In this issue of Blood, Bennett et al1 provide elegant data supporting the mounting evidence of the critical role of hepcidin, the master regulator of iron metabolism, in the pathophysiology of polycythemia vera (PV). Another very recent article by Stetka et al, in Blood, also explored hepcidin and PV.2
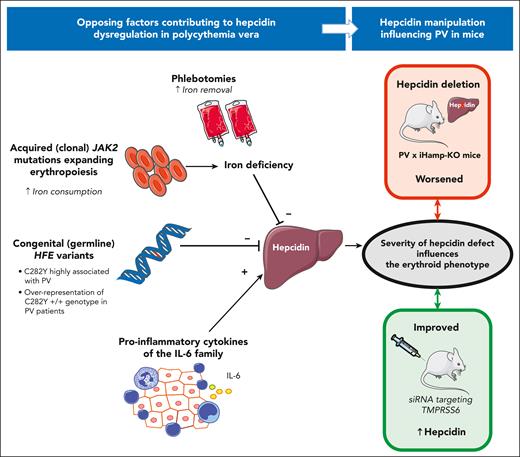
By total number, most of our body’s cells are small red blood cells (RBCs), estimated to represent ∼24.9 trillion of a total of ∼29.6 trillion cells (∼29.6 × 1012), whereas myocytes and adipocytes make up the majority by mass.3 Erythropoiesis produces ∼200 billion RBCs daily (∼2 million per second), requiring ∼80% of circulating iron, of which systemic distribution is finely regulated by the liver hormone hepcidin. Hepcidin acts by occluding the sole cellular iron exporter ferroportin, thus reducing absorption of dietary iron and recycling of iron from macrophages. High levels of hepcidin reduces iron availability, whereas hepcidin deficiency can ultimately lead to iron overload.4 Not surprisingly, the pathological expansion of erythropoiesis in PV is almost invariably associated with iron deficiency (ID) because of exaggerated need, which is further exacerbated by therapeutic phlebotomies. This imbalance results in a complex dysregulation of hepcidin, because of opposing stimuli (see figure). The net effect is a variable degree of hepcidin suppression, predominantly due to ID and the ensuing need to increase iron absorption, partially counteracted by proinflammatory cytokines (reviewed by Ginzburg et al5). The work by Bennett et al identifies hepcidin as a key driver of PV phenotype severity, using 2 complementary approaches. Firstly, unbiased genome-wide association studies (GWASs) on 2 large populations (United Kingdom Biobank and FinnGen) found that single-nucleotide polymorphisms (SNPs) in the HFE gene were strongly associated with the risk of developing PV. This was particularly evident for the SNP corresponding to the common C282Y mutation, whose homozygosity is associated with hepcidin deficiency and clinically overt hemochromatosis, although with a low penetrance.4 Of note, individuals who have homozygous C282Y tend to have slightly higher hemoglobin (Hb) levels than noncarriers.4,6 Common HFE SNPs have also been positively associated with Hb and RBC parameters in large meta-analyses of multiple GWASs.7 Studies on clonal hematopoiesis of indeterminate potential (CHIP) have suggested that the classical acquired somatic JAK2 V617F is far more frequent than PV in the general population (up to 3.1% vs a prevalence of JAK2-associated myeloproliferative neoplasms of 6-8 per 10 000).8 The reasons for this discrepancy are unclear. It has been hypothesized that the acquisition of JAK2 V617F is only a part of the story, with the presence of inherited factors possibly being equally important.9 Until recently, few alleles predisposing to JAK2 V617 CHIP were identified in the JAK2 gene itself and in genes controlling cellular aging (TERT), epigenetic regulation (TET2), and erythroid/megakaryocyte development (GFI1B), among others.9 The work by Bennett et al confirms the strong association of a known germ line JAK2 SNP (tagging the 46/1 haplotype) with PV and adds the identification of HFE as a modifier of PV phenotype severity by predisposing to hepcidin deficiency. Such a genetic background would be likely permissive by favoring iron fueling for exaggerated erythropoiesis when JAK2 somatic mutations are acquired and reach a certain allele burden.
In PV, opposing factors influence hepcidin expression in the liver. Expanded erythropoiesis and phlebotomies contribute to ID, which inhibits hepcidin. Congenital variants in the HFE gene (ie, C282Y known to be associated with hemochromatosis) may further worsen hepcidin deficiency. In contrast, inflammatory cytokines (via the GP130-coupled receptor pathway) stimulate hepcidin. The resulting variable degree of hepcidin suppression influences the phenotype severity, as suggested by manipulation of hepcidin in experimental models.
In PV, opposing factors influence hepcidin expression in the liver. Expanded erythropoiesis and phlebotomies contribute to ID, which inhibits hepcidin. Congenital variants in the HFE gene (ie, C282Y known to be associated with hemochromatosis) may further worsen hepcidin deficiency. In contrast, inflammatory cytokines (via the GP130-coupled receptor pathway) stimulate hepcidin. The resulting variable degree of hepcidin suppression influences the phenotype severity, as suggested by manipulation of hepcidin in experimental models.
To corroborate this hypothesis, Bennett et al manipulated hepcidin in a series of elegant experiments in a PV mouse model. Indeed, genetic ablation of liver hepcidin worsened the erythroid phenotype, whereas a remarkable amelioration was obtained by increasing hepcidin (see figure). From a practical standpoint, such experiments provide a further strong rationale for the use of hepcidin mimetics in patients with PV, which are already under active investigation (recently reviewed by Handa et al10). In particular, the hepcidin mimetic rusfertide has been demonstrated to virtually eliminate the need for phlebotomy in patients with PV in 2 phase 2 clinical trials (NCT04057040 and NCT04767802). Moreover, the drug improved iron parameters and reduced constitutional symptoms likely due to ID in nonerythroid compartments, with no serious safety signals. Results from an ongoing multicenter placebo-controlled phase 3 trial (NCT05210790) are eagerly awaited.10 There is a spectrum of hepcidin mimetics in clinical development, such as oligonucleotides targeting TMPRSS6, a gene encoding for matriptase-2, an upstream negative regulator of hepcidin.4 Silencing TMPRSS6 via an antisense nucleotide (sapablursen) or a small interfering RNA (SLN124, the same used by Bennett et al; see figure) results in significantly increased hepcidin expression lasting for weeks/months after a single subcutaneous dose.10 Whether these drugs will actually have a place in the future treatment of PV, either alone or in combination with other agents, requires further studies confirming the preliminary results and proof that there is robust cost effectiveness compared with current treatments. In particular, it is uncertain whether lowering hematocrit through hepcidin mimetics will mitigate the risk of thrombosis independently associated with the JAK2 V617F by itself.
Finally, the work by Bennett et al provides useful insights on the drivers of hepcidin dysregulation in PV. The authors measured classical serum iron biomarkers (ferritin and transferrin saturation), as well as the circulating levels of hepcidin and erythroferrone (ERFE) in 30 patients with PV. As compared with control study participants, patients with PV had lower hepcidin and increased ERFE, both differences being statistically significant (see Figure 2 in Bennett et al1). However, the degree of hepcidin suppression in patients with PV was lower than expected when compared with that in patients with a similar degree of ID from other causes (in whom hepcidin is usually strongly suppressed or even undetectable). ERFE is a negative regulator of hepcidin that is markedly increased when erythropoiesis is expanded (eg, in the recovery phase after important bleeding) or ineffective (eg, in thalassemic syndromes). Indeed, the ERFE increase in patients with PV was again lower than expected as compared with the increase of the aforementioned conditions. Ablation of ERFE in the PV mouse model by Bennett et al did not significantly alter the erythroid phenotype, confirming that ERFE is not a prominent regulator of hepcidin in PV. Results from Bennett et al point toward an important role of proinflammatory cytokines of the interleukin-6 (IL-6) family (other than simply IL-6 alone) as key factors partially counteracting the absolute hepcidin suppression due to ID in PV (see figure). Further characterization of these cytokines and of their ultimate mechanism leading to hepcidin dysregulation in PV represents a fertile field for future research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal