Visual Abstract
Professional illustration by Patrick Lane, ScEYEnce Studios.
Abstract
Adoptively transferred virus-specific T cells (VSTs) have shown remarkable safety and efficacy for the treatment of virus-associated diseases and malignancies in hematopoietic stem cell transplant (HSCT) recipients, for whom VSTs are derived from the HSCT donor. Autologous VSTs have also shown promise for the treatment of virus-driven malignancies outside the HSCT setting. In both cases, VSTs are manufactured as patient-specific products, and the time required for procurement, manufacture, and release testing precludes their use in acutely ill patients. Further, Good Manufacturing Practices–compliant products are expensive, and failures are common in virus-naive HSCT donors and patient-derived VSTs that are rendered anergic by immunosuppressive tumors. Hence, highly characterized, banked VSTs (B-VSTs) that can be used for multiple unrelated recipients are highly desirable. The major challenges facing B-VSTs result from the inevitable mismatches in the highly polymorphic and immunogenic human leukocyte antigens (HLA) that present internally processed antigens to the T-cell receptor, leading to the requirement for partial HLA matching between the B-VST and recipient. HLA mismatches lead to rapid rejection of allogeneic T-cell products and graft-versus-host disease induced by alloreactive T cells in the infusion product. Here, we summarize the clinical outcomes to date of trials of B-VSTs used for the treatment of viral infections and malignancies and their potential as a platform for chimeric antigen receptors targeting nonviral tumors. We will highlight the properties of VSTs that make them attractive off-the-shelf cell therapies, as well as the challenges that must be overcome before they can become mainstream.
Introduction
T cells expressing native T-cell receptors (TCRs) have exquisite target antigen specificity and can effectively treat viral infections and malignancies without the side effects of conventional therapies.1,2 In contrast to T cells expressing chimeric antigen receptors (CARs) or transgenic TCRs,3-6 virus-specific T cells (VSTs) recognize multiple epitopes in multiple viral antigens, diminishing the likelihood of immune escape.7 VSTs have been most effective in the allogeneic hematopoietic stem cell transplant (HSCT) setting, where HSCT donor–derived (DD)-VSTs can prevent or cure diseases caused by viruses including Epstein-Barr virus (EBV), cytomegalovirus (CMV), adenoviruses (AdVs), BK virus (BKV), JC virus, and human herpesvirus 6 (HHV-6).8-16 Patient-derived (autologous) VSTs have also been successful for the treatment of EBV and human papilloma virus–associated malignancies occurring outside the HSCT setting.17-22
Despite the clinical successes of autologous- and DD-VSTs, major problems limit their broader application.23 First, the time taken to expand VSTs (up to 16 days), combined with up to 14 days for sterility and other quality control testing, means that T cells are not available for acute need. Although VSTs can be selected directly from blood, either using peptides conjugated to recombinant major histocompatibility complex molecules (tetramers, multimers, or streptamers) or by selection of cells that secrete interferon gamma in response to antigen stimulation (γ-capture), the large volumes (>500 mL) of donor blood required to generate sufficient VSTs for a single infusion preclude the generation of banks and effectively make these products patient-specific. Second, the donor must have an immunological memory to the problem virus. Third, unrelated HSCT donors are often unavailable for additional blood draws. Fourth, in the case of autologous products, T cells derived from patients are often damaged by chemotherapy and immunosuppressed by their tumors, resulting in manufacturing failures or low antigen specificity. Fifth, the generation of a separate product for each patient has a high cost. Hence, there is great interest in the use of banked, cryopreserved VSTs (B-VSTs) that can be expanded exponentially in vitro from the blood of selected healthy donors. B-VSTs can be characterized for phenotype, specificity, and function and are immediately available for clinical use. Each B-VST line can be used to treat up to 30 patients, or more if prepared as a “master” cell bank, which requires significant additional testing. B-VSTs are also applicable for viral infections in solid organ transplant (SOT) recipients,24-29 for virus-associated malignancies outside the transplant setting,30 and for lethal viral diseases, such as COVID-19.31,32 Beyond viral targets, the attractive properties of VSTs can be harnessed to target nonviral antigens after modification with CARs.33-37
Here, we explain the challenges to the clinical use of B-VSTs, namely the HLA restriction of antigen recognition and the dual threats of graft-versus-host disease (GVHD) and host-versus-graft rejection, mediated by donor or host-derived alloreactive T cells, respectively. We will then summarize results to date from clinical trials of B-VST treatment in immunocompromised patients with opportunistic viral-driven diseases and patients with chemotherapy-refractory virus expressing cancers. Finally, we will discuss B-VSTs as vehicles for genetic modifications that extend their applicability to nonviral targets by expressing CAR or recombinant TCRs or that enhance their function, for example, by expressing transgenic cytokines or constitutively active cytokine receptors.
Challenges to clinical use of allogeneic B-VSTs
HLA restriction
Viral antigens in infected cells are processed intracellularly into short peptides and carried to the cell surface on HLA class I and class II molecules, where they are recognized by the TCR as a complex of peptides and the polymorphic antigen-presenting domains of HLA molecules.38 Recognition of both peptide and HLA molecule leads to the HLA restriction of TCR recognition, whereby each virus-specific TCR recognizes a peptide in the context of a particular HLA molecule. Therefore, B-VST selected for infusion must recognize viral peptides through “shared” HLA alleles. Because a given VST line is unlikely to recognize viral peptides through all 12 possible surface HLA class I and II alleles, knowledge of which HLA antigens present viral peptides recognized by a B-VST line is critical. For this reason, VST “banks” should include multiple donor products with diverse HLA types that adequately cover the HLA types of the recipient population.
Alloreactivity
Allogeneic HLA molecules are highly immunogenic, resulting in the dual risks of GVHD from alloreactive T cells in the infusion product and graft rejection by alloreactive T cells in the host. The TCR repertoire of VSTs is greatly reduced compared with the CD3-activated T cells generally used to generate CAR T cells. This reduced diversity lowers the potential for alloreactivity,39,40 and indeed, the lack of GVHD reported in multiple clinical trials (>300 recipients) of B-VSTs confirms their suitability as a safe off-the-shelf (OTS) cell therapy.24-32,41-46 However, allogeneic cells are rapidly rejected when administered to HLA-incompatible hosts, and because exponential expansion and persistence of T cells are associated with durable antitumor or antiviral efficacy, host-versus-graft rejection remains a major challenge to be overcome before B-VSTs can enter mainstream use.
VSTs in the immunocompromised host
Immunosuppression increases the risk for viral infection and reactivation
Severely immunosuppressed patients are susceptible to severe infections from common community viruses. Persistent viruses such as the human herpes viruses (HHVs) establish clinically silent, lifelong persistence after acute primary infection but can reactivate, causing lethal disease in immunosuppressed hosts. For example, EBV can transform normal B cells into permanently growing lymphoblastoid cell lines in vitro, and after T-cell depleted allogeneic HSCT, it can produce posttransplant lymphoproliferative disorders (PTLDs) ranging from benign lymphoproliferations to monoclonal lymphoma, which is fatal without intervention.47 Other viral reactivations like CMV or HHV-6 can cause life-threatening multiorgan diseases such as pneumonia, hepatitis, gastroenteritis, or retinitis. Here, we will discuss the clinical experience of using VSTs to treat the most common opportunistic viral diseases of HSCT recipients, including CMV, EBV reactivations and PTLD, HHV-6, AdVs, and BKV.
HSCT DD-VSTs
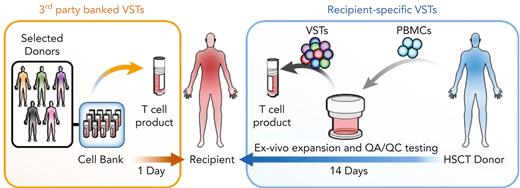
In the earliest clinical trials, EBV-specific T cells (EBVSTs) generated from peripheral blood mononuclear cells of the HSCT donor (DD-VSTs) produced complete and durable responses in patients with EBV reactivations and PTLD.8-11 Because the VSTs were from HLA-compatible stem cell donors, they were not rejected, nor did they produce severe GVHD. These trials were modified to add specificity for CMV and AdV with similar clinical antiviral activity against all 3 viruses.12 Since then, several groups have extended the spectrum of targeted viruses to cover the most problematic posttransplant viral reactivations and infections, adding specificities for HHV-6 and BKV, and with improved culture conditions, manufacturing has been reduced from ∼3 months to 10 days with similar clinical results.13-16,48-50 GVHD after DD multivirus-specific T cells (MVSTs) administration was minimal, and 80% to 90% of patients with drug-resistant viral reactivations and PTLD had clinical responses that coincided with the in vivo expansion of VSTs after infusion.8-16,49,50 This success prompted the development of B-VSTs from virus-immune donors. These are less expensive per patient, are immediately available, and have proven successful in the clinic (Figure 1).
Rapid availability and cost-effectiveness of B-VSTs vs recipient-specific VSTs. B-VSTs from a small number of donors are expanded in vitro and cryopreserved as a bank. They can be thawed and used immediately for multiple recipients. By contrast, recipient-specific VSTs that are manufactured from the HSCT donor, require up to 4 weeks or longer to complete manufacture and quality assurance/quality control (QA/QC) testing and are used only for a single recipient.
Rapid availability and cost-effectiveness of B-VSTs vs recipient-specific VSTs. B-VSTs from a small number of donors are expanded in vitro and cryopreserved as a bank. They can be thawed and used immediately for multiple recipients. By contrast, recipient-specific VSTs that are manufactured from the HSCT donor, require up to 4 weeks or longer to complete manufacture and quality assurance/quality control (QA/QC) testing and are used only for a single recipient.
B-VSTs after solid organ transplantation
B-VSTs were first evaluated by Haque et al24 for the treatment of EBV-driven PTLD in SOT recipients, who have a median survival of 31.5 months after diagnosis.51 Multiple infusions of partially HLA-matched B-VSTs produced complete responses (CRs) in 3 of 8 (37.5%) SOT recipients that had failed conventional measures, including withdrawal of immunosuppression and rituximab. In a follow-up study of 33 patients, response rates were up to 64%, and no cases of GVHD were reported.26 In both trials, patients with “early” PTLD, defined as arising within 2 years of transplant, those with a low burden of disease and those most closely HLA matched with the B-VST line were more likely to respond. When comparing outcomes of B-VST infusions to treat PTLD after SOT and HSCT, Prockop et al showed that 67% of HSCT recipients (22/33) and 54% of SOT recipients (7/13) achieved objective responses (either CR or partial response).29 Only 45% of HSCT recipients and 23% of SOT recipients had responses to a single infusion, suggesting that persistent activity by repeat infusions was required to produce a tumor response. These investigators also showed better outcomes with lower disease burden (<3 sites of involvement) with a response rate of 80% vs 52% for those with >3 sites, or when treated earlier in relation to other therapies (45% when used after rituximab and chemotherapy vs 80% when used after rituximab alone). The better responses of HSCT recipients to B-VSTs may be explained by the more lymphodepleted state of HSCT recipients and that it is rarely possible to completely withdraw active immunosuppression from SOT recipients, limiting both the expansion of the infusion product and the reactivation of endogenous immunity. Common themes that emerged from B-VST trials for PTLD include little or no risk for inducing GVHD and that better outcomes were associated with (1) earlier administration in the disease course, (2) a lower burden of disease, and (3) multiple infusions.28
Banked third-party VSTs in the HSCT setting
In the HSCT setting, allogeneic VSTs not derived from the stem cell donor are commonly referred to as third-party VSTs and may be only partially HLA matched (≥1 matches) between the hematopoietic stem cell donor and recipient. In 2 phase 2 trials, we evaluated B-MVSTs generated using different manufacturing strategies.41,42 In the first multicenter study, 50 HSCT recipients with drug-refractory or drug-intolerant CMV, EBV, or AdV received VSTs generated by stimulation with Ad-pp65–modified EBV–lymphoblastoid cell lines. Complete or partial responses were seen in 74% of patients, and individual viral response rates are listed in Table 1.41 In the second trial, MVSTs targeting HHV-6 and BKV, as well as EBV, CMV, and AdV, were stimulated for 10 days with overlapping peptide libraries representing viral proteins predicted to elicit protective T cells. A single infusion of 2 × 107 B-VSTs produced a cumulative response rate of 92% and rarely induced GVHD (<10% de novo).42 However, not all lines recognized all 5 viruses (range, 0-5), with only 35 of 59 (59%) lines recognizing ≥3 viruses, and the HLA restriction of each virus specificity was not studied. Banks at other centers have reported similarly promising virologic response rates with low associated toxicity43-46 (Table 1). A key observation from studies on VSTs in HSCT recipients is that VSTs targeting BKV and AdV, are often predominantly HLA class II restricted. Thus, HLA matching at both class I and II alleles is important for B-VST line selection.
Clinical studies of allogeneic B-VSTs for opportunistic viral diseases
| Publication . | Target . | Number of patients . | Safety . | Response . |
|---|---|---|---|---|
| EBV | ||||
| Haque et al, 200224 | EBV-PTLD | 8 | 0 | 3 CR |
| Sun et al, 200225 | EBV-PTLD | 2 | 0 | 1 CR |
| Haque et al, 200726 | EBV-PTLD | 33 | 1 aGVHD | 19 CR |
| Gallot et al, 201427 | Opportunistic EBV-lymphoma | 11 | 1 fever | 3 CR |
| Kazi et al, 201928 | EBV-PTLD | 59 | 2 skin only aGVHD | 23 CR |
| Prockop et al, 202029 | EBV-PTLD | 46 | 1 aGVHD | 21 CR |
| Other viruses | ||||
| Leen et al, 201341 | EBV | 9 | 8 aGVHD | EBV: 2 CR |
| AdV | 23 | AdV: 9 CR | ||
| CMV | 18 | CMV: 7 CR | ||
| Tzannou et al, 201742 | EBV | 2 | 3 de novo aGVHD | EBV: 2 CR |
| AdV | 9 | AdV: 5 CR | ||
| CMV | 19 | 3 aGVHD reactivations | CMV: 9 CR | |
| BKV | 20 | BKV: 6 CR | ||
| HHV-6 | 4 | HHV-6: only partial response | ||
| Withers et al, 201743 | CMV | 28 | 2 de novo aGVHD | CMV: 22 CR |
| EBV | 1 | EBV: 0 CR | ||
| AdV | 1 | AdV: 1 CR | ||
| Tzannou et al, 201952 | CMV | 10 | 0 | CMV: 7 CR |
| Nelson et al, 202044 | BKV | 24 | 1 de novo aGVHD | BK: 77% CR |
| Rubinstein et al, 202145 | AdV | 23 | 1 de novo aGVHD | AdV: 42% CR |
| Olson et al, 202146 | BKV | 59 | 1 de novo aGVHD | BK: 67% CR |
| 1 aGVHD reactivation | ||||
| Jiang et al, 202253 | CMV | 27 | 4 aGVHD | CMV: 25 CR |
| EBV | 3 | EBV: 3 CR | ||
| Pei et al, 202254 | CMV | 31 | 3 aGVHD | CMV: 80.6% CR |
| Publication . | Target . | Number of patients . | Safety . | Response . |
|---|---|---|---|---|
| EBV | ||||
| Haque et al, 200224 | EBV-PTLD | 8 | 0 | 3 CR |
| Sun et al, 200225 | EBV-PTLD | 2 | 0 | 1 CR |
| Haque et al, 200726 | EBV-PTLD | 33 | 1 aGVHD | 19 CR |
| Gallot et al, 201427 | Opportunistic EBV-lymphoma | 11 | 1 fever | 3 CR |
| Kazi et al, 201928 | EBV-PTLD | 59 | 2 skin only aGVHD | 23 CR |
| Prockop et al, 202029 | EBV-PTLD | 46 | 1 aGVHD | 21 CR |
| Other viruses | ||||
| Leen et al, 201341 | EBV | 9 | 8 aGVHD | EBV: 2 CR |
| AdV | 23 | AdV: 9 CR | ||
| CMV | 18 | CMV: 7 CR | ||
| Tzannou et al, 201742 | EBV | 2 | 3 de novo aGVHD | EBV: 2 CR |
| AdV | 9 | AdV: 5 CR | ||
| CMV | 19 | 3 aGVHD reactivations | CMV: 9 CR | |
| BKV | 20 | BKV: 6 CR | ||
| HHV-6 | 4 | HHV-6: only partial response | ||
| Withers et al, 201743 | CMV | 28 | 2 de novo aGVHD | CMV: 22 CR |
| EBV | 1 | EBV: 0 CR | ||
| AdV | 1 | AdV: 1 CR | ||
| Tzannou et al, 201952 | CMV | 10 | 0 | CMV: 7 CR |
| Nelson et al, 202044 | BKV | 24 | 1 de novo aGVHD | BK: 77% CR |
| Rubinstein et al, 202145 | AdV | 23 | 1 de novo aGVHD | AdV: 42% CR |
| Olson et al, 202146 | BKV | 59 | 1 de novo aGVHD | BK: 67% CR |
| 1 aGVHD reactivation | ||||
| Jiang et al, 202253 | CMV | 27 | 4 aGVHD | CMV: 25 CR |
| EBV | 3 | EBV: 3 CR | ||
| Pei et al, 202254 | CMV | 31 | 3 aGVHD | CMV: 80.6% CR |
aGVHD, acute graft-versus-host disease.
Lessons learned when using B-VSTs after transplant
The requirement for partial HLA matching poses challenges to B-VST trials. First, VST banks must be large enough to provide partial HLA compatibility with all potential recipients. In a trial of B-CMV–specific T cells (B-CMVSTs), we could cover the HLA types of recipients in our catchment area with ≥8 HLA-diverse B-VST products.41,42,52 However, when using B-MVSTs, not all lines recognize all the viruses targeted because of the common immunological dominance of T cells specific for CMV and EBV. Further, VSTs may be restricted by only a few of the 12 possible HLA class I (A, B, and C) and class II (DR, DP, and DQ) alleles. If the restricting alleles are not known, the HLA matching may not be appropriate. Identifying HLA restriction is very complex for MVSTs with multiple virus specificities, especially when the frequency of T cells specific for a particular target virus is low. Although many studies select lines based solely on HLA matching, better characterization of VST products to identify the restricting alleles would enable more appropriate VST line selection, likely with a better outcome. Second, in transplant recipients, viral diseases may occur in either donor or recipient tissues. For example, EBV-derived PTLD occurs in donor B cells after HSCT but in recipient B cells after SOTs, whereas CMV, AdV, and BKV cause disease in recipient tissues. Hence, knowledge of both donor and recipient HLA types is required to select the most suitable VST line.
To fully evaluate the mode of action of allogeneic VSTs, it is important to track their persistence after infusion, but because B-VSTs have thus far not been gene marked, persistence is challenging to confirm. Immunoassays can monitor the frequency of VSTs before and after infusion but cannot identify the source of this activity. In a study of streptamer-selected CMVSTs, Neuenhahn et al55 compared the persistence of DD-CMVSTs and third-party donor CMVSTs by TCR sequencing of the infused product. DD-CMVST TCRs were detected in 8 of 8 recipients 7 days after infusion, whereas only 1 of 8 third-party CMVST TCRs were detected, and this CMVST product was closely HLA matched (>7 major HLA matches) with the recipient. This patient and 2 others were responders, and in these the reconstituting CMVSTs were endogenous (from the recipient). Similarly, Haque et al and Gallot et al rarely detected infused B-VSTs 7 to 10 days after infusion using either TCR spectratyping or quantitative polymerase chain reaction for infused TCRs, even though functional EBV-directed responses were detected for months after infusion.24,26,27 These findings suggest that B-VSTs were rejected and that clinical responses rely on the reactivation of recipient VSTs by the infused VST rather than on the long-term persistence of the B-VSTs. This phenomenon, known as antigen or epitope spreading, results from the release of cytokines and chemokines by the infused T cells after antigen ligation at the site of infection or tumor. These cytokines may reactivate local antigen-specific T cells, convert inhibitory myeloid cells into a more activating phenotype, and/or recruit both professional antigen-presenting cells and T cells to the disease site, creating a more favorable environment for endogenous T-cell activation. Host alloreactive T cells recognizing and rejecting the infusion product within tumors or infected tissues may enhance this effect. Notably, epitope spreading is less frequently reported after CAR T-cell infusion, likely because of the invariable and necessary lymphodepletion that precedes CAR T-cell infusions. Hence, strategies that ensure the expansion of B-VSTs after infusion while preserving epitope spreading, would be of great benefit.
B-VSTs for acute, lethal virus infections
The success of B-VSTs for viral infections and reactivations in transplant recipients has led investigators to study their therapeutic potential in other problematic viral infections, such as influenza, human metapneumovirus, respiratory syncytial virus, and COVID-19, in both immunocompromised and immunocompetent hosts.31,32,56,57 Because infections with these viruses are generally short-lived, B-VSTs may have both antiviral activity and the ability to boost the patient’s immune system before they are rejected. B-MVSTs targeting respiratory viruses (NCT04933968) and COVID-19 are currently in clinical trials in many countries, including the United States, Singapore, and Greece. Larger trials are needed to determine the success of B-VSTs in these settings as well as their mechanism of action.
B-VSTs to treat virus-associated malignancies outside the transplant setting
EBV-PTLD develops only in individuals who are severely immunosuppressed, but EBV is associated with a variety of other neoplasms such as nasopharyngeal carcinoma (NPC), leiomyosarcoma, gastric cancer, and lymphoma in individuals who are not obviously immunosuppressed.58 Six other human viruses, human papilloma virus, hepatitis B virus, hepatitis C virus, Kaposi sarcoma–associated herpesvirus, human T-lymphotrophic virus 1, and Merkel cell polyomavirus can also induce cancers by direct oncogene expression or by inducing chronic inflammation.59-61 Regardless of their contribution to carcinogenesis, viral proteins expressed in tumor cells provide unique target antigens for cancer immunotherapy. Thus, VSTs may provide a safe and effective treatment against virus-associated cancers, and in the next section we describe examples of their use in autologous settings and their potential as allogeneic therapies.
EBV-expressing cancers in immunocompetent hosts
To evade endogenous T cells in immunocompetent hosts, EBV-expressing (EBV+) tumors express only a fraction of the 9 EBV latent cycle proteins that are expressed by PTLDs. EBV+ lymphoma and NPC express LMP1, LMP2, EBNA1, and BARF1, a pattern of gene expression termed type 2 latency, whereas EBV+ Burkitt lymphoma and gastric carcinoma only express EBNA1 and BARF1 (type 1 latency).47 Most of these proteins are poorly immunogenic and provide a reduced pool of target antigens for VSTs. Smith et al reported 16 patients with NPC who showed longer overall survival after receipt of EBVSTs compared with a retrospective comparator (523 days vs 220 days).62 We showed that autologous EBVSTs focused on LMP1- and LMP2-induced CRs in 11 of 21 patients with EBV+ lymphoma who had failed at least 2 lines of conventional therapy.18 In the allogeneic arm of the same trial, we demonstrated the safety of LMP-directed DD-VSTs in 26 patients who had undergone an allogeneic hematopoietic cell transplant for lymphoma.63 Of the 7 patients with active lymphoma at the time of infusion, 2 had objective responses, whereas the 2-year overall survival for the remaining 19 adjuvant patients was 78%, which was superior to the expected outcomes for this cohort (up to 50% for lymphomas after allogeneic hematopoietic cell transplant). With the safety of DD-VSTs demonstrated, we initiated a phase 1 clinical trial of B-EBVSTs targeting LMP1, LMP2, BARF1, and EBNA1 in patients with EBV+ lymphomas (#NCT02287311) and reported preliminary results in abstract form.30 None of the 19 B-VST recipients experienced GVHD or any other infusion-related toxicity. Of the 14 evaluable patients, 8 had objective clinical responses, and the trial is now open for any EBV+ malignancy.
Malignancies associated with other viruses
Virus-associated solid tumors present greater challenges to immunotherapy than B-cell malignancies, and few robust successes have been reported, even in the autologous setting.22,64-71 Therefore, it is not surprising that trials of B-VSTs targeting these malignancies have not yet been reported. Any cellular immunotherapy for a solid tumor must contend with a tumor microenvironment (TME) that is “coinhibitory” instead of “costimulatory,” local fibrosis preventing T-cell trafficking and immunosuppressive cytokines and cellular infiltrates that inhibit T-cell function. Before implementing banked approaches, overcoming these barriers in solid tumors will be necessary, and we will further discuss how genetic modification of B-VSTs may improve their function and address potential obstacles.
The potential of B-VSTs to treat nonviral malignancies
Only 15% to 20% of all cancers are virus associated, but VSTs are interesting candidate hosts for CARs, which allow them to target nonviral tumor antigens in an HLA-unrestricted manner. To overcome exhaustion and inadequate stimulation in the TME, CAR-VSTs may receive additional stimulation via their native TCR. This could come from endogenous viruses like EBV, viral vaccines, or oncolytic viruses. Because viruses potently activate the innate immune response, this stimulation may overcome the anergy induced by tumors.37 Autologous and DD-VSTs have been tested clinically as hosts for GD2.CARs and CD19.CARs for the treatment of neuroblastoma and B-cell acute lymphocytic leukemia, respectively. In patients receiving both EBVSTs and CD3-activated T cells expressing a first-generation GD2.CAR,72 5 of 11 patients had tumor responses, including 3 CRs.73 However, it was not possible to determine which product produced the tumor responses. Studies with DD-MVSTs expressing a CD19.CAR to treat patients with relapsed B-cell acute lymphocytic leukemia after allogeneic HSCT33 suggested that EBV reactivation could drive the expansion of CD19.CAR-MVSTs, resulting in the elimination of normal B cells, which did not occur in patients without virus reactivation.34 This supports the hypothesis that the expansion, function, and persistence of CAR T cells could be driven through the endogenous TCR. VSTs have also been modified with transgenic TCRs to target intracellular tumor antigens.74,75 However, the studies have been small and the tumor responses inconsistent, so improvements are required before such studies can be moved to the banked allogeneic setting.
Improving the efficacy of B-VSTs
Although unmodified allogeneic VSTs are attractive because of their relative simplicity, they are unlikely to be effective long term in immunocompetent patients with cancers that do not express viral epitopes. Thus, strategies to prevent rejection and improve their activity and longevity will likely be required.
Overcoming GVHD and graft rejection
The most common approach to preventing GVHD from an allogeneic T-cell product is to knock out the endogenous αβ-TCR, the main effector molecule of alloreactivity.76 Caveats to this approach include the necessity for near complete depletion of TCR-expressing cells because patients infused with <1% residual αβ-TCR–positive T cells have developed GVHD,77 the increased manufacturing complexity of gene editing, and the associated regulatory burden. Further, there have been reports of reduced function of CAR T cells lacking TCRs.78 An alternative approach is to use cell products that naturally lack alloreactivity, such as natural killer (NK) T cells that have invariant TCRs recognizing lipids presented by nonpolymorphic CD1d molecules, γδ-TCRs whose limited array of TCRs recognize a range of pathogen and cellular stress–related proteins independent of polymorphic major histocompatibility complex molecules,79 or αβ T-cell populations with a limited TCR repertoire, such as VSTs as described above.39,40
Preventing rejection is more difficult and complex. Because it is impossible to eliminate all alloreactive T cells and NK cells in the recipient, the most common approach is to prevent recognition of the infused cells by eliminating HLA expression on the cell surface. Surface expression of HLA class I molecules can be prevented by knockout of β2 microglobulin, the common β chain of all HLA class I molecules.80 Class II molecules can be knocked out individually or in combination by deletion of CIITA, the master regulator of class II gene expression.81-83 Whether these modifications affect the function or persistence of T cells is not yet clear, but HLA class I–negative cells become targets for NK cells, so that additional expression of nonpolymorphic HLA class I molecules like HLA-E becomes necessary.84 Because NK cells heterogeneously express a range of activating and inhibitory receptors,85 this modification may not inhibit all NK cells and again the extensive gene editing required to produce immunologically invisible effector cells poses regulatory issues.
We took an alternative approach of actively eliminating recipient alloreactive T cells using a chimeric protein that fused β2 microglobulin to the cytotoxic CD3 zeta chain of the TCR,86 so that allogeneic VSTs expressing this chimeric HLA accessory receptor (CHAR) could kill host alloreactive T cells that engage their class I alloantigens. We have extended this concept by developing alloimmune defense receptors comprising CARs directed against molecules such as 4-1BB or CD30 that are upregulated on activated T cells. CD3-activated T cells expressing alloimmune defense receptors are protected from allorejection both in vitro and in murine models.87 EBVSTs expressing a CD30.CAR have bispecificity for CD30 and EBV and can eliminate both alloreactive T cells and CD30+ lymphoma. Because autologous CD30.CAR CD3-activated T cells have already been proved safe and effective for the treatment of CD30+ lymphoma,88,89 we are now evaluating a bank of CD30.CAR-EBVSTs manufactured from healthy EBV-seropositive donors as an OTS treatment for CD30+ lymphoma in a phase 1 trial (Figure 2) (#NCT04288726) with promising early results.90
Development and clinical testing of a bank of CD30.CAR-EBVSTs. Allogeneic CD30.CAR transduced EBVSTs (CD30.CAR-EBVSTs) will kill (1) recipient activated alloreactive T cells that upregulate CD30 upon activation and (2) CD30-expressing lymphoma cells. EBVSTs will not cause GVHD.
Development and clinical testing of a bank of CD30.CAR-EBVSTs. Allogeneic CD30.CAR transduced EBVSTs (CD30.CAR-EBVSTs) will kill (1) recipient activated alloreactive T cells that upregulate CD30 upon activation and (2) CD30-expressing lymphoma cells. EBVSTs will not cause GVHD.
Improving the function of B-VST
VSTs have advantages as a platform for banked allogeneic cell therapies because they have been proved safe in the clinic, lack alloreactivity, and have long-term memory potential. They are amenable to genetic modification, allowing them to target additional tumor antigens, be protected from rejection, and providing enhanced function in the immunosuppressive TME.91-94 Examples tested clinically include dominant-negative receptors to counteract inhibitory cytokines, such as transforming growth factor β, secreted and tethered cytokines, and constitutively active cytokine receptors to prolong cytokine signaling.92-98 Many gene modifications have been proposed in preclinical models, but relatively few have been tested clinically, and thus far only in the autologous setting.
Conclusion
The adoptive transfer of B-VSTs has demonstrated efficacy comparable to recipient-specific products in the treatment of viral infections of transplant recipients, leading to ongoing Food and Drug Administration registration trials. Their efficacy in this setting may be owing to reactivation of endogenous immunity rather than to the persistence of the infused product. Regardless, this success has led to their evaluation for the treatment of problematic viral diseases and malignancies outside the immunocompromised setting. Challenges to B-VSTs outside the HSCT setting is limited persistence because of rejection and the almost universal use of lymphodepletion that may prevent the reactivation of endogenous immunity. Despite these obstacles, VSTs offer advantages as a candidate T-cell platform, including their demonstrated lack of alloreactivity and amenability to gene modifications to provide additional anticancer specificity, improve their persistence, and overall efficacy. Although B-VSTs have yet to demonstrate their potential in large late-phase studies, efforts to provide well-characterized, immediately available, and cost-effective OTS T-cell therapies that are safe, clinically effective, and provide durable responses are warranted.
Acknowledgments
This work was supported in part by grants from National Cancer Institute and National Heart, Lung, Blood Institute, National Institutes of Health (grants NCI-P50CA126752, NCI-P01CA094237, and NHLBI HHSN-268201600015I-75N9020F00001), Cancer Prevention and Research Institute of Texas (grant RP190067 and RP200584 [P.D.L.]), and Leukemia & Lymphoma Society SCOR (C.M.R.).
Authorship
Contribution: All authors conducted the research and wrote and edited the manuscript.
Conflict-of-interest disclosure: D.H.Q. and C.M.R. receive research support from Tessa Therapeutics. C.M.R. and spouse are founder members of Marker Therapeutics and AlloVir, receive royalties, and are on the scientific advisory board of Marker Therapeutics. C.M.R.'s spouse is on the scientific advisory board of and or has stock options in Bellicum Pharmaceuticals, bluebird bio Inc, Memgen, LLC, Tscan, Turnstone Biologics Ltd, Walking Fish, Abintus, Allogene, Adaptimmune Therapeutics PLC, and Coya. P.L. receives research funding from Marker Therapeutics and AlloVir.
Correspondence: David H. Quach, Center for Cell and Gene Therapy, Baylor College of Medicine, 1102 Bates Ave, St 1770, Houston, TX 77030; e-mail: dhquach@bcm.edu; Cliona M. Rooney, Center for Cell and Gene Therapy, Baylor College of Medicine, 1102 Bates Ave, St 1770, Houston, TX 77030; e-mail: crooney@bcm.edu; and Premal Lulla, Center for Cell and Gene Therapy, Baylor College of Medicine, 1102 Bates Ave, St 1770, Houston, TX 77030; e-mail: lulla@bcm.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal