In this issue of Blood, Xie et al1 describe a novel role for interleukin 34 (IL-34) in promoting hematopoietic progenitor and myeloid leukemia differentiation by binding to a previously unrecognized receptor for IL-34, triggering receptor expressed on myeloid cells 2 (TREM2).
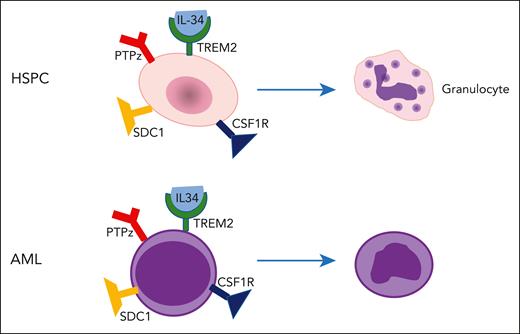
Extending their prior observation that osteoclast-specific deletion of tuberous sclerosis 1 (Tsc1) results in increased numbers of osteoclasts,2 the authors evaluated the hematopoietic system and observed increased granulocytic differentiation and enhanced osteoclast production of IL-34, a cytokine previously shown to promote myeloid maturation.3 The authors investigated whether IL-34 may promote maturation of acute myeloid leukemia (AML) blasts and found that IL-34 induced differentiation of human AML cell lines and primary mouse and human blasts by binding to TREM2 (see figure). In addition, AML cells engrafted into osteoclast-specific Tsc1-deleted mice resulted in reduced disease aggressiveness. Impressively, IL-34 treatment of mice engrafted with primary human AML cells or human and mouse AML cell lines resulted in enhanced blast differentiation, reduced disease burden, and improved survival.
IL-34 induces differentiation. IL-34 induces differentiation of normal hematopoietic/stem progenitor cells (HSPCs) as well as acute myeloid leukemia (AML) blast by binding to its previously unrecognized receptor, TREM2, instead of one of its known receptors: CSF1R, PTPz, or syndecan 1 (SDC1). Although osteoclasts likely represent an important source of IL-34 production, the sources of IL-34 production in patients with AML are unclear, as are the potential consequences of long-term IL-34 administration.
IL-34 induces differentiation. IL-34 induces differentiation of normal hematopoietic/stem progenitor cells (HSPCs) as well as acute myeloid leukemia (AML) blast by binding to its previously unrecognized receptor, TREM2, instead of one of its known receptors: CSF1R, PTPz, or syndecan 1 (SDC1). Although osteoclasts likely represent an important source of IL-34 production, the sources of IL-34 production in patients with AML are unclear, as are the potential consequences of long-term IL-34 administration.
Although IL-34 has been reported to bind to receptors including colony stimulating factor 1 receptor (CSF1R), protein tyrosine phosphatase receptor type Z1 (PTPz), and syndecan 1 (CD138), deletion of these receptors did not attenuate the differentiation activity of IL-34. Using a combination of approaches, the authors identified TREM2 as a novel receptor required for IL-34’s differentiation activity and found that IL-34 binding to TREM2 inhibits mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling by inducing phosphorylation of RAS protein activator like 1. Notably, IL-34 induced blast differentiation more robustly than small-molecule inhibitors of MAPK/ERK, suggesting that IL-34 regulates additional, as of yet, uncharacterized signaling events. This finding is consistent with prior observations that MEK inhibitors reduce blast proliferation or cell cycle arrest, rather than inducing differentiation.4,5
The authors showed that IL-34 serum levels are reduced in patients with AML compared with controls, but given that elevated levels of IL-34 are observed in numerous inflammatory disorders and cancers,3 and AML and preleukemic states are associated with increased systemic inflammation,6 it is unclear why serum IL-34 is reduced in patients with AML. We speculate that such differences may be due to AML blast suppression of IL-34 production in the marrow or systemically, or the ability of IL-34 to promote the development of myeloid cells into distinct subtypes that reduce IL-34 production during disease progression.7 Related to the latter possibility, IL-34 has been reported to have disease-promoting or protective effects depending on cancer type,3 and thus it would be important to determine its effects during AML pathogenesis. To address these issues, it will be important to determine the source/s of IL-34 production in patients with AML, and such a search should interrogate the various components of the marrow microenvironment as well as tissues outside the marrow because prior studies have shown IL-34 is present in nearly all tissues and is expressed at highest levels in the brain, skin, and lymphoid tissues.3 In addition, it would be important to determine if IL-34 expression is altered during aging or in preleukemic states such as clonal hematopoiesis and myelodysplastic syndrome, and to determine if IL-34 is required for the outgrowth of mutant clones. Such studies could be complemented by investigations using mouse models of AML that allow systematic evaluation of disease initiation and progression.
Although the IL-34–TREM2 signaling axis appears to be a promising target for the development of novel differentiation therapies in AML, several important questions remain regarding the therapeutic efficacy of targeting this pathway. Although the authors demonstrated that IL-34 can reduce AML clonogenicity, formal experiments testing effects on leukemia stem cells (eg, limiting dilution transplant studies following treatment) were not performed. Concerns also remain regarding whether long-term IL-34 treatment would be possible in patients because although no major toxicities were observed in these studies, the treatment periods were short (14-21 days). In addition, prolonged IL-34 treatment may result in signal attenuation through receptor downregulation or negative signaling feedback mechanisms, which may limit the efficacy of IL-34. Determining which patients may be suitable candidates for IL-34/TREM2-targeted therapies is also an important consideration. Although the authors show that TREM2 is required for the differentiation activity of IL-34, TREM2 expression is heterogeneous among patients with AML, and it remains unclear if differences in TREM2 expression levels impact IL-34 responses. Finally, given the importance of IL-34 in promoting myeloid differentiation and osteoclastogenesis, it would be important to determine if prolonged IL-34 treatment promotes diseases of osteogenesis or inflammation, because recent studies point to both protective effects of low-dose IL-348 as well as potential bone toxicities of high levels of IL-34.9 Although these concerns may ultimately limit the use of IL-34 as a differentiation therapy, direct manipulation of TREM2 signals using other approaches may provide additional potential strategies to induce AML differentiation.
Overall, these studies are notable for their comprehensive evaluation of IL-34 function in normal and malignant hematopoiesis, identification of a novel receptor for IL-34, and exciting proof-of-concept studies indicating that IL-34–based differentiation strategies are a promising strategy to treat AML. Given the relatively limited success of differentiation therapy as stand-alone therapy in AML,10 it will be important to determine if IL-34 can be used in combination with frontline or maintenance therapies. Although such studies could be performed using preclinical in vitro and in vivo models, ultimately the answer regarding the efficacy of these strategies likely will require clinical trials. We look forward to future studies that further explore the potential of using IL-34 or other TREM2-targeting strategies to treat patients with AML.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal