Key Points
Murine allogeneic anti-CD19 CAR T cells integrated before or early after PTCy for allo-HCT can clear leukemia without added toxicity.
Clinical translation of this approach would combine the activity of both CAR T cells and polyclonal alloreactive T cells to reduce relapse.
Abstract
Relapse limits the therapeutic efficacy both of chimeric antigen receptor (CAR) T cells and allogeneic hematopoietic cell transplantation (allo-HCT). Patients may undergo these therapies sequentially to prevent or treat relapsed malignancy. However, direct integration of the 2 therapies has been avoided over concerns for potential induction of graft-versus-host disease (GVHD) by allogeneic CAR T cells. We have shown in murine T-cell-replete MHC-haploidentical allo-HCT that suppressive mechanisms induced immediately after posttransplant cyclophosphamide (PTCy), given on days +3/+4, prevent GVHD induction by alloreactive T cells infused as early as day +5. Therefore, we hypothesized that allogeneic CAR T cells given in a similarly integrated manner in our murine MHC-haploidentical allo-HCT model may safely exert antitumor effects. Indeed, allogeneic anti-CD19 CAR T cells given early after (day +5) PTCy or even prior to (day 0) PTCy cleared leukemia without exacerbating the cytokine release syndrome occurring from the MHC-haploidentical allo-HCT or interfering with PTCy-mediated GVHD prevention. Meanwhile, CAR T-cell treatment on day +9 or day +14 was safe but less effective, suggesting a limited therapeutic window. CAR T cells infused before PTCy were not eliminated, but surviving CAR T cells continued to proliferate highly and expand despite PTCy. In comparison with infusion on day +5, CAR T-cell infusion on day 0 demonstrated superior clinical efficacy associated with earlier CAR T-cell expansion, higher phenotypic CAR T-cell activation, less CD4+CD25+Foxp3+ CAR T-cell recovery, and transcriptional changes suggesting increased activation of CD4+ CAR T cells and more cytotoxic CD8+ CAR T cells. This study provides mechanistic insight into PTCy’s impact on graft-versus-tumor immunity and describes novel approaches to integrate CAR T cells and allo-HCT that may compensate for deficiencies of each individual approach.
Introduction
The efficacy of chimeric antigen receptor (CAR) T cells and allogeneic hematopoietic cell transplantation (allo-HCT) each is limited by relapse. To try to achieve long-term cure, many CAR T cell–treated patients proceed to allo-HCT either before or after relapse.1,2 Direct integration of these 2 therapies has been avoided due to the potential of allogeneic CAR T cells to cause graft-versus-host disease (GVHD), as seen in murine allo-HCT models.3,4 Clinically, GVHD has been infrequently seen with allogeneic CAR T cells, but generally allogeneic CAR T cells have been implemented later post transplant and using HLA-matched donors,5,6 wherein the risk for GVHD may be lower.
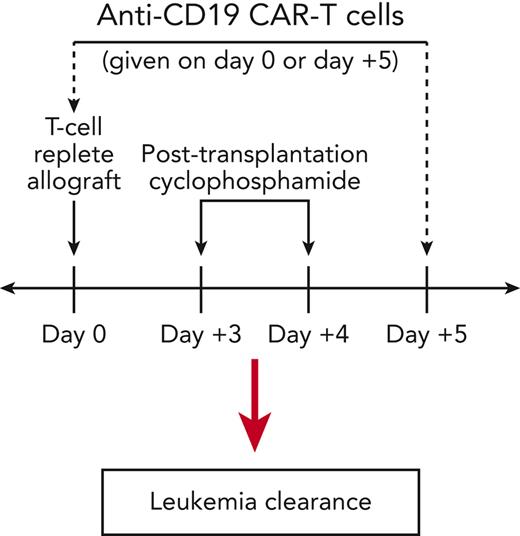
Posttransplant cyclophosphamide (PTCy) substantially reduces the risk of severe GVHD after clinical T-cell-replete allo-HCT without seemingly compromising antitumor immunity.7-11 The clinical efficacy of PTCy is in part mediated by the rapid induction of highly active suppressive mechanisms12-14; indeed, we previously showed that very high doses of additional donor splenocytes given as early as 24 hours after the last PTCy dose (ie, day +5) do not aggravate GVHD.12 Therefore, we hypothesized that donor CAR T cells given early after allo-HCT using PTCy would not cause GVHD but may retain an antitumor effect; the premise for clinical application is that the CAR T cells would induce an initial deep remission, and the polyclonal graft-versus-tumor (GVT) T-cell responses derived from the allograft would effect long-term cure. To test this hypothesis, we modified our previously described T-cell-replete murine MHC-haploidentical allo-HCT model (B6C3F1→B6D2F1)12 by inoculating recipient mice pretransplant with a radioresistant and chemotherapy-resistant pre-B-cell acute lymphoblastic leukemia (ALL) cell line (E2a-PBX)3,15 and testing the safety and efficacy of administering allogeneic anti-CD19 CAR T cells either before or after PTCy.
Methods
Mice
B6C3F1/Crl, B6D2F1/Crl, C3H/HeNCrl, and B6-Ly5.1/Crl mice were obtained from the Charles River Laboratories. B6C3F1 CD45.1+CD45.2+ mice were bred at the National Cancer Institute from B6-Ly5.1/Crl mothers and C3H/HeNCrl fathers. B6C3F1 (donor) and B6D2F1 (recipient) mice were females aged 10 to 12 weeks at HCT. All mice were housed in specific pathogen-free conditions and provided food and water ad libitum.
Tumor cell line
CAR T-cell generation
As previously described,3 donor splenocytes were T-cell enriched (∼65%-80% T cells), stimulated with anti-CD3/CD28 beads (1 bead-to-cell ratio), transduced via spinoculation on culture days 2 and 3 with a second-generation anti-CD19 CAR retroviral vector with a CD28 costimulatory domain,3,16 and infused fresh on culture day 6 at 1 × 106 transduced T cells per mouse. Transduction efficiency was median 73.6% (interquartile range 62.9%-82.9%) for CD4+ T cells and median 71.2% (interquartile range 64.4%-78.2%) for CD8+ T cells. Nontransduced (NT) cells were processed identically in parallel except were spinoculated with media not containing vector. NT cells were dosed at the same total number of T cells per mouse as mice receiving CAR T cells. For experiments using cryopreserved cells, CAR T-cell or NT-cell products were aliquoted for cryopreservation such that identical products could be thawed and administered on each treatment day of a given experiment.
Hematopoietic cell transplantation
Transplantation, PTCy administration, clinical scoring, histopathologic assessments, and flow cytometry (see supplemental Methods, available on the Blood website for a list of specific antibodies) were performed as previously described.12 For day 0 CAR T-cell experiments, 1 × 106 transduced T cells per mouse (or equivalent NT-cell numbers) were admixed with the graft immediately before transplantation.
Cytokine analysis
Cryopreserved plasma specimens were analyzed in duplicate using ProcartaPlex (Thermo Fisher) custom 10-plex assays according to the manufacturer’s instructions with analysis by a Luminex Flexmap 3D.
Single-cell partitioning, library preparation, and sequencing
Cells were captured with the 10× Genomics Chromium platform using the 5′ v2 gene expression chemistry targeting 6000 cells when sufficient cell numbers allowed. Preparation of libraries was performed according to the vendor’s recommendations. Sequencing was performed on an Illumina NextSeq 2000 instrument with P3 100-cycle kits with a 26-base pair (bp) read to identify cell barcodes and unique molecular indices, dual 10-bp reads for sample indices, and a 90-bp read to identify cDNA inserts. Samples were multiplexed for sequencing, and reads were combined from multiple sequencing runs to achieve at least 50 000 reads per cell on average for all samples.
Single-cell data processing and analysis
Raw sequence data were processed using 10× Genomics cellranger (version 6.0.0). Sequenced reads were aligned based on the mouse reference gex-mm10-2020-A. Bioinformatics analysis and visualization were performed in the NIH Integrated Analysis Portal using R programs developed on the Foundry platform (Palantir Technologies, Inc). Processed sequence data were analyzed using Seurat 3.1.5 single-cell RNA workflow.17 Cells containing <200 genes, <500 RNA counts, or gene-to-unique molecular identifier ratio <0.8 were filtered from the data. Seurat SCTransform function was used to regress out the effect of RNA counts on sample heterogeneity, and the top 3000 variable features were selected using the variance stabilizing transformation function.
T cells were hierarchically classified according to expression of CD3D, CD3E, and CD3G, followed by CD4 and CD8A, using an algorithm that utilizes thresholding using bimodal distributions from the Seurat AddModuleScore function. Within the CD4 and CD8 T-cell populations, Seurat FindMarker function was used to find differentially expressed genes between day 0 and day +5 treatment groups across corresponding time points. Pathway analysis was performed on each comparison using the l2p (version 0.1) R package (https://github.com/CCBR/l2p). Significantly regulated pathways were selected from the GO, REACTOME, and KEGG libraries. T cells were further subclassified using ProjectTILs (version 2.0.0)18 and partitioned into clusters through Seurat FindClusters function with 0.4 resolution.
Statistics
Survival distributions were compared using the exact log-rank test. Weight and clinical score area-under-the curve comparisons were performed using the Wilcoxon rank sum test. Other data analysis and data presentation were performed using GraphPad Prism. Data underwent natural logarithmic (numbers) or arcsine (percentages) transformation before t test or one-way analysis of variance (ANOVA). Numerical values of 0 were assigned a value of 0.1 before transformation. All analyses were 2-tailed, and all the statistical results should be interpreted as exploratory including P values <.05, which are described as statistically significant.
Results
Allogeneic CAR T cells given on day +5 to PTCy-treated mice can clear leukemia
The E2a-PBX tumor cell line has a B6 background and thus does not express major histocompatibility antigens different from those expressed on either recipient or donor in our B6C3F1→B6D2F1 model; hence, only anti-CD19 CAR T cells can treat E2a-PBX (no major histocompatibility antigen-driven allogeneic response against it; minor antigens are insufficient to control it3). Mice receiving E2a-PBX leukemia, syngeneic or allogeneic HCT, and PTCy all died of leukemia (supplemental Figure 1, Figure 1A) when receiving NT cells on day +5 but cleared leukemia when receiving CAR T cells (Figure 1A-E). This effect occurred without appreciable toxicity (Figure 1C-E, supplemental Table 1). At day +21 in PTCy-treated allo-HCT mice, CAR T cells persisted and no measurable leukemia was seen (Figures 1F-H, supplemental Figure 2). In mice surviving to day +120, we found CAR T-cell persistence and absence of E2a-PBX and donor B cells in all groups (Figure 1I-L, supplemental Figure 2, supplemental Table 2).
Integration of anti-CD19 CAR T cells early after PTCy (day +5). (A) Donor (B6C3F1), E2a-PBX leukemia (B6), and recipient (B6D2F1) cells have differing MHC haplotypes, allowing their delineation based on expression of H2kb, H2kk, and H2kd. (B) Treatment schema. Mice in treatment groups not receiving leukemia, CAR T cells, NT T cells, or PTCy received vehicle on the indicated day (D). (C) Histopathologic scores of GVHD severity at day +21 post transplant. Statistical testing was performed with one-way ANOVA followed by the Holm-Sidak post hoc test on natural logarithmically transformed data using the Allo BM/Splen, PTCy, CAR D5 group as the reference. ∗P < .05. (D-E) Survival, weights, and clinical scores for (D) syngeneic (B6C3F1→B6C3F1) or (E) allogeneic (B6C3F1→B6D2F1) HCT. (F-G) Total numbers at day +21 of (F) CD4+ or (G) CD8+ T cells that were CAR+ as measured by Protein-L staining and gated on viable donor T-cell subsets. (H) Percentages at day +21 of viable cells that were E2a-PBX leukemia. (I-J) All mice in D-E surviving to day +120 were assessed for the frequency of (I) CD4+ and (J) CD8+ CAR T cells, (K) E2a-PBX leukemia, and (L) donor (H2kk+H2kb+) CD19+ B cells, revealing persistence of CAR T cells, clearance of leukemia, and persistent donor B-cell aplasia. Combined results from 4 independent experiments are shown for panels D, E, and I to L (numbers/group shown in supplemental Table 2) and 2 independent experiments (n = 3 per group per experiment) for panels C, F, G, and H. BM, bone marrow; D, day; IP, intraperitoneal; IV, intravenous; ns, not statistically significant (P ≥ .05); Splen, splenocytes; TBI, total body irradiation; TCD, T-cell–depleted.
Integration of anti-CD19 CAR T cells early after PTCy (day +5). (A) Donor (B6C3F1), E2a-PBX leukemia (B6), and recipient (B6D2F1) cells have differing MHC haplotypes, allowing their delineation based on expression of H2kb, H2kk, and H2kd. (B) Treatment schema. Mice in treatment groups not receiving leukemia, CAR T cells, NT T cells, or PTCy received vehicle on the indicated day (D). (C) Histopathologic scores of GVHD severity at day +21 post transplant. Statistical testing was performed with one-way ANOVA followed by the Holm-Sidak post hoc test on natural logarithmically transformed data using the Allo BM/Splen, PTCy, CAR D5 group as the reference. ∗P < .05. (D-E) Survival, weights, and clinical scores for (D) syngeneic (B6C3F1→B6C3F1) or (E) allogeneic (B6C3F1→B6D2F1) HCT. (F-G) Total numbers at day +21 of (F) CD4+ or (G) CD8+ T cells that were CAR+ as measured by Protein-L staining and gated on viable donor T-cell subsets. (H) Percentages at day +21 of viable cells that were E2a-PBX leukemia. (I-J) All mice in D-E surviving to day +120 were assessed for the frequency of (I) CD4+ and (J) CD8+ CAR T cells, (K) E2a-PBX leukemia, and (L) donor (H2kk+H2kb+) CD19+ B cells, revealing persistence of CAR T cells, clearance of leukemia, and persistent donor B-cell aplasia. Combined results from 4 independent experiments are shown for panels D, E, and I to L (numbers/group shown in supplemental Table 2) and 2 independent experiments (n = 3 per group per experiment) for panels C, F, G, and H. BM, bone marrow; D, day; IP, intraperitoneal; IV, intravenous; ns, not statistically significant (P ≥ .05); Splen, splenocytes; TBI, total body irradiation; TCD, T-cell–depleted.
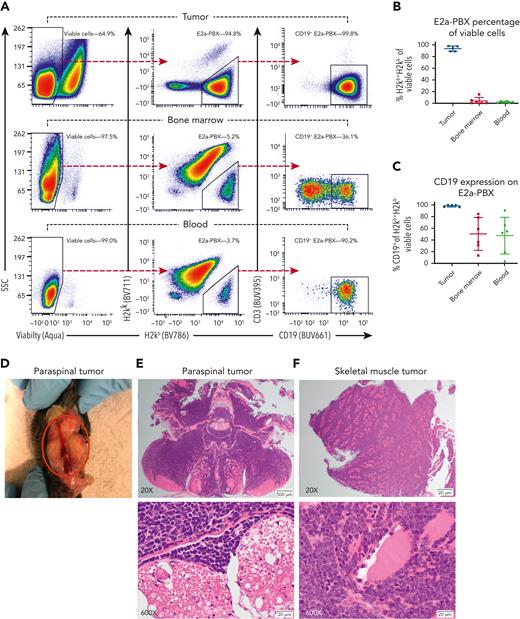
E2a-PBX relapse did occur in some CAR T cell–treated mice. Early relapses were most commonly seen after syngeneic transplants without PTCy, reiterating the importance of lymphodepletion before adoptive cell therapy.19,20 Late relapses were more frequently observed in mice treated with allo-HCT/PTCy; leukemia antigen loss21 and CAR T-cell exhaustion22 both were potential contributors. Relapse after allo-HCT/PTCy was associated with CD19+ extramedullary tumors but partial loss of CD19 on E2a-PBX in hematopoietic organs (Figure 2). CAR T cells given after allo-HCT/PTCy remained proliferative, but had increased expression of coinhibitory molecules (supplemental Figure 3). Attempts to assess the impact of having both CAR T cells and allogeneic T cells able to treat the leukemia (to mirror the intended clinical use) in a C3H→B6D2F1 allo-HCT model were limited by permanent clearance of E2a-PBX by the allogeneic response alone.
In mice receiving allogeneic HCT using PTCy and CAR T cells on day +5, E2a-PBX relapses uniquely occurred as CD19+extramedullary tumors with partial loss of CD19+on leukemia within hematopoietic organs. (A) Flow cytometric schema for a representative mouse. (Left) Viable cells were gated off singlets. (Center) From viable cells, H2kb+H2kk− E2a-PBX leukemia cells were gated and (right) assessed for CD19 expression. (B-C) E2a-PBX leukemia composed nearly all cells in the extramedullary tumors wherein it was uniformly CD19+. By contrast, E2a-PBX was only a small fraction of cells in hematopoietic organs wherein it was CD19− in a subset of cells. (D-F) Photograph and histopathology of paraspinal extramedullary tumors for the mouse in panel A with the histopathology and flow cytometric analyses all coming from different sections of the same tumor. (E) Cross section of vertebral column in the lumbosacral region, demonstrating effacement of paraspinal muscles by lymphoma. Higher magnification shows infiltration of paraspinal muscles by a uniform population of lymphoblasts and invasion of the spinal canal adjacent to spinal nerve roots. (F) Section of skeletal muscle severely infiltrated by a uniform population of lymphoblasts. Higher magnification demonstrates individual myofibers separated by a uniform population of neoplastic lymphoblasts.
In mice receiving allogeneic HCT using PTCy and CAR T cells on day +5, E2a-PBX relapses uniquely occurred as CD19+extramedullary tumors with partial loss of CD19+on leukemia within hematopoietic organs. (A) Flow cytometric schema for a representative mouse. (Left) Viable cells were gated off singlets. (Center) From viable cells, H2kb+H2kk− E2a-PBX leukemia cells were gated and (right) assessed for CD19 expression. (B-C) E2a-PBX leukemia composed nearly all cells in the extramedullary tumors wherein it was uniformly CD19+. By contrast, E2a-PBX was only a small fraction of cells in hematopoietic organs wherein it was CD19− in a subset of cells. (D-F) Photograph and histopathology of paraspinal extramedullary tumors for the mouse in panel A with the histopathology and flow cytometric analyses all coming from different sections of the same tumor. (E) Cross section of vertebral column in the lumbosacral region, demonstrating effacement of paraspinal muscles by lymphoma. Higher magnification shows infiltration of paraspinal muscles by a uniform population of lymphoblasts and invasion of the spinal canal adjacent to spinal nerve roots. (F) Section of skeletal muscle severely infiltrated by a uniform population of lymphoblasts. Higher magnification demonstrates individual myofibers separated by a uniform population of neoplastic lymphoblasts.
Limited therapeutic window for efficacy of CAR T cells given after PTCy
To investigate the time dependency of PTCy’s impact on the relative safety and efficacy of CAR T cells given early post transplant and thereby also to inform clinical translation, we directly compared CAR T-cell infusion on day +5 vs day +9 vs day +14 in our B6C3F1→B6D2F1 model. To remove potential confounders, we administered aliquots of the same cryopreserved CAR T cells at each time point within the same experiments (Figure 3A). Cryopreserved CAR T cells retained high viability and efficacy in eradicating syngeneic donor B cells in vitro (supplemental Figure 4).
Limited therapeutic window to give anti-CD19 CAR T cells after PTCy. (A) Treatment schema that is identical to Figure 1B except that cryopreserved CAR T (or NT) cells were thawed and administered intravenously either on day +5, day +9, or day +14 post transplant to remove any potential confounding effects of variability in different CAR T-cell products or other aspects of the transplantation platform if these were done in parallel experiments. Splenocytes for transduction also came from CD45.1+CD45.2+ B6C3F1 mice to most definitively separate CAR+ (Protein-L+CD45.1+) from graft-derived (CD45.1−) T cells as wild-type (CD45.1−CD45.2+) B6C3F1 mice were used for the splenocytes and bone marrow for transplant. Mice not receiving cells on a given day received RPMI vehicle intravenously. (B) CAR T-cell administration on day +5, day +9, or day +14 to PTCy-treated mice did not aggravate histopathologic GVHD at day +21 compared with mice treated with PTCy without CAR T cells. (C) CAR T cells on day +5 significantly prolonged survival compared with mice not receiving CAR T cells (hazard ratio [HR] 0.29, P = .0022), mice receiving CAR T cells on day +9 (HR 0.33, P = .009) or day +14 (HR 0.19, P = .0003), or mice receiving NT cells on day +5 (HR 0.37, P = .02; NT-cell groups are shown in supplemental Figure 5). This prolonged survival was achieved without any significant difference in weights or clinical scores in the CAR T cells on day +5 group compared with these other groups. Meanwhile, CAR T cells administered on day +9 or day +14 did not prolong survival compared with the no CAR T or NT-cell groups. (D) Successful clearance of both benign and malignant CD19+ targets at day +21 was achieved only when CAR T cells were given on day +5. (E-F) Despite superior efficacy, viable donor CAR T cells were lowest in (E) total numbers and (F) percentages at day +21, particularly within the spleen, for the group administered CAR T cells on day +5. (F) Despite being the most highly proliferative, the lack of clinical activity of CAR T cells given on day +14 was associated with these CAR T cells at day +21 being (H-I) significantly less differentiated and (J-K) having significantly higher expression of coinhibitory molecules PD1 and LAG3 compared with CAR T cells administered on day +5 or day +9. Combined results of 3 independent experiments are shown for all parts with 5 mice per group per experiment for panel C and 4 mice per group per experiment for all other parts. Data underwent natural logarithmic (total numbers) or arcsin (percentages) transformation prior to one-way ANOVA followed by the Holm-Sidak post hoc test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Limited therapeutic window to give anti-CD19 CAR T cells after PTCy. (A) Treatment schema that is identical to Figure 1B except that cryopreserved CAR T (or NT) cells were thawed and administered intravenously either on day +5, day +9, or day +14 post transplant to remove any potential confounding effects of variability in different CAR T-cell products or other aspects of the transplantation platform if these were done in parallel experiments. Splenocytes for transduction also came from CD45.1+CD45.2+ B6C3F1 mice to most definitively separate CAR+ (Protein-L+CD45.1+) from graft-derived (CD45.1−) T cells as wild-type (CD45.1−CD45.2+) B6C3F1 mice were used for the splenocytes and bone marrow for transplant. Mice not receiving cells on a given day received RPMI vehicle intravenously. (B) CAR T-cell administration on day +5, day +9, or day +14 to PTCy-treated mice did not aggravate histopathologic GVHD at day +21 compared with mice treated with PTCy without CAR T cells. (C) CAR T cells on day +5 significantly prolonged survival compared with mice not receiving CAR T cells (hazard ratio [HR] 0.29, P = .0022), mice receiving CAR T cells on day +9 (HR 0.33, P = .009) or day +14 (HR 0.19, P = .0003), or mice receiving NT cells on day +5 (HR 0.37, P = .02; NT-cell groups are shown in supplemental Figure 5). This prolonged survival was achieved without any significant difference in weights or clinical scores in the CAR T cells on day +5 group compared with these other groups. Meanwhile, CAR T cells administered on day +9 or day +14 did not prolong survival compared with the no CAR T or NT-cell groups. (D) Successful clearance of both benign and malignant CD19+ targets at day +21 was achieved only when CAR T cells were given on day +5. (E-F) Despite superior efficacy, viable donor CAR T cells were lowest in (E) total numbers and (F) percentages at day +21, particularly within the spleen, for the group administered CAR T cells on day +5. (F) Despite being the most highly proliferative, the lack of clinical activity of CAR T cells given on day +14 was associated with these CAR T cells at day +21 being (H-I) significantly less differentiated and (J-K) having significantly higher expression of coinhibitory molecules PD1 and LAG3 compared with CAR T cells administered on day +5 or day +9. Combined results of 3 independent experiments are shown for all parts with 5 mice per group per experiment for panel C and 4 mice per group per experiment for all other parts. Data underwent natural logarithmic (total numbers) or arcsin (percentages) transformation prior to one-way ANOVA followed by the Holm-Sidak post hoc test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Regardless of administration on day +5, day +9, or day +14, treatment with CAR T cells did not induce appreciable toxicity (Figure 3B-C). However, CAR T-cell treatment on day +5 resulted in significantly prolonged survival compared with mice receiving CAR T cells on day +9 or day +14, NT cells, or no additional cells, whereas survival was not significantly prolonged with treatment with CAR T cells on day +9 or day +14 (Figure 3C, supplemental Figure 5). Consistent with enhanced clinical efficacy, reduction of target cells (donor B cells and E2a-PBX leukemia) was most effectively achieved with CAR T-cell treatment on day +5 (Figure 3D). Even so, CAR T-cell expansion at day +21 was least robust for the day +5 infusion group (Figure 3E-F). Conversely, despite their poor activity, CAR T cells given on day +14 were highly proliferative in the bone marrow (Figure 3G); nevertheless, they also were the least differentiated (Figure 3H-I) and had significantly higher expression of PD1 and LAG3 at day +21 (Figure 3J-K) particularly in comparison with the day +5 treatment group, potentially explaining in part the inferior efficacy of treatment on day +14. Overall, these results suggest that CAR T cells are more efficacious at eradicating target cells when given very early after PTCy, whereas waiting until day +9 or day +14 to administer CAR T cells leads to better CAR T-cell expansion but reduced efficacy.
CAR T cells given prior to PTCy are not eliminated but exert a potent antitumor response
Given our previous findings that alloreactive T cells are not selectively eliminated by PTCy,12 we explored the direct impact of PTCy on graft-versus-tumor immunity by giving CAR T cells on day 0 (Figure 4A). PTCy-treated mice receiving CAR T cells survived without E2a-PBX relapse (Figure 4B-C, supplemental Table 3) and without increased toxicity (Figure 4C-D, supplemental Figure 6, supplemental Table 4). E2a-PBX and donor B cells both were markedly reduced between day +3 and day +7 (Figure 4E), suggesting that CAR T cells retained functionality despite PTCy administration on day +3 or day +4.
Integration of anti-CD19 CAR T cells prior to PTCy (day 0). (A) Treatment schema. Mice in treatment groups not receiving E2a-PBX leukemia, CAR T cells, NT cells, or PTCy received vehicle on the indicated day. (B-C) Survival, weights, and clinical scores for (B) syngeneic (B6C3F1→B6C3F1) or (C) allogeneic (B6C3F1→B6D2F1) HCT. (D) Histopathologic scores of GVHD severity on day +7 post transplant for allogeneic transplant groups. Comparisons were made using the Allo BM/Splen, PTCy, CAR D0 group as the reference group. (E-G) Splenocytes for transduction came from CD45.1+CD45.2+ B6C3F1 mice to most definitively separate CAR+ (Protein-L+CD45.1+) from graft-derived (CD45.1−) T cells as wild-type (CD45.1−CD45.2+) B6C3F1 mice were used for the splenocytes and bone marrow for transplant. (E) Total numbers of (left) E2a-PBX leukemia or (right) donor B cells on day +3 and day +7 in mice treated with CAR T cells (CAR) or NT cells on day 0 and receiving vehicle (no PTCy) or PTCy on days +3 and +4. (F) Total numbers of viable donor (left) CD4+ or (right) CD8+ T cells that were CAR+, showing numerical expansion within the bone marrow despite PTCy treatment. (G) At day +7, there was continued high-level proliferation (Ki-67+) of CAR T cells surviving PTCy. Combined results from 2 independent experiments are shown for all parts; n = 5 per group per experiment for panels B and C and n = 3 to 4 per group per experiment for panels D to G. Data underwent natural logarithmic (total numbers) or arcsin (percentages) transformation prior to t test or one-way ANOVA followed by the Holm-Sidak post hoc test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Integration of anti-CD19 CAR T cells prior to PTCy (day 0). (A) Treatment schema. Mice in treatment groups not receiving E2a-PBX leukemia, CAR T cells, NT cells, or PTCy received vehicle on the indicated day. (B-C) Survival, weights, and clinical scores for (B) syngeneic (B6C3F1→B6C3F1) or (C) allogeneic (B6C3F1→B6D2F1) HCT. (D) Histopathologic scores of GVHD severity on day +7 post transplant for allogeneic transplant groups. Comparisons were made using the Allo BM/Splen, PTCy, CAR D0 group as the reference group. (E-G) Splenocytes for transduction came from CD45.1+CD45.2+ B6C3F1 mice to most definitively separate CAR+ (Protein-L+CD45.1+) from graft-derived (CD45.1−) T cells as wild-type (CD45.1−CD45.2+) B6C3F1 mice were used for the splenocytes and bone marrow for transplant. (E) Total numbers of (left) E2a-PBX leukemia or (right) donor B cells on day +3 and day +7 in mice treated with CAR T cells (CAR) or NT cells on day 0 and receiving vehicle (no PTCy) or PTCy on days +3 and +4. (F) Total numbers of viable donor (left) CD4+ or (right) CD8+ T cells that were CAR+, showing numerical expansion within the bone marrow despite PTCy treatment. (G) At day +7, there was continued high-level proliferation (Ki-67+) of CAR T cells surviving PTCy. Combined results from 2 independent experiments are shown for all parts; n = 5 per group per experiment for panels B and C and n = 3 to 4 per group per experiment for panels D to G. Data underwent natural logarithmic (total numbers) or arcsin (percentages) transformation prior to t test or one-way ANOVA followed by the Holm-Sidak post hoc test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
CAR T-cell numbers were slightly reduced in PTCy-treated compared with vehicle-treated mice, but CAR T cells remained highly proliferative and expanded in the bone marrow from day +3 to day +7 despite PTCy (Figure 4F-G, supplemental Figure 7). Furthermore, different from administration of CAR T cells on day +5, mice receiving allogeneic CAR T cells before PTCy did not relapse with E2a-PBX, even with extended follow-up (supplemental Table 3). Instead, very late deaths occurring in this model frequently were due to donor-derived T-cell precursor leukemias (supplemental Figure 8, supplemental Table 3), likely related to transformation of hematopoietic stem cells contained in the transduced splenocytes as has been reported in human transplants using gamma-retrovirally transduced stem cells,23-25 and here was independent of syngeneic/allogeneic transplant, T-cell-replete/T-cell-deplete grafts, or the administration or timing of PTCy.
CAR T cells given before PTCy have better early expansion, particularly within CD8+ CAR T cells, and have a more activated phenotype when compared with CAR T cells given after PTCy
To understand better why CAR T cells given before PTCy (day 0) were more successful in fully eradicating leukemia compared with CAR T cells given early after PTCy (day +5), we directly compared the phenotypic and transcriptional profiles of the CAR T cells and levels of plasma inflammatory cytokines that resulted from administration on day 0 vs day +5 (Figure 5A). We limited the investigation to the first 3 weeks post transplant when sufficient numbers of CAR T cells would be retrievable for assessment within the spleen and when the CAR T cells in both groups had appeared to clear leukemia before later relapse in some mice treated on day +5.
CAR T cells given on day 0 compared with day +5 have higher early expansion associated with a more activated phenotype. (A) To study fresh CAR T cells but also remove any confounder of differences between different CAR T-cell products, 2 separate transplantations were aligned such that on the same day mice in 1 transplantation received CAR T cells on day 0 and mice in the other transplantation received on day +5 cells from the same CAR T-cell product. Splenocytes for transduction came from CD45.1+CD45.2+ B6C3F1 mice to most definitively separate CAR+ (Protein-L+CD45.1+) from graft-derived (CD45.1−) T cells as wild-type (CD45.1−CD45.2+) B6C3F1 mice were used for the splenocytes and bone marrow for transplant. Recipient mice were euthanized and assessed by flow cytometry 3, 8, or 16 days post infusion of CAR T cells. (B-D) Shown are (B) total numbers and (C) percentages of viable donor CAR T cells at each time point as well as (D) the relative distribution of CD4+ vs CD8+ CAR T cells. CAR T cells administered on day 0 had better early expansion 3 days post infusion, whereas CAR T cells administered on day +5 had better expansion at 8 and 16 days post infusion, particularly within the CD4+ CAR T cells. (E) More robust recovery of CD4+ CAR T cells with a regulatory T-cell phenotype (CD25+Foxp3+) was seen in mice receiving CAR T cells on day +5. (F) CAR T cells given on day 0 had similar to higher proliferation at all time points. (G-H) Most consistently seen within the spleen, there was significantly higher PD1 and LAG3 expression at early time points for CAR T cells given on day 0 compared with on day +5. (I) Greater differentiation (higher CD62L−) was seen at 8 and 16 days after infusion for CAR T cells given on day 0 vs CAR T cells given on day +5. Combined results of 2 independent experiments are shown with n = 5 mice per group per experiment. Data underwent natural logarithmic (total numbers) or arcsin (percentages) transformation prior to t test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
CAR T cells given on day 0 compared with day +5 have higher early expansion associated with a more activated phenotype. (A) To study fresh CAR T cells but also remove any confounder of differences between different CAR T-cell products, 2 separate transplantations were aligned such that on the same day mice in 1 transplantation received CAR T cells on day 0 and mice in the other transplantation received on day +5 cells from the same CAR T-cell product. Splenocytes for transduction came from CD45.1+CD45.2+ B6C3F1 mice to most definitively separate CAR+ (Protein-L+CD45.1+) from graft-derived (CD45.1−) T cells as wild-type (CD45.1−CD45.2+) B6C3F1 mice were used for the splenocytes and bone marrow for transplant. Recipient mice were euthanized and assessed by flow cytometry 3, 8, or 16 days post infusion of CAR T cells. (B-D) Shown are (B) total numbers and (C) percentages of viable donor CAR T cells at each time point as well as (D) the relative distribution of CD4+ vs CD8+ CAR T cells. CAR T cells administered on day 0 had better early expansion 3 days post infusion, whereas CAR T cells administered on day +5 had better expansion at 8 and 16 days post infusion, particularly within the CD4+ CAR T cells. (E) More robust recovery of CD4+ CAR T cells with a regulatory T-cell phenotype (CD25+Foxp3+) was seen in mice receiving CAR T cells on day +5. (F) CAR T cells given on day 0 had similar to higher proliferation at all time points. (G-H) Most consistently seen within the spleen, there was significantly higher PD1 and LAG3 expression at early time points for CAR T cells given on day 0 compared with on day +5. (I) Greater differentiation (higher CD62L−) was seen at 8 and 16 days after infusion for CAR T cells given on day 0 vs CAR T cells given on day +5. Combined results of 2 independent experiments are shown with n = 5 mice per group per experiment. Data underwent natural logarithmic (total numbers) or arcsin (percentages) transformation prior to t test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
CAR T cells given on day 0 had better expansion 3 days post infusion, whereas CAR T cells given on day +5 expanded better at 8 and 16 days post infusion particularly within the CD4+ T-cell compartment (Figure 5B-D). This higher CD4+ CAR T-cell numerical recovery when given on day +5 occurred despite more robust CD4+CD25+Foxp3+ CAR T-cell recovery and less robust CD4+ CAR T-cell proliferation at the later time points (Figure 5E-F). The earlier expansion of CAR T cells when given on day 0 was associated with a more activated phenotype (higher PD1 and LAG3 expression 3 and 8 days post infusion) (Figure 5G-H) and at 8 and 16 days post infusion a subsequent higher degree of differentiation (Figure 5I, supplemental Figure 9). These CAR T-cell kinetics did not overall disturb normal recovery of CD4+CD25+Foxp3+ non-CAR T cells (supplemental Figure 10).
CAR T cells given on day 0 have a transcriptional profile consistent with increased activation of CD4+ CAR T cells and higher cytotoxicity of CD8+ CAR T cells compared with when given on day +5
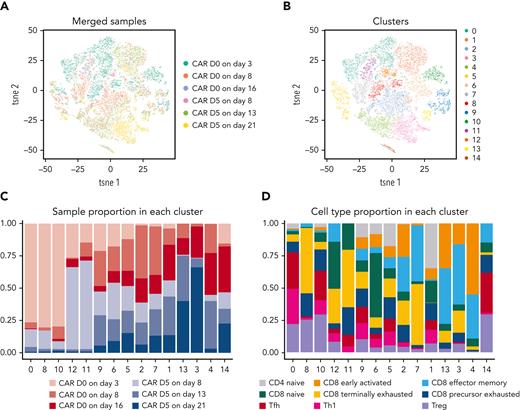
To further investigate the enhanced efficacy of CAR T cells when administered on day 0 compared with day +5, we performed single-cell RNA sequencing of flow cytometrically sorted CAR T cells at 3, 8, and 16 days post infusion (supplemental Figure 11). The overall transcriptional profile suggested globally different fates for CAR T cells given on day 0 vs day +5 (Figure 6).
Single-cell transcriptional profiles of CAR T cells given either on day 0 or on day +5 and assessed 3, 8, and 16 days post infusion. Mice were transplanted as in Figure 5. At 3, 8, or 16 days after CAR T-cell infusion, recipient mice were euthanized and splenocytes pooled from 5 to 11 mice per group were flow cytometrically sorted for viable (LIVE/DEAD−) CAR T cells (Thy1.2+CD45.1+Protein-L+). Flow cytometrically purified CAR T cells underwent single-cell RNA sequencing with the 10× Genomics Chromium platform using the 5′ v2 gene expression chemistry targeting up to 6000 cells captured per sample. Combined data from 2 independent experiments are shown. (A-B) tSNE plots grouped by (A) treatment and time point or (B) unsupervised clustering are shown. (C-D) Clusters from panel B are shown subsetted based on (C) treatment and time point group or (D) putative T-cell differentiation type based on a prior publication.18 Th, T helper; Tfh, T follicular helper; Treg, T regulatory; tsne, t-distributed stochastic neighbor embedding.
Single-cell transcriptional profiles of CAR T cells given either on day 0 or on day +5 and assessed 3, 8, and 16 days post infusion. Mice were transplanted as in Figure 5. At 3, 8, or 16 days after CAR T-cell infusion, recipient mice were euthanized and splenocytes pooled from 5 to 11 mice per group were flow cytometrically sorted for viable (LIVE/DEAD−) CAR T cells (Thy1.2+CD45.1+Protein-L+). Flow cytometrically purified CAR T cells underwent single-cell RNA sequencing with the 10× Genomics Chromium platform using the 5′ v2 gene expression chemistry targeting up to 6000 cells captured per sample. Combined data from 2 independent experiments are shown. (A-B) tSNE plots grouped by (A) treatment and time point or (B) unsupervised clustering are shown. (C-D) Clusters from panel B are shown subsetted based on (C) treatment and time point group or (D) putative T-cell differentiation type based on a prior publication.18 Th, T helper; Tfh, T follicular helper; Treg, T regulatory; tsne, t-distributed stochastic neighbor embedding.
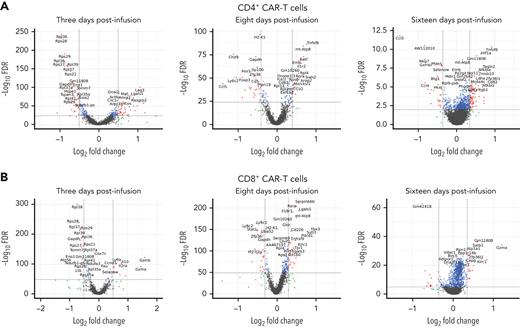
The assessments across all 3 time points suggested better activation of CD4+ CAR T cells given on day 0 compared with those given on day +5. Three days post infusion, CD4+ CAR T cells given on day 0 had increased expression of genes involved in activation such as Lag3 and Icos but decreased ribosomal-related genes compared with CD4+ CAR T cells given on day +5 (Figure 7A, supplemental Figure 12); correspondingly, gene pathways involved in pathogen response were upregulated while translational gene pathways were downregulated in the CD4+ CAR T cells given on day 0 (supplemental Figure 13). Eight days post infusion, CD4+ CAR T cells given on day 0 had increased expression of some genes involved in T-cell proliferation and differentiation, such as Tnfsf8, Batf, and Rora, with concomitant decrease in regulatory Foxp3 and Cd5 and substantial decrease in Ccl5 expression, compared with CD4+ CAR T cells given on day +5 (Figure 7A); upregulated pathways in CD4+ CAR T cells given on day 0 included cytokine-mediated signaling, and downregulated pathways included immune response including to interferon gamma (supplemental Figure 13). Sixteen days post infusion, CD4+ CAR T cells given on day 0 had increased expression of Itgb1 involved in IL1b and CD40/CD40L responses, Cd82 involved in costimulation of the T-cell receptor in association with CD4, and Tnfsf8, Nfkbia, and Nfkbiz involved in regulating immune responses, whereas some cytotoxic molecules (Nkg7, Gzmb, and Hcst) and chemokines (Ccl4, Ccl5) were downregulated (Figure 7A); upregulated pathways in the CD4+ CAR T cells given on day 0 included cytokine signaling, and downregulated pathways included interleukin-10 signaling and negative regulation of cell activation (supplemental Figure 13).
Day 0 administration results in transcriptional profiles consistent with more activated CD4+CAR T cells and more cytotoxic CD8+CAR T cells compared with administration on day +5. Volcano plots show differential gene expression for day 0 compared with day +5 CAR T-cell administration for viable (A) CD4+ and (B) CD8+ CAR T cells at (left) 3, (center) 8, and (right) 16 days after CAR T-cell infusion. Combined data from 2 independent experiments are shown. FDR, false discovery rate.
Day 0 administration results in transcriptional profiles consistent with more activated CD4+CAR T cells and more cytotoxic CD8+CAR T cells compared with administration on day +5. Volcano plots show differential gene expression for day 0 compared with day +5 CAR T-cell administration for viable (A) CD4+ and (B) CD8+ CAR T cells at (left) 3, (center) 8, and (right) 16 days after CAR T-cell infusion. Combined data from 2 independent experiments are shown. FDR, false discovery rate.
Meanwhile, results within CD8+ CAR T cells suggested greater cytotoxicity and potentially greater activation and differentiation when given on day 0 compared with when given on day +5 (Figure 7B, supplemental Figure 14). At 3 days post infusion, CD8+ CAR T cells given on day 0 had greatly increased expression of Gzma and Gzmb but decreased expression of ribosomal-related genes. At 8 days post infusion, CD8+ CAR T cells given on day 0 had increased expression of genes involved in T-cell activation and differentiation including Rora, Il18r1, Cd160, Cd226, and Tnfrsf9 but also increased expression of regulatory Klrc1 and Pdcd1. At 16 days post infusion, CD8+ CAR T cells given on day 0 again had greatly increased expression of Gzma, which was the primary gene responsible for differential clustering at that time point (Figure 6, supplemental Figure 15).
Allogeneic CAR T cells integrated before or after PTCy do not exacerbate inflammatory cytokines beyond those induced by the ongoing allogeneic response
Levels of plasma cytokines over the first 3 posttransplant weeks seemed to be driven by the allogeneic response from the graft itself and not the CAR T cells as levels were not substantively different compared with levels in mice receiving allo-HCT without CAR T cells at the same posttransplant day (supplemental Figure 16); there were no substantial differences between groups treated with CAR T cells on day 0 vs day +5 not obviously attributable to differences in posttransplant day.
Discussion
Herein we found that PTCy can serve dually as GVHD prophylaxis and as an immunomodulating and lymphocyte-reducing agent to facilitate the safe and effective integration of other adoptive T-cell therapies with T-cell-replete MHC-haploidentical allo-HCT. Particularly when administered before or very early after PTCy, CAR T cells given in this context substantially reduce and often eradicate leukemia. This therapeutic effect is achieved without substantive increase in inflammatory cytokines beyond those already occurring from the normal allo-HCT response and no loss of PTCy’s protection against GVHD inducible by the transplanted alloreactive T cells.
We also have provided the first data examining the direct impact of PTCy on GVT-mediating T cells. Similar to its effect on alloreactive T cells,12 PTCy does not eliminate GVT-mediating T cells, but they persist, continue proliferating, and expand despite PTCy. Importantly, neither leukemia nor donor B cells are eliminated by day +3, suggesting that CAR T-cell functionality persists after PTCy. The superiority of long-term leukemia clearance when CAR T cells are given before, rather than after, PTCy is unexpected. It is impossible to rule out that total body irradiation may provide better lymphodepletion than PTCy or that the higher CD19 antigen density (from normal donor or host B cells) but slightly lower tumor burden on day 0 may contribute. Furthermore, it is unclear whether the lack of PTCy for the first 3 posttransplant days or the PTCy itself is what modulates CAR T cells to improve their functionality. Recent data suggest that resting CAR T cells during generation or immune responses may improve their overall functionality.26 It is possible that PTCy dampens or restrains T-cell responses as it does for alloreactive T cells,12 such that these CAR T cells are not becoming overly stimulated or exhausted; this hypothesis may be supported by pathway analyses showing decreased immune response at 8 days post infusion in CAR T cells given on day 0 compared with CAR T cells given on day +5 (supplemental Figures 13 and 14). Regardless of whether it is a direct or indirect effect of PTCy, CAR T cells given before PTCy are modulated such that they are more activated and cytotoxic compared with when given after PTCy even though the latter are not directly exposed to PTCy. This may explain the absence of any apparent diminution in GVT immunity from the allograft observed clinically in PTCy-treated patients,8,10,11,27 suggesting that the functional impairment induced by PTCy12 is not severe enough to ablate antitumor responses.
Nevertheless, relapses still occur in this model, particularly when CAR T cells are given after PTCy. CAR T cells given on day +14 did not eliminate donor B cells, suggesting that compromised CAR T-cell functionality was responsible. Although initial remissions were uniform in mice treated with CAR T cells on day +5, late relapses were frequently seen. It is possible that PTCy may differentially affect immune control in hematopoietic vs nonhematopoietic compartments, potentially explaining both effective GVHD prevention and the higher risk for extramedullary relapse we observed, but this hypothesis requires further study. It is important to recall though that only the CAR T cells can treat the leukemia in this model. The clinical application, not testable here, also would involve polyclonal alloreactive T cells infused within the transplanted allograft that could exert broader GVT immunity and hopefully eliminate resistant clones. Thus, this integrated CAR T-cell/allo-HCT approach may be synergistic, compensating for deficiencies of each therapy and hopefully resulting in higher long-term curability. Additional potential clinical advantages of this approach would be decreasing the amount of chemotherapy and duration of therapy required, allowing CAR T cells to persist post transplant, and providing deeper immediate remissions to allow allo-HCT to work more effectively.
Whether this approach is superior to other possible strategies to give allogeneic CAR T cells is unknown. Outcomes in our study appear similar or even better when using T-cell-depleted allo-HCT and CAR T cells, and GVHD may be attenuated when CAR T cells are given with T-cell-depleted MHC-mismatched allo-HCT.4 However, polyclonal T cells in allo-HCT are critical not just for GVT and GVHD, but also for control of infection, a major driver of nonrelapse mortality after clinical T-cell-depleted allo-HCT.7 This may counterbalance clinically the less robust CAR T-cell expansion when given after PTCy in the T-cell-replete allo-HCT setting compared with syngeneic HCT using PTCy or T-cell-depleted allo-HCT, likely driven by the suppressive mechanisms induced by PTCy after allo-HCT.12-14,28 Furthermore, having polyclonal alloreactive T cells beyond the CAR T cells, as intended with clinical translation of these findings, may create broader and more potent alloimmunity that may produce more durable remissions than when relying on CAR T cells or polyclonal alloreactive T cells alone to control tumor. Nevertheless, the additional immunosuppression beyond PTCy required clinically to control GVHD after T-cell-replete allo-HCT may prevent the CAR T cells from exerting a maximal antitumor effect. In that case, the adjunct immunosuppression exposure or type may require modification, sirolimus-resistant29 or genetically modified (for immunosuppression resistance) T cells potentially may be used for transduction, or the approach may be restricted to HLA-matched bone marrow transplant in which PTCy can be used as single-agent GVHD prophylaxis and there is no immunosuppression after day +4.9,10,30,31
Additional questions remain as to how best translate these results. Our data suggest that the greatest efficacy may be achieved if CAR T cells are given on day 0 rather than early after PTCy; yet, this lower relapse potential may be abated clinically wherein polyclonal alloreactive T cells also can respond to and eliminate residual malignancy. Regardless, the first few posttransplant weeks are associated with sequential inflammatory states that may make clinical CAR T-cell integration challenging. HLA-haploidentical allo-HCT already is associated with high risk for “haplo fevers,” cytokine release syndrome (CRS) in the first posttransplant week due to the high frequency of alloreactive T cells after HLA partially mismatched allo-HCT. Haplo fevers can be substantial and sometimes life-threatening32 but generally respond well to PTCy and abate by day +5 to day +7.33 Whether overlaying the potential for severe CRS and neurotoxicity from CAR T cells themselves34 would be tolerable in such a setting is unclear. This concern is heightened given higher numbers of CD19-expressing cells present peritransplant from a combination of tumor and recipient B cells surviving conditioning and donor B cells given within the allograft. Even so, our data did not show a marked exacerbation of inflammatory cytokines, suggesting that the haplo fever response may be more dominant than that of the CAR T cells. Moreover, it is possible that PTCy may control CRS from CAR T cells given on day 0 in a favorable way as it does for the CRS from haplo fevers, but the first few posttransplant days still might be clinically tenuous. One might expect that CAR T cells may be better tolerated when not overlaid with haplo fevers, but the second week post transplant also is a challenging period, including dense neutropenia, common posttransplant complications such as mucositis, infection, and organ toxicity, and also the beginning of engraftment, which itself can be a high inflammatory state.35 This risk profile would be expected to be different for HLA-matched allo-HCT, and so it remains to be seen whether the feasibility and optimal timing of such integration may vary by donor type.
An important limitation of our study is that the CAR T cells themselves do not seem to induce substantive GVHD in this MHC-haploidentical HCT model (supplemental Figure 17). Although initially surprising given that CAR T cells generated with this identical construct caused fatal GVHD in an MHC-matched HCT model,3 this finding is consistent with the lack of substantive GVHD inducible in MHC-disparate or MHC-haploidentical HCT models when using constructs with a CD28 costimulatory domain4 and low risk of GVHD in patients receiving allogeneic CAR T cells across donor types.5,6 Furthermore, we observed reduction in percentages of alloreactive CAR T cells in mice treated with or without PTCy (supplemental Figure 18), consistent with prior observation4; interestingly, the kinetics of alloreactive CAR T-cell expansion and contraction early post transplant varied based on T-cell-deplete vs T-cell-replete allografts. Future studies should carefully assess the impact of different costimulatory domains on the relative safety and efficacy of CAR T cells integrated before or after PTCy, whether such effects differ depending on the HCT model and its degree of MHC matching or allograft type, and most critically whether such findings are conserved in patients. Nevertheless, based on available data, first-in-human studies translating our approach can proceed using constructs with a CD28 costimulatory domain.
Overall, we have shown that engineered adoptive cellular therapies can be integrated with PTCy-based allo-HCT in a way that is safe and effective. Such novel treatment platforms combining allo-HCT with CAR T cells require testing and refinement clinically in an effort to improve outcomes for patients with high-risk hematologic malignancies. Our findings also provide important mechanistic insights into the direct and indirect impact of PTCy on allogeneic GVT-mediating cells. Whether the positive effects of PTCy shown here in modulating CAR T-cell responses also translate to the use of cyclophosphamide to reduce toxicities of autologous CAR T-cell therapies requires further study.
Acknowledgments
The authors thank Jon Inglefield and Yanyu Wang of the Clinical Support Laboratory of the Frederick National Laboratory for Cancer Research for performing the cytokine analyses and Devorah Gallardo and Nga Hawk for technical assistance.
This work was supported by the Intramural Research Program of the National Cancer Institute (NCI), National Institutes of Health (NIH). This work was made possible by support from the National Cancer Institute Center for Cancer Research Single Cell Analysis Facility and Sequencing Facility, which are funded by the Frederick National Laboratory for Cancer Research contract HHSN261200800001E.
This work utilized the computational resources of the NIH High Performance Computing Biowulf cluster (http://hpc.nih.gov).
Authorship
Contribution: C.G.K designed the research; M.T.P., S.M.K., and T.J.F. contributed to the study design; M.T.P., S.M.K., N.S.N., R.E.F., A.D.H., S.K.M., A.d.P.P., and C.G.K. performed experiments; M.T.P., S.M.K., and C.G.K. analyzed data, interpreted data, and prepared figures and tables; J.B. and M.C. performed analyses of the single-cell RNA sequencing data; M.A.E. performed blinded histopathologic assessments and photographed the pathologic slides; D.J.V. and H.C.-W. performed statistical analyses; K.I., H.Q., and T.J.F. provided critical reagents and technical assistance; C.G.K. wrote the manuscript; and all authors revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christopher G. Kanakry, Building 10-CRC, Room 4-3142, 10 Center Dr, Bethesda, MD 20892; e-mail: christopher.kanakry@nih.gov.
References
Author notes
The single-cell RNA sequencing data have been deposited in GEO under the accession number GSE214788. The code for the single-cell RNA sequencing has also been published at: GitHub - NIDAP-Community/Murine-Allogeneic-CAR-T-Cells (https://github.com/NIDAP-Community/Murine-Allogeneic-CAR-T-Cells).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
M.T.P. and S.M.K. contributed equally to this work.


![Limited therapeutic window to give anti-CD19 CAR T cells after PTCy. (A) Treatment schema that is identical to Figure 1B except that cryopreserved CAR T (or NT) cells were thawed and administered intravenously either on day +5, day +9, or day +14 post transplant to remove any potential confounding effects of variability in different CAR T-cell products or other aspects of the transplantation platform if these were done in parallel experiments. Splenocytes for transduction also came from CD45.1+CD45.2+ B6C3F1 mice to most definitively separate CAR+ (Protein-L+CD45.1+) from graft-derived (CD45.1−) T cells as wild-type (CD45.1−CD45.2+) B6C3F1 mice were used for the splenocytes and bone marrow for transplant. Mice not receiving cells on a given day received RPMI vehicle intravenously. (B) CAR T-cell administration on day +5, day +9, or day +14 to PTCy-treated mice did not aggravate histopathologic GVHD at day +21 compared with mice treated with PTCy without CAR T cells. (C) CAR T cells on day +5 significantly prolonged survival compared with mice not receiving CAR T cells (hazard ratio [HR] 0.29, P = .0022), mice receiving CAR T cells on day +9 (HR 0.33, P = .009) or day +14 (HR 0.19, P = .0003), or mice receiving NT cells on day +5 (HR 0.37, P = .02; NT-cell groups are shown in supplemental Figure 5). This prolonged survival was achieved without any significant difference in weights or clinical scores in the CAR T cells on day +5 group compared with these other groups. Meanwhile, CAR T cells administered on day +9 or day +14 did not prolong survival compared with the no CAR T or NT-cell groups. (D) Successful clearance of both benign and malignant CD19+ targets at day +21 was achieved only when CAR T cells were given on day +5. (E-F) Despite superior efficacy, viable donor CAR T cells were lowest in (E) total numbers and (F) percentages at day +21, particularly within the spleen, for the group administered CAR T cells on day +5. (F) Despite being the most highly proliferative, the lack of clinical activity of CAR T cells given on day +14 was associated with these CAR T cells at day +21 being (H-I) significantly less differentiated and (J-K) having significantly higher expression of coinhibitory molecules PD1 and LAG3 compared with CAR T cells administered on day +5 or day +9. Combined results of 3 independent experiments are shown for all parts with 5 mice per group per experiment for panel C and 4 mice per group per experiment for all other parts. Data underwent natural logarithmic (total numbers) or arcsin (percentages) transformation prior to one-way ANOVA followed by the Holm-Sidak post hoc test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/6/10.1182_blood.2022016660/6/m_blood_bld-2022-016660-gr3.jpeg?Expires=1764959891&Signature=xnoisInDUuSdcIPiQZyLHl8YfFA4ZNaKDUSSXTEMa-iC3ltPkkvPoVgjYQaYEHM-g89r-9eYpw3tNCTWpr8b29DYRNJ-9ST1-T-hCkylyf05YM5MJBOC03rao3Y6Lm8xYiIbphFbUZG77DsK2F2vK~iuj31SQG2jBuY8AACb-V9m~Evkyy3gIpecdEpNLCqSQIJN2n-qxE7I~4BSyOZUaNTZqd918Qn9FdyWzpQb3MW204am71NZ4lPVTpgdwTb3XX6pnrmjOmg-zdRtrB2FCG~UjTqD0dc0Kj1v9WltxnX1ZvJ5F6oOr~Tdgza7NR9QH-k6dKo7708J-v4eA25ZWQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal