In this issue of Blood, Moujalled et al1 identify loss-of-function BAX mutations in 17% of patients with acute myeloid leukemia (AML), relapsing after treatment with the BH3-mimetic venetoclax, providing a novel explanation for venetoclax resistance in AML.
The BH3-mimetic venetoclax has transformed the management of patients with AML who are ineligible for intensive induction therapy, and has quickly become the standard of care treatment in combination with hypomethylation agents or low-dose cytarabine for patients with newly diagnosed AML.2,3 At the same time, progression of disease upon treatment with venetoclax is frequent and associated with dismal outcomes. As such, understanding the mechanisms of resistance to venetoclax-based regimens in AML is of utmost importance.
Resistance to targeted therapies, such as venetoclax frequently occurs because of mutations in the drug target itself. For example, in the setting of chronic lymphocytic leukemia (CLL), another disease where venetoclax-based therapy has been incredibly successful, acquired BCL2 G101V and D103Y mutations have been identified in a number of patients with CLL with resistance to venetoclax, and impair venetoclax binding to BCL2.4,5 However, recurrent mutations in BCL2 have not been identified in patients with AML with resistance to venetoclax. Instead, previous genomic analyses of patients with AML receiving venetoclax-based regimens have identified both primary and acquired mutations in TP53 and/or genes in mitogenic signaling pathways (such as FLT3 and K/NRAS), which are associated with reduced response to venetoclax.6-8 Orthogonal functional genomic screens in AML model systems have also found that deletions of apoptotic regulators such as TP53, BAX, or NOXA induce venetoclax resistance.9 However, of these genes, to date, only mutations in TP53 have been identified in patients with AML.
One clue that previous genomic efforts might have missed crucial resistance mutations to venetoclax came from a recent study from the same working group of Moujalled et al. Their previous work demonstrated that patients with CLL treated with long-term venetoclax acquired not only well-described clonal hematopoiesis mutations such as DNMT3A and TET2, but also loss-of-function mutations in BAX, specifically in the myeloid compartment.10
BAX is a proapoptotic effector protein, which upon activation by BH3-only proteins, induces apoptosis by porating the outer mitochondrial membrane (OMM) (see figure). Venetoclax, whose mechanism of action involves releasing BH3 proteins from BCL2 to activate BAX, therefore, requires functional BAX. Genetic selection of BAX mutant myeloid clones in patients with CLL upon prolonged venetoclax treatment strongly suggested that BAX inactivation might be another potential mechanism of acquired resistance to venetoclax in AML.
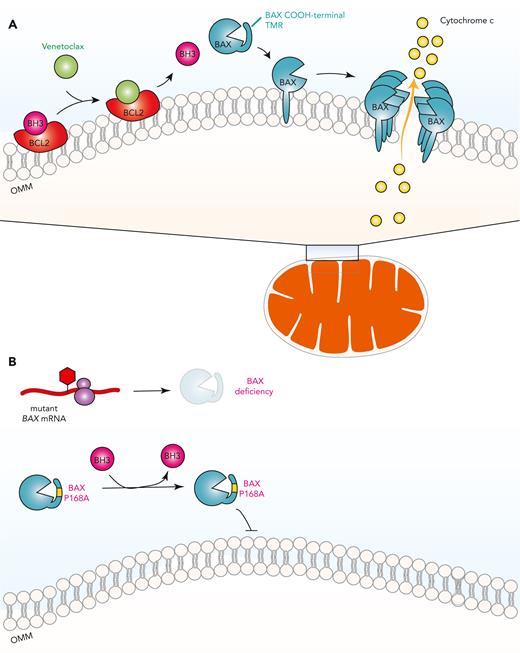
Dependence of venetoclax response on BAX and development of venetoclax resistance through BAX mutations. (A) Venetoclax, by engaging BCL2 as an alternative ligand, releases proapoptotic BH3-only proteins from BCL2, which then activates apoptosis effector proteins such as BAX. BAX is found predominantly in the cytosol with its COOH-terminal transmembrane region (TMR) sequestered within its hydrophobic pocket. Once activated, BAX releases its TMR to be inserted into the OMM, where BAX oligomerizes to permeabilize the OMM and induce release of cytochrome c. (B) Acquired frameshift/nonsense mutations in BAX reduce its protein expression while inactivating missense mutations in BAX such as P168A compromises its effector functions by preventing BAX TMR release and insertion into the OMM. mRNA, messenger RNA.
Dependence of venetoclax response on BAX and development of venetoclax resistance through BAX mutations. (A) Venetoclax, by engaging BCL2 as an alternative ligand, releases proapoptotic BH3-only proteins from BCL2, which then activates apoptosis effector proteins such as BAX. BAX is found predominantly in the cytosol with its COOH-terminal transmembrane region (TMR) sequestered within its hydrophobic pocket. Once activated, BAX releases its TMR to be inserted into the OMM, where BAX oligomerizes to permeabilize the OMM and induce release of cytochrome c. (B) Acquired frameshift/nonsense mutations in BAX reduce its protein expression while inactivating missense mutations in BAX such as P168A compromises its effector functions by preventing BAX TMR release and insertion into the OMM. mRNA, messenger RNA.
Now, work from Moujalled et al definitively confirms that somatic mutations in BAX do occur in patients with AML upon relapse following venetoclax therapy and are quite common, occurring in 17% of patients with AML in this study. The BAX variants discovered in this study fall into the following 2 mechanistic classes: mutations resulting in nonsense-mediated decay of BAX mRNA, or mutations causing structural alteration of the BAX protein (see figure). Mutations resulting in decreased BAX protein levels attenuate apoptotic signals unleashed by venetoclax, a finding consistent with previous work using BAX knockout cells. The authors also identified missense mutations concentrated in the BAX COOH-terminal domain, which impair the ability of BAX to translocate from the cytosol to the OMM to induce apoptosis. A BAX P168 hinge residue was previously characterized to be crucial for BAX translocation to the OMM.11 Consistent with this, BAX P168A mutation was seen in patients with AML and was functionally found to confer resistance to venetoclax.
The finding that acquired BCL2-resistant mutations occur in CLL, whereas acquired BAX mutations occur in AML raise important questions about lineage specific differences in venetoclax adaptation. Cell-type specific differences in response to venetoclax may be because of distinct cell-lineage specific dependencies in prosurvival proteins such as MCL1, BCL2, and BCL-xL, and/or differences in the mechanisms for generating mutations in distinct cell types. Emerging data on clonal mosaicism in solid tissue compartments also beg the question of how nonhematopoietic tissues may be genetically affected by venetoclax exposure.
Given that venetoclax is often given without a fixed end point in patients with AML initially responding to therapy, it will be important to delineate how early BAX mutations arise in the setting of venetoclax treatment and how long BAX mutants persist after discontinuation of venetoclax. From a clinical perspective, it may be helpful to consider altering the schedule of venetoclax to determine if this might prevent the emergence of BAX mutant clones, while still allowing clinical benefit from venetoclax administration. These data also highlight more broadly that the genomics of AML upon exposure to therapeutics may have critically important differences compared with patients with newly diagnosed AML. Thus, the genomic landscape of AML must be continually reevaluated in the context of the changing treatment landscape.
The discovery of acquired venetoclax-resistant BAX mutations in patients with AML has many important future therapeutic implications. Firstly, expression of BAX mutations conferred resistance not only to venetoclax, but also to other BCL2 inhibitors such as S55746 as well as to the MCL1 inhibitor S63845. These data suggest the possibility that BAX loss-of-function may underlie therapeutic resistance to multiple classes of BH3-mimetic compounds in the treatment of AML. Furthermore, BAX mutations were almost never seen at baseline in patients with AML or in the setting of relapse following cytotoxic chemotherapy, indicating that these mutations are acquired because of a selective advantage conferred in the setting of venetoclax therapy specifically. Consistently, BAX-deficient AML cells in vitro, although resistant to venetoclax and other BH3 mimetics, were still sensitive to conventional cytotoxic agents such as cytarabine or anthracyclines. These findings support the emerging concepts of combining venetoclax with cytotoxic chemotherapy, and it would be interesting to see if BAX mutations also arise in this drug combination that elicits distinct mechanisms of leukemic cell death. Lastly, it would be important to identify genetic and pharmacological vulnerabilities in BAX-deficient AML cells, with the goal of identifying novel targets for patients with AML upon relapse to venetoclax.
Conflict-of-interest disclosure: O.A.-W. has served as a consultant for H3B Biomedicine, Foundation Medicine Inc, Merck, Prelude Therapeutics, and Janssen; is on the scientific advisory board of Envisagenics Inc, Alchemy, Harmonic Discovery Inc, and Pfizer Boulder; and has received prior research funding from H3B Biomedicine, Nurix Therapeutics, and LOXO Oncology, unrelated to the current manuscript. W.J.K. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal