In this issue of Blood, Largeot et al1 show that flavagline FL3-mediated inhibition of enhanced messenger RNA (mRNA) translation in chronic lymphocytic leukemia (CLL) cells has multiple effects, including blocking proliferation, rewiring MYC-driven metabolism, and controlling CLL growth in a murine model.
CLL is the most frequent leukemia in older people in the Western world. The disease now may often be controlled for years or sometimes may not even require treatment for extended periods. However, CLL still can not be cured, and aggressive forms, such as a transformation into a diffuse large B-cell lymphoma (Richter transformation) or the immunodeficiency caused by CLL, can be rapidly life threatening. Thus, there is a continuing search for cellular vulnerabilities that can be therapeutically exploited. Largeot et al focused on the prior observation that CLL cells have an enhanced protein translation rate compared with normal B cells2 and on indications that inhibiting mRNA translation may be toxic for CLL cells.3 The authors first validated an increased translation rate in human CLL cells and then showed that this is also a feature of murine CLL cells in the TCL1-driven murine CLL model. Triggering B-cell receptor (BCR) or Toll-like receptor pathways further increased translation in CLL cells. A known translation inhibitor and modifier, the synthetic flavagline compound FL3, inhibited translation in CLL cells. FL3 was active at a low concentration, at which most other immune cells were not affected. Thus, CLL cells seem particularly sensitive to translation inhibition, opening a promising therapeutic window. Treatment of activated CLL cells with FL3 modified several pathways, including a reduction of MYC activity. Importantly, FL3 treatment also decreased proliferation of CLL cells, rewired multiple metabolic pathways, and induced apoptosis in these cells. MYC seems to play a major role in the metabolic rewiring, as direct MYC inhibition showed similar effects as FL3 treatment. This may be linked to a positive feedback loop, as strong translation initiation promotes MYC activity, and MYC itself promotes translation.
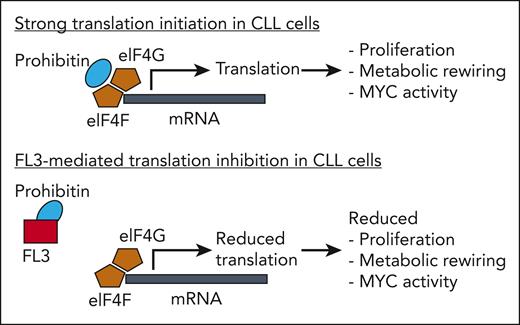
The striking effects of FL3 on CLL cells prompted the authors to investigate its mechanism of action in more detail. An unexpected finding from these studies was that the primary effect of FL3 on its prohibitin targets was not the known impairment of the RAS/RAF pathway. Rather, in CLL cells prohibitins bind directly to the eukaryotic translation initiation factor complex involving eIF4A, eIF4G, and eIF4F, and this binding is inhibited by FL3 (see figure). Thus, a novel mechanism of FL3-mediated inhibition of translation initiation was identified for CLL cells. The important role of prohibitins for translation initiation in CLL cells was confirmed by showing that silencing of prohibitin expression had similar inhibitory effects on translation as FL3 treatment. Finally, in the CLL mouse model, the authors showed that FL3 treatment of transplanted CLL cells slowed CLL growth and prolonged survival of the mice. Notably, FL3 treatment in this model not only inhibited translation in CLL cells (while not affecting normal B cells) but also in regulatory T cells, which also have a relatively high translation rate. As regulatory T cells promote CLL development,4 the additional effect of FL3 on these cells may be of added value for FL3 treatment of CLL.
Prohibitins directly binding to the eukaryotic translation initiation complex (shown here are the factors eIF4G and eIF4F) promote strong translation initiation, which results in CLL cell proliferation, metabolic rewiring, MYC activity, and other effects (not shown). FL3 directly binds to prohibitins, thereby replacing them from the translation initiation factors. As a consequence, translation initiation is reduced, causing reduced proliferation, metabolic rewiring, and MYC activity in CLL cells.
Prohibitins directly binding to the eukaryotic translation initiation complex (shown here are the factors eIF4G and eIF4F) promote strong translation initiation, which results in CLL cell proliferation, metabolic rewiring, MYC activity, and other effects (not shown). FL3 directly binds to prohibitins, thereby replacing them from the translation initiation factors. As a consequence, translation initiation is reduced, causing reduced proliferation, metabolic rewiring, and MYC activity in CLL cells.
The obvious question is whether these in vitro findings are relevant in vivo. Indeed, most CLL proliferation takes place in proliferation centers in lymph nodes, where CLL cells are in contact with T helper cells and presumably receive autoantigen triggering of their often autoreactive BCRs. In these proliferation centers, a fraction of the CLL cells shows MYC expression.5 Hence, FL3 may specifically target these proliferating and metabolically active tumor cells, thereby attacking the root of CLL tumor clone expansion.
Several aspects deserve further considerations and study. First, CLL is presumably derived from mature CD5+ B cells, and MYC expression and activity were reported for a subset of these normal CD5+ B cells.6 Hence, a moderate propensity for increased translation may already be an inherent property of the normal counterparts of CLL cells. Second, CLL is usually preceded by the premalignant condition monoclonal B-cell lymphocytosis, in which expanding B-cell clones already carry genetic lesions typical for CLL cells. It would be interesting to know whether these premalignant cells also already show increased translation initiation and metabolic rewiring. If this is the case, would treatment with a translation initiation inhibitor such as FL3 (assuming it has minimal adverse effects) prevent evolution to CLL? Third, there are 2 forms of CLL, 1 with mutated BCR variable region genes and 1 with unmutated BCR. The latter has an unfavorable clinical behavior, and it shows stronger BCR signaling upon activation and also ZAP70-mediated MYC activation.7,8 Hence, are unmutated CLL cells more vulnerable to translation inhibition than BCR-mutated CLL, so that such a therapy may be useful only for unmutated CLL? Fourth, Richter transformation of CLL, which has a particularly poor prognosis, is often associated with genetic lesions of MYC causing increased MYC activity.9 This points to an even more pronounced translational dysregulation and metabolic rewiring in this transformed form of CLL. Even though translation inhibition with FL3 alone is unlikely to control this aggressive lymphoma, it is certainly worthwhile to study whether Richter transformation is particularly sensitive to inhibition of translation. In that case, this could become a valuable component of a multimodality treatment of Richter transformation.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal