animals will not be used for commercial purposes. Requests for mice from for-profit corporations to use the mice commercially will be negotiated by our institution's technology transfer office. All licensing shall be subject to distribution pursuant to Washington University policies and procedures on royalty income. The technology transfer office will report any invention disclosure submitted to them to the appropriate Federal Agency. For the mice we generate, we will use standard nomenclature and receive approval from the Mouse Genome Informatics nomenclature committee (http://www.informatics.jax.org/mgihome). We will provide relevant genotyping protocols upon request.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal