An increasing number of patients with relapsed or refractory B-cell leukemia and lymphoma receive both allogeneic hematopoietic cell transplantation (allo-HCT) and chimeric antigen receptor (CAR)-T cell therapy over the course of their disease. Yet little is known about the optimal sequence and how to best integrate these 2 treatment modalities. In this issue of Blood, Patterson et al1 demonstrate in murine studies that allo-HCT with posttransplantation cyclophosphamide (PTCy) can effectively be combined with allogeneic CD19 CAR-T cell treatment. Surprisingly, CAR-T cells given just before or shortly after cyclophosphamide graft-versus-host disease (GVHD) prophylaxis exert stronger antileukemic effects than CAR-T cells administered later.
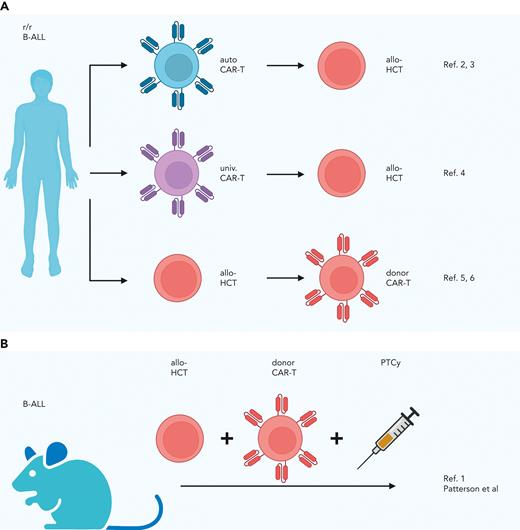
The many possibilities for combining allo-HCT and CAR-T cells to treat aggressive hematologic malignancies are currently best illustrated by relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL). Data from early clinical trials and clinical series in this patient population indicate that the following combined approaches may be safe and effective: first, consolidative allo-HCT in patients who have achieved complete remission after autologous CD19 CAR-T but remain at high risk of relapsing2,3; second, allo-HCT in patients who have responded to bridging therapy with universal CAR-T cells4; and third, donor CD19 CAR-T in patients who relapse after allo-HCT.5,6 In their preclinical study, Patterson et al investigated a different integrated approach in which allo-HCT and donor CD19 CAR-T cells are given in short succession (see figure). To study how to prevent the allogeneic CAR-T cells from causing GVHD, they explored a major histocompatibility complex (MHC)-haploidentical allo-HCT model with PTCy prophylaxis. To discern the effects of PTCy on CAR-T cells, they developed a model of pre-B-ALL (E2a-PBX tumor cell line with a B6 background not expressing major histocompatibility antigens different from those expressed on either recipient or donor strain) in which only the donor CD19 CAR-T cells can exert an antileukemic effect but not the allogeneic T cells in the graft. Testing different administration schedules, they found CAR-T cells given before or shortly after PTCy eradicate leukemia more efficiently than CAR-T cells given later after PTCy. The finding that direct in vivo exposure of the CAR-T cells to cyclophosphamide not only spares but enhances their antileukemic effect was unexpected and prompted the authors to study the possible underlying mechanisms. The investigators found that administration of CAR-T cells before PTCy resulted in earlier CAR-T cell expansion, higher phenotypic CAR-T cell activation, and fewer CAR-T regulatory cells compared with administration after PTCy. In addition, transcriptional profiling indicated increased activation of CD4+ CAR-T cells and more cytotoxic CD8+ CAR-T cells for CAR-T cell application before PTCy as opposed to a later application. A possible explanation for this observed PTCy effect is that it transiently rests the CAR-T cells, resulting in better long-term antileukemia potency, as reported for other rest-inducing modalities in CAR-T cells.7
Combination strategies for allogeneic hematopoietic cell transplantation and CAR-T cells previously reported (A) and developed by Patterson et al (B). allo, allogeneic; auto, autologous; r/r, relapsed or refractory; univ., universal.
Combination strategies for allogeneic hematopoietic cell transplantation and CAR-T cells previously reported (A) and developed by Patterson et al (B). allo, allogeneic; auto, autologous; r/r, relapsed or refractory; univ., universal.
A few critical points remain that will be relevant for a future clinical translation of this approach combining allo-HCT and donor CAR-T cells. A limitation of the MHC-haploidentical HCT model used is that the CAR-T cells themselves do not induce GVHD. Although the data clearly indicate that PTCy does not ablate the CAR-T cells’ antileukemia effect, it remains to be assessed whether PTCy can prevent human donor CAR-T cells from inducing GVHD. Furthermore, the authors used a CD19 CAR with a CD28 costimulatory domain throughout the study. Because the potential of donor-derived CD19 CAR-T cells to induce GVHD has been shown to differ depending on their costimulatory domain, it is important to evaluate the feasibility of integrating CAR-T cells with different costimulatory domains into allo-HCT strategies with PTCy prophylaxis in future studies.8
In conclusion, this study identifies a promising strategy for leveraging the complementary antileukemic effects of polyclonal alloreactive T cells and antigen-specific CAR-T cells. The data support the clinical translation of allo-HCT with PTCy in combination with donor CD19 CAR-T cells to evaluate its safety and efficacy. Integration of these 2 immune-based approaches shows potential to advance relapse prevention and treatment for patients with aggressive hematologic malignancies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal