Key Points
RUNX1-deficient megakaryocytes exhibit thrombopoietic and platelet defects in NSG/VWFR1326H mice.
Preexposure of RUNX1-deficient megakaryocytes to a TGFβ1-pathway inhibitor ameliorated both defects, correcting hemostasis.
Abstract
Heterozygous defects in runt-related transcription factor 1 (RUNX1) are causative of a familial platelet disorder with associated myeloid malignancy (FPDMM). Because RUNX1-deficient animal models do not mimic bleeding disorder or leukemic risk associated with FPDMM, development of a proper model system is critical to understanding the underlying mechanisms of the observed phenotype and to identifying therapeutic interventions. We previously reported an in vitro megakaryopoiesis system comprising human CD34+ hematopoietic stem and progenitor cells that recapitulated the FPDMM quantitative megakaryocyte defect through a decrease in RUNX1 expression via a lentiviral short hairpin RNA strategy. We now show that shRX-megakaryocytes have a marked reduction in agonist responsiveness. We then infused shRX-megakaryocytes into immunocompromised NOD scid gamma (NSG) mice and demonstrated that these megakaryocytes released fewer platelets than megakaryocytes transfected with a nontargeting shRNA, and these platelets had a diminished half-life. The platelets were also poorly responsive to agonists, unable to correct thrombus formation in NSG mice homozygous for a R1326H mutation in von Willebrand Factor (VWFR1326H), which switches the species-binding specificity of the VWF from mouse to human glycoprotein Ibα. A small-molecule inhibitor RepSox, which blocks the transforming growth factor β1 (TGFβ1) pathway and rescued defective megakaryopoiesis in vitro, corrected the thrombopoietic defect, defects in thrombus formation and platelet half-life, and agonist response in NSG/VWFR1326H mice. Thus, this model recapitulates the defects in FPDMM megakaryocytes and platelets, identifies previously unrecognized defects in thrombopoiesis and platelet half-life, and demonstrates for the first time, reversal of RUNX1 deficiency–induced hemostatic defects by a drug.
Introduction
Lineage-specific gene regulation by key transcription factors is critical for hematopoiesis. Failures of such key transcription factors to express at the appropriate level and time can broadly affect downstream pathways of differentiation, likely at multiple levels, and the final phenotypes would involve multiple pathways in multiple lineages.1-4 Runt-related transcription factor 1 (RUNX1) is an essential transcriptional factor in hematopoietic stem cells and is involved in multiple lineages, including megakaryocyte formation.5-9RUNX1 haploinsufficiency (RUNX1+/−) causes a described syndrome, familial platelet disorder with associated myeloid malignancy (FPDMM).7,10 Usually, patients with FPDMM have symptoms of mild to moderate qualitative and quantitative platelet defects with increased risk of myelodysplastic syndrome (MDS) and acute myeloblastic leukemia (AML).11-16
Unlike human FPDMM, RUNX1+/− mice do not have a bleeding diathesis nor do they develop either MDS or AML.6,16,17 These studies suggest that there are significant species-to-species differences in phenotypes of RUNX1 deficiency that limit usage of these nonhuman animal models. Without a small animal model mimicking the main features of human FPDMM, it has been a challenge to advance our understanding of functional mechanisms of the observed phenotype seen in RUNX1+/− and in testing therapeutics.
As an alternative to animal models, human induced pluripotent stem cells (iPSCs) have served as a resource for modeling human hematopoietic diseases, including transcription factors central to megakaryopoiesis.18 RUNX1+/− human iPSCs and iPSCs established from affected individuals recapitulate critical features of patients with FPDMM, for example, reduced megakaryocyte yield and functionality.19-21 We showed that there was an accompanying depletion of megakaryocyte-biased subpopulations of hematopoietic progenitors.22 In addition, single-cell RNA sequencing data suggest that RUNX1+/− induces inflammatory-related pathways such as the transforming growth factor β 1 (TGFβ1) signaling pathway. By blocking these pathways with a small-molecule inhibitor, we observed rescue of the FPDMM phenotypes, which may have therapeutic implications.22 Because iPSC-derived megakaryocytes are likely embryonic in nature23 and FPDMM is a disease affecting adult hematopoiesis, we treated CD34+-derived adult hematopoietic stem and progenitor cells (HSPCs) using lentiviral short hairpin RNA (shRNA) targeting RUNX1 (shRX), reducing RUNX1 expression by ∼50% to 70%.22 After differentiation, the resulting shRX-megakaryocytes recapitulated the decrease in megakaryocyte yield, which could be corrected by the same small molecule used in the iPSC studies.
Herein, we extended these shRX studies to better understand the effects on platelet yield and on circulating platelet half-life and functionality in a murine host model. We then examined potential drug intervention to correct these defects. We began these studies in vitro, utilizing these shRX-megakaryocytes to examine agonist responsiveness and the quantitative and qualitative responsiveness of the shRX-megakaryocytes yield to potential therapeutics. Next, we focused on in vivo studies of released platelets from CD34+-derived megakaryocytes infused into immunocompromised NOD scid gamma (NSG) mice, having previously shown that infused megakaryocytes release near-physiologic platelets after entrapment in the lungs;24,25 similar to what has been proposed by other investigators that marrow-derived megakaryocytes that have traveled to the lungs are a potential source of platelet production, at least in the mouse.26 We found that shRX-megakaryocytes infused into NSG mice released fewer platelets per megakaryocyte and the resultant platelets had a shortened half-life. Released shRX-platelets also poorly responded to agonists. To study overall hemostatic functionality of the platelets released from shRX-megakaryocytes, we used NSG mice that were homozygous for an R1326H substitution in von Willebrand factor (VWF), switching species specificity of VWF from mice to human glycoprotein Ibα.27,28 These mice have a bleeding diathesis unless infused with sufficient numbers of functional human platelets. We found that shRX-megakaryocyte–released platelets poorly improved hemostasis; however, shRX-megakaryocytes grown in the presence of a small-molecule inhibitor, RepSox, that blocks the TGFβ1 pathway,29 improved shRX-platelet yield, half-life, and agonist responsiveness, correcting thrombus formation in a carotid artery photochemical injury model. Thus, this developed system offers a model for studies of modified human megakaryocytes and the resultant platelets, allowing detailed analysis of the released platelets, and offering a platform for preclinical screening of potential therapeutics.
Materials and methods
CD34+ cells, ex vivo culture, and megakaryopoiesis
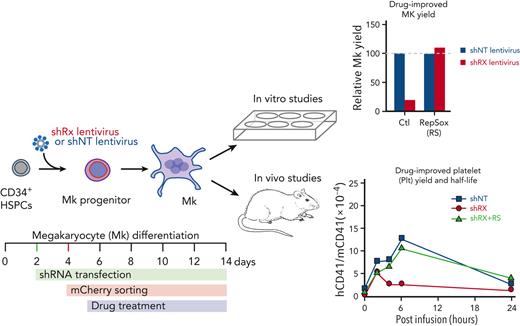
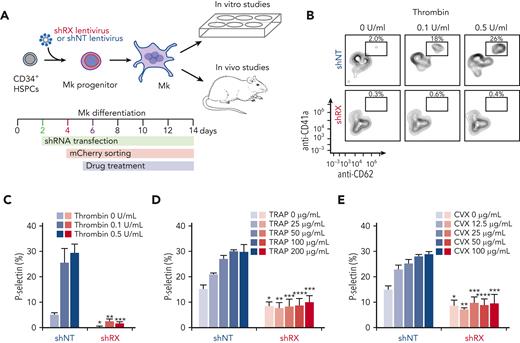
Mobilized human CD34+–derived HSPCs were purchased from the Fred Hutchinson Cancer Research Center. Thawed CD34+ HSPCs were cultured in differentiation medium consisting of 20% BIT 9500 Serum Substitute (STEMCELL Technologies, #09500), 55 μM 2-mercaptoethanol (Gibco, #2198523), 40 μg/mL low-density lipoprotein (STEMCELL Technologies, #02698), human cytokines (1 ng/mL stem cell factor, 100 ng/mL thrombopoietin, 13.5 ng/mL interleukin 9, and 10 ng/mL interleukin 6; all cytokines from R&D Systems), and 1× penicillin-streptomycin (Gibco, #1514022) in Iscove modified Dulbecco medium with GlutaMAX (Gibco, #31980030), as previously described.22 Cells were grown at 37°C in a 5% CO2 incubator for 11 to 14 days before study of the terminally differentiated megakaryocytes (Figure 1A).
Analysis of in vitro–grown megakaryocytes derived from human CD34+cells after RUNX1 suppression. (A) Experimental schema of the studies performed. To mimic FPDMM disease, CD34+ cells were infected with shRX- or shNT-lentiviruses on day 2 of differentiation. Infected cells expressing mCherry (mCherry+) that were sorted on day 4 of differentiation, were the focus of these studies. From day 5 of differentiation, cells were treated with drugs until day 11 or day 13 to 14 of differentiation. These matured megakaryocytes (Mk) were used for either in vitro or in vivo experiments. (B) Representative flow cytometric data on day 11 of differentiation for agonist-induced surface P-selectin exposure. After stimulation of mCherry+ megakaryocytes with indicated doses of thrombin, cells were stained with both anti-hCD41a and hCD62P (P-selectin). (C) The mean ± 1 standard deviation (SD) levels of surface P-selectin were quantified in megakaryocytes stimulated by increasing doses of thrombin as indicated from lighter to darker color. Blue indicates shNT-megakaryocytes; red, shRX-megakaryocytes. In panels D and E, similar studies as in panel C, but shNT- or shRX-megakaryocytes were exposed to TRAP (D) or convulxin (CVX) (E). In panels C-E, N = 3 separate studies, each in duplicate. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ∗∗∗∗P ≤ .0001. P values were calculated by 1-way analysis of variance (ANOVA) comparing shRX-megakaryocyte to each shNT-megakaryocyte sample. Also, see supplemental Figure 5 for all 3 agonists on day 14 megakaryocytes showing that all megakaryocytes that were mCherry− for shRX-lentivirus were agonist responsive.
Analysis of in vitro–grown megakaryocytes derived from human CD34+cells after RUNX1 suppression. (A) Experimental schema of the studies performed. To mimic FPDMM disease, CD34+ cells were infected with shRX- or shNT-lentiviruses on day 2 of differentiation. Infected cells expressing mCherry (mCherry+) that were sorted on day 4 of differentiation, were the focus of these studies. From day 5 of differentiation, cells were treated with drugs until day 11 or day 13 to 14 of differentiation. These matured megakaryocytes (Mk) were used for either in vitro or in vivo experiments. (B) Representative flow cytometric data on day 11 of differentiation for agonist-induced surface P-selectin exposure. After stimulation of mCherry+ megakaryocytes with indicated doses of thrombin, cells were stained with both anti-hCD41a and hCD62P (P-selectin). (C) The mean ± 1 standard deviation (SD) levels of surface P-selectin were quantified in megakaryocytes stimulated by increasing doses of thrombin as indicated from lighter to darker color. Blue indicates shNT-megakaryocytes; red, shRX-megakaryocytes. In panels D and E, similar studies as in panel C, but shNT- or shRX-megakaryocytes were exposed to TRAP (D) or convulxin (CVX) (E). In panels C-E, N = 3 separate studies, each in duplicate. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ∗∗∗∗P ≤ .0001. P values were calculated by 1-way analysis of variance (ANOVA) comparing shRX-megakaryocyte to each shNT-megakaryocyte sample. Also, see supplemental Figure 5 for all 3 agonists on day 14 megakaryocytes showing that all megakaryocytes that were mCherry− for shRX-lentivirus were agonist responsive.
Lentiviral infection
At 24 hours after seeding, CD34+ HSPCs at 1 × 106 per well in a 6-well plate (ultralow attachment surface, Corning #3471) were exposed to viral supernatant containing shRX-lentivirus (micro RNA: 5′-CCTACGATCAGTCCTACCAAT-3′; supplemental Figure 1A, available on the Blood website, and Estevez et al22) or a nontargeting, control (shNT) lentivirus to luciferase (micro RNA: 5′-CCGCCTGAAGTCTCTGATTAA-3′; supplemental Figure 1A and Estevez et al22) with 400 μg/mL poloxamer-407 (Sigma, #9003-11-6) and incubated at 37°C in a CO2 incubator for an additional 24 hours (Figure 1A). To calculate infection efficiencies, mCherry expression was measured at 72 hours postinfection using a CytoFlex 6 (BD Biosciences). Determination of RUNX1 messenger RNA levels was by quantitative reverse transcription polymerase chain reaction as described in the supplemental Materials and Methods.
Agonist-induced activation assays of megakaryocytes
CD34+-derived human megakaryocytes differentiated on days 13 to 14 were washed in phosphate-buffered saline (PBS, Gibco) and then resuspended as previously described to study agonist responsiveness.30 A series of agonists, that is, convulxin (Santa Cruz, #SC-202554), thrombin (Sigma), or the thrombin receptor–activating peptide (TRAP, Sigma) was added to each sample and incubated at room temperature for 15 minutes. After washing the sample with PBS, allophycocyanin-labeled polyclonal rabbit anti-CD41 (BD Pharmingen, 1:100) and BV421-labeled polyclonal rabbit anti-CD62P (BD Pharmingen, 1:100) were added, and activation of human megakaryocyte was determined by surface expression of P-selectin. Flow cytometric analysis was done and analyzed using FlowJo software version 10.6 (BD Biosciences).
Infused megakaryocyte murine studies
Immunocompromised NSG mice were used as previously described for studies of infused CD34+-derived human megakaryocytes to determine platelet yield and half-life.26 At 48 hours after infection, an mCherry+ sorted population, performed as described in the supplemental Materials and Methods, was cultured. Equal numbers (3 × 106 per mouse) of shNT- and shRX-megakaryocytes in 200 μL PBS were infused via the tail vein over 3 to 5 minutes on days 13 to 14. Blood samples (∼100 μL) containing 0.38% sodium citrate were then obtained from the contralateral tail vein to measure human platelets released from the infused megakaryocytes over the subsequent 48 to 72 hours. Human platelets in the obtained blood were identified using allophycocyanin-labeled polyclonal rabbit antihuman CD41 and/or CD42b (1:100), and fluorescein isothiocyanate–labeled polyclonal rabbit antimouse CD41 (1:100) was used to detect murine platelets. The ratio of human to murine platelets were then determined and used to calculate yield of human platelets released per infused megakaryocytes as well as human platelet half-life. This half-life was compared with tail vein–infused donor-derived human platelets (200 × 106 per mouse) studied as with the infused human megakaryocytes as previously described.24
Agonist responsiveness of the released human platelets was done as described for in vitro–generated human megakaryocytes, with the exception that it was performed in 100 μL of murine blood obtained by retro-orbital puncture. For in vivo hemostatic studies, previously generated NSG mice homozygous for VWFR1326H were studied in a Rose Bengal-Photochemical carotid artery injury model.28 At 4 hours after infusion of 3 × 106 human megakaryocytes, carotid artery thrombosis was induced in mice anesthetized with sodium pentobarbital (80 mg/kg) injected intraperitoneally. Under anesthesia, Rose Bengal (75 mg/kg, Sigma) was injected via the tail veil and the right carotid was exposed. After mounting a Doppler Flowprobe (Transonic Systems) on the site of carotid artery, a 540-nm laser (50 mW, Edmonds Optical) was used to injure the artery. Residual blood flow was monitored for an hour. After recording the blood flow data, all mice were euthanized.
Institutional approval and statistical analysis
For studies of donor-derived platelets, platelets were obtained from healthy donors not on aspirin with no bleeding/thrombotic history. The Children’s Hospital of Philadelphia Institutional Human Subjects Review Board approved these studies, and informed consent was obtained from anonymized donors. Studies were done in accord with the Declaration of Helsinki. The institutional animal care and use committee approved the murine studies and animals were euthanized in accord with the American Veterinary Medical Association.
Differences between 2 groups were compared using a 2-tailed Student t test. For multiple comparisons of >2 groups, statistical analysis was performed by 1-way analysis of variance using GraphPad Prism version 6.07. Differences were considered significant when P < .05.
Results
Recapitulation of human FPDMM disease in in vitro–grown CD34+-derived megakaryocytes
Prior studies by our group included CD34+-derived shRX-megakaryocytes in vitro studies demonstrating a deficiency of both megakaryocyte-biased hematopoietic progenitors and terminally differentiated megakaryocytes as well as alterations in the TGFβ1 signaling pathway.22 The overall schematic of further in vitro studies of these megakaryocytes studies is shown in Figure 1A. In using the described lentiviruses (supplemental Figure 1) and studying sorted mCherry+ cells, we found that mCherry positivity was preserved through differentiation with >97% of control shNT lentivirus–infected cells retaining positivity on day 11, as did >90% of shRX-transfected cells (supplemental Figure 2). We confirmed our prior findings of a ∼50% decrease in both RUNX1 messenger RNA and protein levels (supplemental Figure 3A-B) and a greater than fourfold decrease in mature CD41+CD42+ megakaryocyte yield (supplemental Figure 3C). We also observed that immature CD41−CD42− and CD41+CD42− cell populations were increased, whereas mature CD41+CD42+ megakaryocyte yield was markedly decreased by shRX (supplemental Figure 4A-B). Ploidy of mature megakaryocytes was also decreased by shRX (supplemental Figure 4C). These results are consistent with RUNX1-deficiency blocking terminal megakaryocyte differentiation. However, even with this blockage in our differentiation system, there were few cells in other lineages (eg, erythroid and myeloid; supplemental Figure 4B).
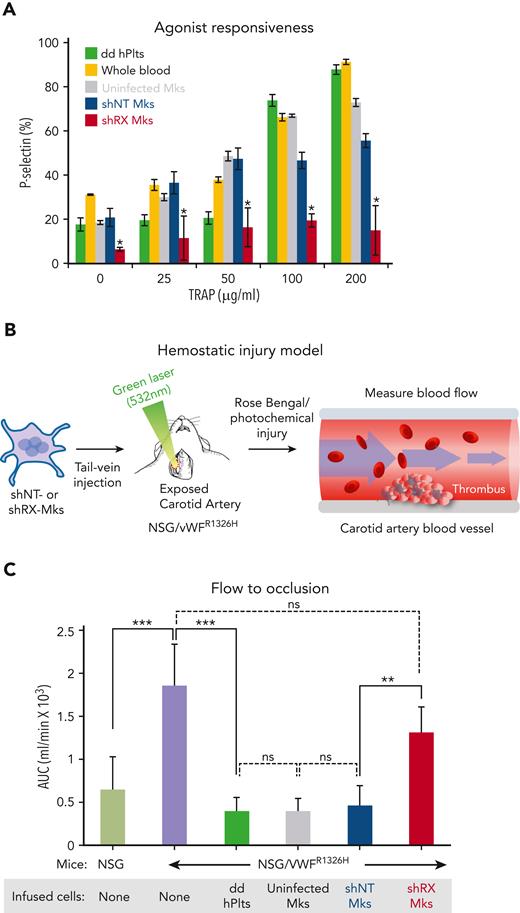
We then extended these studies to look at megakaryocyte agonist responsiveness by flow cytometry. Megakaryocyte agonist responsiveness studies (shRX- vs shNT-megakaryocytes) were done on day 11 of differentiation for thrombin (Figure 1B-C); TRAP, which activates megakaryocytes in a PAR1-dependent manner31,32 (Figure 1D); and convulxin, a collagen receptor glycoprotein VI agonist33,34 (Figure 1E). These data are consistent with multiple receptor pathways being defective in shRX-megakaryocytes, supporting the conclusion that RUNX1 levels in CD34+ megakaryopoiesis are critical for not only maturation of megakaryocyte, but also agonist-induced activation.
Decreased human platelet yield and half-life from infused shRX-megakaryocytes in NSG mice
Next, we were interested in measuring in vivo human shRX-platelet yield per megakaryocyte, released platelet half-life, and their responsiveness to agonists. We therefore combined the in vitro production of RUNX1-deficient megakaryocytes with our prior studies of infused human megakaryocytes releasing functional human platelets intrapulmonary in immunocompromised mice.22,24 We previously showed that such released human platelets in mice had similar size, half-life, and agonist responsiveness to that of freshly drawn, healthy donor-derived platelets.24 We measured both quantity and quality of human platelets released from infused shRX-megakaryocytes compared with shNT-megakaryocytes and donor-derived platelets. We infused 3 × 106 of uninfected or shNT- or shRX-lentivirus–infected megakaryocytes or 4 × 108 donor-derived platelets into NSG mice. After infusion, we monitored circulating human platelets by flow cytometry for 24 hours using the hCD41+:mCD41+ ratio as a comparative measure of circulating human platelets in the recipient mice (Figure 2A). Infused donor-derived platelet numbers peaked almost immediately. After infusion of uninfected megakaryocytes, circulating human platelets peaked 4 to 6 hours after infusion, as we reported previously.24 This delay is likely owing to the time needed to release platelets from megakaryocytes entrapped in the lungs.24,26 Next, we infused the same number of mCherry+ shNT- and shRX-megakaryocytes into NSG mice (Figure 2B). The release of platelets from shNT-megakaryocytes was similar to uninfected megakaryocytes with a peak platelet count at 4 to 6 hours after megakaryocyte infusion. In contrast, in mice infused with a similar number of shRX-megakaryocytes, the number of circulating human platelets was ∼30% of the shNT-megakaryocyte platelet number and peaked earlier at ∼2 hours.
Number and half-life of circulating human platelets after donor-derived platelets or megakaryocytes were infused into NSG mice. (A) 4 × 108 donor-derived (dd) human platelets or 3 × 106 CD34+ megakaryocytes were infused into NSG mice. At each time point, peripheral blood was withdrawn to measure circulating human platelet (hPlts) numbers relative to murine platelets after staining with hCD41 and mCD41 antibodies. Mean ± 1 SD is shown. N = 3 per arm. ∗∗∗P ≤ .001 by 1-way ANOVA. (B) Same as in panel A, but for 9 × 106 infused mCherry+ shNT- and shRX-megakaryocytes. N = 3 per arm. ∗P ≤ .05 and ∗∗P ≤ 0.01 by 1-way ANOVA comparing shNT-platelets released vs shRX-platelets released. (C) Similar to panel B, but after 9 × 106 of shNT- or shRX-megakaryocytes were infused and shown to display a drop from peak platelet count, with the 50% and 25% levels indicated. N = 3 per arm. ∗P ≤ .05 by 1-way ANOVA comparing shNT-platelets released vs shRX-platelets released.
Number and half-life of circulating human platelets after donor-derived platelets or megakaryocytes were infused into NSG mice. (A) 4 × 108 donor-derived (dd) human platelets or 3 × 106 CD34+ megakaryocytes were infused into NSG mice. At each time point, peripheral blood was withdrawn to measure circulating human platelet (hPlts) numbers relative to murine platelets after staining with hCD41 and mCD41 antibodies. Mean ± 1 SD is shown. N = 3 per arm. ∗∗∗P ≤ .001 by 1-way ANOVA. (B) Same as in panel A, but for 9 × 106 infused mCherry+ shNT- and shRX-megakaryocytes. N = 3 per arm. ∗P ≤ .05 and ∗∗P ≤ 0.01 by 1-way ANOVA comparing shNT-platelets released vs shRX-platelets released. (C) Similar to panel B, but after 9 × 106 of shNT- or shRX-megakaryocytes were infused and shown to display a drop from peak platelet count, with the 50% and 25% levels indicated. N = 3 per arm. ∗P ≤ .05 by 1-way ANOVA comparing shNT-platelets released vs shRX-platelets released.
To compare shNT- and shRX-platelet half-life, we increased the number of infused shNT- and shRX-megakaryocytes to 9 × 106 per recipient mouse and monitored the mice more frequently and for up to 48 hours (Figure 2C). Within the first 6 hours after reaching peak platelet count, the initial half-life of shRX-platelets was ∼2 hours compared with ∼6 hours of shNT-platelets (P < .01); a subsequent half-life for shRX-platelets was ∼5 hours compared with ∼12 hours for shNT-platelets. The shorter initial half-life of shRX-platelets likely affected the time and height of the peak of released platelets relative to that of shNT-platelets. The basis for the shortened half-life is unclear and whether it involves intravascular or extravascular destruction of the human platelets in the liver or spleen is also unclear at present.
Decreased agonist responsiveness by released shRX-platelets in NSG mice
To examine whether circulating human shRX-platelets are responsive to agonist, we isolated total platelets from the peripheral blood of NSG mice infused with uninfected or shNT- or shRX-megakaryocytes or with donor-derived platelets 2 hours after infusion. TRAP, which is human platelet-specific,35 was added to the samples at increasing concentrations. In line with our observation of in vitro megakaryocyte activation with agonists shown in Figure 1D and supplemental Figure 5C, circulating shRX-platelets had impaired activation in response to TRAP (Figure 3A).
Studies of released human platelets, agonist responsiveness, and hemostatic efficacy. (A) Flow cytometric studies of removed murine blood at 2 hours after infusion of donor-derived platelets or uninfected, shNT-, or shRX-megakaryocytes into NSG mice for P-selectin level analysis after activation with various concentrations of TRAP. Mean ± 1 SD is shown. N = 3 per arm. ∗P ≤ .05 by 1-way ANOVA comparing shNT-platelets released vs shRX-platelets. (B) Schematic of the Rose Bengal photochemical carotid artery thrombotic challenge with NSG/VWFR1326H mice and infused human megakaryocytes or platelets. (C) Same as in panel A except that NSG mice were studied as a hemostatic control because the untreated NSG/VWFR1326H mice had a hemostatic defect. Studies were done 4 hours after infusion of human platelets or megakaryocytes. Mean ± 1 is shown for residual blood flow after carotid artery injury. N = 4 to 6 animals per arm. ∗∗P ≤ .01, ∗∗∗P ≤ .001, and not significant (ns) by 1-way ANOVA. AUC, area under the curve.
Studies of released human platelets, agonist responsiveness, and hemostatic efficacy. (A) Flow cytometric studies of removed murine blood at 2 hours after infusion of donor-derived platelets or uninfected, shNT-, or shRX-megakaryocytes into NSG mice for P-selectin level analysis after activation with various concentrations of TRAP. Mean ± 1 SD is shown. N = 3 per arm. ∗P ≤ .05 by 1-way ANOVA comparing shNT-platelets released vs shRX-platelets. (B) Schematic of the Rose Bengal photochemical carotid artery thrombotic challenge with NSG/VWFR1326H mice and infused human megakaryocytes or platelets. (C) Same as in panel A except that NSG mice were studied as a hemostatic control because the untreated NSG/VWFR1326H mice had a hemostatic defect. Studies were done 4 hours after infusion of human platelets or megakaryocytes. Mean ± 1 is shown for residual blood flow after carotid artery injury. N = 4 to 6 animals per arm. ∗∗P ≤ .01, ∗∗∗P ≤ .001, and not significant (ns) by 1-way ANOVA. AUC, area under the curve.
Impaired thrombus formation by infused shRX-megakaryocytes in NSG/VWFR1326H mice
To better study the hemostatic effects of the various quantitative and qualitative defects in RUNX1-deficient megakaryocytes and platelets, NSG/VWFR1326H mice were infused with donor-derived platelets or with uninfected or shNT- or shRX-megakaryocytes and monitored during thrombus formation for residual blood flow after a Rose Bengal-photochemical carotid artery injury (Figure 3B). We generated these NSG/VWFR1326H mice previously to characterize human platelet function in vivo.28 These NSG mice are homozygous for VWFR1326H, leading to a switch in species specificity of VWF binding from mouse to human platelets. Compared with NSG mice, NSG/VWFR1326H mice have a bleeding diathesis if not infused with functional human platelets (Figure 3C). In the various arms of this study, initial blood flow was nearly identical (supplemental Figure 6), supporting the concept that animals were studied under similar conditions. Infusion of 3 × 106 uninfected or shNT-megakaryocytes into NSG/VWFR1326H mice was sufficient to decrease the time to occlusion and total blood flow to that seen in NSG mice and in NSG/VWFR1326H mice after infusion of 3 × 108 donor-derived platelets (Figure 3C). In contrast, infusion of 3 × 106 shRX-megakaryocytes resulted in little decrease in total blood flow.
Drug screening of shRX-HSPCs to correct megakaryocyte yield and agonist responsiveness
We screened several lead drugs and pathways that may improve outcome in FPDMM, both for megakaryocyte yield and agonist responsiveness.21,22,36 On day 5 of differentiation (Figure 1A), shNT- or shRX-lentivirus–infected cells were exposed to each drug at doses based on prior studies.21,22,29,37-46 We focused our studies on yield on days 11 and 14 megakaryocytes (supplemental Figure 7; Figure 4, respectively) because megakaryocytes at both these days of differentiation have been studied in other drug-screening studies.22,30,47,48 At both days 11 and 14 of differentiation, megakaryocyte yield decreased to ∼20% in RUNX1-deficient megakaryocytes, in the absence of drug treatment, compared with shNT controls. At both time points, most tested drugs showed either modest enhancements of megakaryocyte yield or no effect on shNT-megakaryocyte yield, whereas treatment of shNT-HSPCs with 1 μM or 10 μM ruxolitinib suppressed megakaryocyte yield (Figure 4A; supplemental Figure 7A). In studies of shRX-megakaryocytes, the TGFβ1 pathway inhibitor RepSox was the only compound that improved outcome up to that seen in control cells for both days of differentiation (Figure 4B; supplemental Figure 7B). The NOTCH pathway inhibitors, DAPT and avagacestat; JNK2 inhibitors, JIN8 and JIN10; and the mTOR inhibitor, MHY had more modest positive effects on day 14, which were not apparent on day 11. RepSox reversed the blockade in megakaryocyte differentiation with a decrease in observed CD41−CD42− and CD41+CD42− cells on day 11 of differentiation, in addition to increasing the number of mature megakaryocytes and returning ploidy to that seen in shNT-megakaryocytes (supplemental Figures 3C and 4).
Drug screening to rescue RUNX1-deficient megakaryocyte yield. Studies of shNT- (A) and shRX-megakaryocytes (B), exposed to the indicated drugs from day 5 to day 14 of differentiation. Megakaryocyte yield was calculated by flow cytometric analysis stained for an anti-hCD42b antibody and for mCherry. The dashed line represents yield of megakaryocytes from uninfected HSPCs not exposed to any drug. Mean ± SD is shown. N = 3 to 5 separate studies, each in duplicate. ∗P ≤ .05, ∗∗∗P ≤ .001, and ∗∗∗∗P ≤ .0001 by 1-way ANOVA compared to each dimethyl sulfoxide control sample.
Drug screening to rescue RUNX1-deficient megakaryocyte yield. Studies of shNT- (A) and shRX-megakaryocytes (B), exposed to the indicated drugs from day 5 to day 14 of differentiation. Megakaryocyte yield was calculated by flow cytometric analysis stained for an anti-hCD42b antibody and for mCherry. The dashed line represents yield of megakaryocytes from uninfected HSPCs not exposed to any drug. Mean ± SD is shown. N = 3 to 5 separate studies, each in duplicate. ∗P ≤ .05, ∗∗∗P ≤ .001, and ∗∗∗∗P ≤ .0001 by 1-way ANOVA compared to each dimethyl sulfoxide control sample.
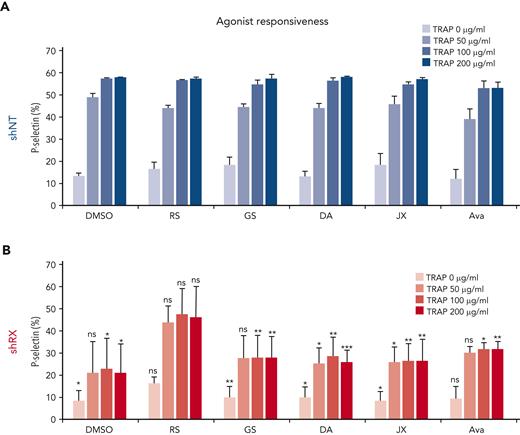
We then tested small-molecule drugs (RepSox, galunisertib, DAPT, JIN10, and avagacestat) that had moderate or greater effects in our assay systems or in prior studies21,22,38,44,46 and examined agonist-induced megakaryocyte responsiveness to various concentrations of TRAP by measuring P-selectin expression on day 11 (Figure 5). Studies of agonist responsiveness in megakaryocytes have been done by many groups since first described in an analysis of integrin intracellular signaling in comparison with platelets.49 Day 11 shNT-megakaryocytes had a high baseline level of P-selectin expression that further increased with exposure to TRAP (Figure 5A), whereas shRX-megakaryocytes had a lower baseline P-selectin expression level and a minimal increase after TRAP (Figure 5B). Of the various drug exposures, RepSox treatment had the greatest effect on TRAP response, increasing it near to that seen in shNT-megakaryocytes (Figure 5A vs Figure 5B).
Drug screening to rescue RUNX1-deficient megakaryocyte agonist response. shNT- (A) and shRX (B) megakaryocytes were treated with the indicated drugs from day 5 to day 11 of differentiation. Expression of P-selectin levels was measured by flow cytometry after staining with human CD62P in day 11 megakaryocytes exposed to various concentrations of TRAP as indicated. Mean ± 1 SD is shown. N = 3. ∗P ≤ .05, ∗∗P ≤ .01, and ∗∗∗P ≤ .001 by 1-way ANOVA comparing each shRX data point to its shNT comparative.
Drug screening to rescue RUNX1-deficient megakaryocyte agonist response. shNT- (A) and shRX (B) megakaryocytes were treated with the indicated drugs from day 5 to day 11 of differentiation. Expression of P-selectin levels was measured by flow cytometry after staining with human CD62P in day 11 megakaryocytes exposed to various concentrations of TRAP as indicated. Mean ± 1 SD is shown. N = 3. ∗P ≤ .05, ∗∗P ≤ .01, and ∗∗∗P ≤ .001 by 1-way ANOVA comparing each shRX data point to its shNT comparative.
RepSox as a model of therapeutic intervention in RUNX1+/−
We propose a strategy using shRNA suppression of RUNX1 in CD34+-derived HSPCs with subsequent differentiation into megakaryocytes followed by their infusion into NSG/VWFR1326H mice as a model that would facilitate testing of potential therapies to correct the platelet-related bleeding diathesis in patients with FPDMM (Figure 3B). Of the various drugs tested, RepSox was most effective at correcting shRX-megakaryocyte yield and agonist responsiveness (Figures 4 and 5; supplemental Figure 7). When shRX-HSPCs were differentiated in the presence of RepSox and then infused into mice, the drug partially to completely corrected platelet yield and half-life and agonist responsiveness (Figure 6A-B). We then asked whether RepSox-exposed shRX-megakaryocytes infused into NSG/VWFR1326H mice would correct the bleeding diathesis in the photochemical carotid artery injury model and observed near-complete correction of both total blood flow until occlusion and time to occlusion (Figure 7A-B).
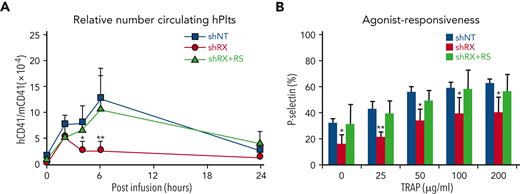
Drug correction of function and platelet-release defects with RepSox. (A) 3 × 106 shNT-, shRX-, or shRX+RepSox (shRX+RS)–megakaryocytes were infused into NSG mice. At each time point, mouse peripheral blood was withdrawn to monitor human platelet level. The peripheral blood samples were stained with hCD41 and mCD41 antibodies and analyzed by flow cytometry for human vs mouse platelets as in Figure 2. Mean ± 1 SD are shown. N = 3 per arm. ∗P < .05 and ∗∗P < .001 comparing shRX vs shRX+RS studies. (B) Study as in panel A. P-selectin levels on released human platelets in mouse blood were measured by flow cytometry under activation with various concentrations of TRAP. N = 3 per arm. ∗P ≤ .05 and ∗∗P ≤ .001 by 1-way ANOVA comparing shRX vs shRX+RS studies.
Drug correction of function and platelet-release defects with RepSox. (A) 3 × 106 shNT-, shRX-, or shRX+RepSox (shRX+RS)–megakaryocytes were infused into NSG mice. At each time point, mouse peripheral blood was withdrawn to monitor human platelet level. The peripheral blood samples were stained with hCD41 and mCD41 antibodies and analyzed by flow cytometry for human vs mouse platelets as in Figure 2. Mean ± 1 SD are shown. N = 3 per arm. ∗P < .05 and ∗∗P < .001 comparing shRX vs shRX+RS studies. (B) Study as in panel A. P-selectin levels on released human platelets in mouse blood were measured by flow cytometry under activation with various concentrations of TRAP. N = 3 per arm. ∗P ≤ .05 and ∗∗P ≤ .001 by 1-way ANOVA comparing shRX vs shRX+RS studies.
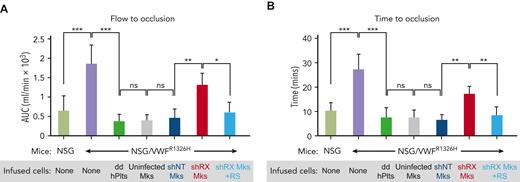
In vivo hemostatic correction of shRX-platelets by RepSox exposure of developing megakaryocytes. Thrombus formation studies as in Figure 3 with infused shRX-megakaryocytes in a Rose Bengal-photochemical carotid injury model system in NSG/VWFR1326H with NSG mice used as a positive control. To determine in vivo functionalities of infused RepSox-treated shRX-megakaryocytes, we monitored thrombus formation by measuring total blood flow (A) and time to occlusion (B). Mean ± SD are shown. N = 4 to 6 per arm. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ns = not significant by 1-way ANOVA comparing indicated matches.
In vivo hemostatic correction of shRX-platelets by RepSox exposure of developing megakaryocytes. Thrombus formation studies as in Figure 3 with infused shRX-megakaryocytes in a Rose Bengal-photochemical carotid injury model system in NSG/VWFR1326H with NSG mice used as a positive control. To determine in vivo functionalities of infused RepSox-treated shRX-megakaryocytes, we monitored thrombus formation by measuring total blood flow (A) and time to occlusion (B). Mean ± SD are shown. N = 4 to 6 per arm. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ns = not significant by 1-way ANOVA comparing indicated matches.
Discussion
The bleeding manifestations in FPDMM are often present since birth and lead to life-long risk of acute bleedings, chronic injuries like joint deformities, and even death.7,10,50 Moreover, although patients’ platelet counts are often >50 000/μL3, patients often require platelet transfusions to prevent traumatic and surgical bleeding. There are challenges associated with treating patients with FPDMM. Their higher baseline platelet count interferes with the physician’s ability to estimate response to a platelet transfusion as opposed to the more common cause of acquired platelet disorders associated with severe thrombocytopenia. Also, as this study clearly demonstrates, RUNX1 deficiency is associated with a combination of significant qualitative and quantitative defects in the megakaryocytes and released platelets that affect multiple pathways and result in pleiotropic defects. Thus, although the focus of finding therapeutic interventions for patients with FPDMM has been on the disease’s leukemic propensity, which approaches 50% by the age of 40 years,51 the bleeding manifestation is a significant medical challenge in these patients, and a better understanding of the pathogenesis of these defects and better therapeutics are needed.
Our studies provide insights into the defects in the megakaryocyte/platelet axis in individuals with RUNX1 deficiency. Not only is there the well-recognized defect in megakaryocyte yield, but also in the ability of these megakaryocytes to release platelets when entrapped in the pulmonary bed in recipient immunocompromised mice. Furthermore, the released platelets have a decreased half-life. Thus, there are at least 3 reasons for the observed thrombocytopenia in patients with FPDMM; defects in (1) megakaryopoiesis, (2) thrombopoiesis, and (3) platelet half-life. Neither the importance of each defect in the final thrombocytopenia nor the mechanistic bases of each defect has yet been defined. Moreover, RUNX1 haploinsufficiency also decreases responsiveness to multiple agonists. The finding that multiple agonist receptor pathways are defective in RUNX1-deficient megakaryocytes/platelets is not surprising given the key role that this transcription factor plays in both hematopoiesis and megakaryopoiesis.5,6 It would also not be surprising that the observed phenotype varies not only based on the degree of residual effective RUNX1 but likely also by different susceptibilities of various pathways to low RUNX1 levels based on an individual’s genotypic background.
Unlike developing therapeutics intended to decrease the long-term risk of leukemic progression, drug correction of the qualitative and quantitative platelet defects in FPDMM should have rapid onset of action. Measurements of platelet counts and platelet aggregation are standard clinical laboratory studies, and a short therapeutic intervention should ideally generate platelets that circulate and maintain function from days to weeks, be fully functional, and thus, be useful for hemostatic prophylaxis. We envision short-term perioperative drug treatment to be a useful first application of such platelet-correcting drugs in patients with FPDMM, which allows not only coverage of the immediate operative period, but also extended coverage until full healing has occurred.
We present studies of candidate drugs for therapeutic intervention in RUNX+/−. Megakaryocyte yield and responsiveness to agonist are the most straightforward parameters to measure in drug efficacy evaluation; however, the xenotransfusion model using shRX-megakaryocytes from healthy adult donor CD34+-derived HSPCs infused into NSG/VWFR1326H mice allows a more detailed look at important aspects of correcting platelet biology and correcting the hemostatic defects in these mice. A thrombosis challenge, as represented by our photochemical injury studies, could be applied as a potential preclinical study for FPDMM. Our studies clearly showed a positive effect of RepSox on each defined defect, owing to RUNX1 deficiency, in the megakaryocyte/platelet axis and a strong correction of overall hemostasis. RepSox is thought to inhibit TGFβ1 uptake by the TGF receptor 1β,27 a pathway that we had previously shown to be defective in RUNX1 deficiency. However, there are limited published in vitro and animal studies on RepSox27,52; therefore, it is clearly possible that RepSox targets an alternative pathway. Studies to define candidate pathways in RUNX1-deficient megakaryocytes after RepSox treatment are underway.
The presented xenoinfusion model is dependent on prior observations made by our group24,25 and others26 that marrow-derived megakaryocytes contribute to physiologic platelet production after becoming entrapped in the lungs. As discussed in a recent review on adult megakaryopoiesis, the degree to which the lung contributes to thrombopoiesis remains a matter of debate.53 However, even if lung-released platelets from marrow megakaryocytes is not a major source of circulating platelets, we have shown that size distribution, half-life, and function of human platelets released from infused human megakaryocytes are nearly on par with infused donor-derived human platelets.24 We propose that this model would be useful to study other acquired and inherited megakaryocyte/platelet disorders and could provide important in vivo insights into such disorders. There are important limitations to this xenoinfusion shRX-megakaryocyte model, including the fact that this is a short-term model. Drug exposure in this model is of committed differentiating human hematopoietic cells. The effects of more chronic drug exposure on stem and progenitor cells, and the effects of drugs on supporting stromal cells are not examined in this model. We are presently trying to develop a human xenotransplantation system beginning with shRX-HSPCs to overcome these limitations. An additional advantage of developing such a xenotransplant model would be that this system may allow studies of therapeutic interventions in impeding clonal progression to MDS or leukemia.
In summary, we developed a model system using shRX suppression of RUNX1 expression in differentiating CD34+ HSPCs to study the qualitative and quantitative defects in the final differentiated megakaryocytes and showed previously unrecognized defects in thrombopoiesis and circulating platelet half-life as well as significant defects in agonist responsiveness. Upon testing the effects of potential therapeutics on differentiating shRX-megakaryocytes, only treatment with RepSox, a TGFβ1 pathway inhibitor, had a clear effect on all the defects in the shRX-megakaryocytes and platelets, and was able to correct a hemostatic defect in recipient mice. We propose that this xenotransfusion model may be useful preclinically for drug testing before committing to a clinical trial for correcting the bleeding manifestations of FPDMM and perhaps other inherited platelet disorders.
Acknowledgments
The authors thank Nancy A. Speck (University of Pennsylvania) for helpful discussions and Douglas B. Cines (University of Pennsylvania) for reviewing the manuscript.
This work was supported by grants from the RUNX1 Research Program in association with the Alex’s Lemonade Foundation (M.P.) and from the National Heart, Lung, and Blood Institute, National Institutes of Health (grant R35 HL150698) (M.P.).
Authorship
Contribution: K.L. designed new experimental systems, prepared the first draft of the manuscript, and carried out most of the described studies with advice and assistance from H.S.A. in the in vitro and murine xenotransfusion studies; B.E. developed the short hairpin RNA study and carried out initial drug therapy studies; and M.P. developed the initial overall study, performed data interpretation with K.L., and assisted K.L. in preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mortimer Poncz, Department of Pediatrics, Children’s Hospital of Philadelphia, Abramson Pediatric Research Center, Room 317, 1 Civic Center Blvd, Philadelphia, PA 19104; e-mail: poncz@chop.edu.
References
Author notes
Data are available on request from the corresponding author, Mortimer Poncz (poncz@chop.edu).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal