In this issue of Blood, Sharma et al show that hepatic damage in sickle cell disease (SCD) is aggravated by the limited ability of phagocytes to clear apoptotic cells, a process known as efferocytosis, and to resolve inflammation.1 Hemolysis plays a major role by reprogramming macrophages towards highly inflammatory and poorly efferocytic cells through the coordinated suppression of efferocytosis receptors and induction of a metabolic shift towards aerobic glycolysis.
SCD is a debilitating disorder with significant morbidity due to organ damage. The liver is affected by SCD, resulting in the so-called sickle hepatopathy. Sickle hepatopathy encompasses a wide range of acute and chronic liver pathologies, the pathogenesis of which have not been fully characterized. The clinical spectrum of liver disease ranges from mild abnormalities of liver function in patients who are asymptomatic to dramatic acute crises associated with liver failure and eventual cirrhosis. Although hepatic dysfunction affects up to 10% to 40% of patients with SCD, the molecular mechanisms promoting progressive liver injury in SCD remain poorly understood, and therapeutic approaches to prevent it are suboptimal.2 Sharma et al provide novel insight into the cellular and molecular mechanisms underlying liver damage in SCD, showing that hepatic injury is aggravated by defective apoptotic cell clearance by resident and recruited macrophages.
Macrophages are responsible for the removal of senescent and/or damaged erythrocytes and for the clearance of free hemoglobin and heme through receptor-mediated endocytosis of hemoglobin/haptoglobin and heme/hemopexin complexes, respectively.3,4 In SCD, the macrophages are stressed owing to the shortened half-life of red blood cells. Moreover, the macrophages are exposed to excess heme owing to the saturation of both haptoglobin and hemopexin binding capacity. In addition, both hepatic and recruited macrophages actively participate in the resolution of tissue damage by removing dead and dying cells.5
Sharma et al show that efferocytosis is impaired by hemolysis in SCD. Heme scavenging by the heme carrier hemopexin is sufficient to improve apoptotic cell removal by resident and recruited hepatic phagocytes, thus indicating that excess heme actively contributes to impaired efferocytosis. Importantly, heme was found to drive a complex functional reprogramming of macrophages, whereby cell efferocytic and reparative capacities are suppressed in favor of a marked proinflammatory profile. This effect is in line with heme action as a damage-associated molecular pattern (DAMP) and with its ability to promote sterile inflammation through inflammatory cytokine release and impaired resolution of tissue damage.6
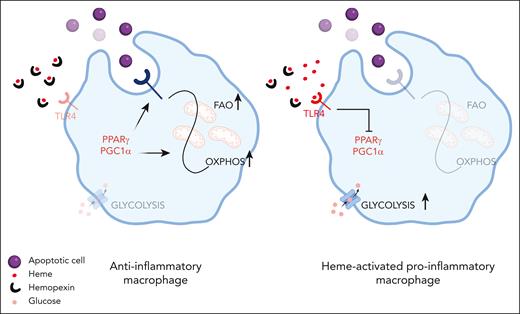
Sharma et al show that heme reprograms macrophages through a coordinated functional and metabolic adaptation achieved via suppression of efferocytosis and mitochondrial remodeling. Normally, in the presence of apoptotic cells, macrophages enhance mitochondrial biogenesis to meet the metabolic needs of efferocytosis and catabolize, via mitochondrial fatty acid β-oxidation and oxidative phosphorylation, the excess membrane lipids of the ingested apoptotic cells. These events are all controlled by the transcription factors PPARγ and PGC1α, key regulators of the expression of efferocytosis receptors and mitochondrial biogenesis.7 Heme-mediated suppression of PPARγ and PGC1α, via Toll-like receptor 4 (TLR4) activation, reduces efferocytosis receptors on the cell plasma membrane and alters mitochondrial dynamics. Thus, heme-induced mitochondrial remodeling acts as an adaptive metabolic response to reduced cell lipid load and apoptotic cell engulfment. As a result of diminished efferocytosis and mitochondrial remodeling, heme-activated macrophages do not increase mitochondrial respiration and adenosine triphosphate production upon apoptotic cell or lipid exposure (see figure).
Heme excess impairs macrophage phagocytic capacity. In anti-inflammatory macrophages, effective efferocytosis is achieved through PPARγ/PCG1α that induce the expression of efferocytic receptors and promote a metabolic rewiring toward oxidative phosphorylation (OXPHOS) sustained by fatty acid oxidation (FAO). In SCD, heme excess, by inhibiting PPARγ/PCG1α, leads to the diminished expression of efferocytic receptors and favors glycolytic metabolism that in turn sustains a proinflammatory phenotype. Both heme scavenging and pharmacological modulation of PPARγ/PCG1α improve efferocytosis in heme overloaded macrophages. Created with BioRender.com.
Heme excess impairs macrophage phagocytic capacity. In anti-inflammatory macrophages, effective efferocytosis is achieved through PPARγ/PCG1α that induce the expression of efferocytic receptors and promote a metabolic rewiring toward oxidative phosphorylation (OXPHOS) sustained by fatty acid oxidation (FAO). In SCD, heme excess, by inhibiting PPARγ/PCG1α, leads to the diminished expression of efferocytic receptors and favors glycolytic metabolism that in turn sustains a proinflammatory phenotype. Both heme scavenging and pharmacological modulation of PPARγ/PCG1α improve efferocytosis in heme overloaded macrophages. Created with BioRender.com.
Whereas anti-inflammatory macrophages rely on fatty acid degradation and metabolic shift to oxidative phosphorylation, proinflammatory macrophages usually adopt a metabolic program fueled by glucose, key to supporting the synthesis of inflammatory mediators.8 Metabolome analysis shows that in SCD, macrophage metabolism is supported by aerobic glycolysis and pentose phosphate pathway shunt with enhanced pyruvate/lactate production. Thus, the metabolic adaptation resulting from heme-driven mitochondria remodeling is part of the sterile inflammation activation program induced by hemolysis in macrophages.
Efferocytosis is a key process for the resolution of tissue damage, through the immunologically silent removal of apoptotic cells, and the resolution of inflammation, through the production of the anti-inflammatory cytokines interleukin-4 (IL-4) and IL-10.9 The study by Sharma et al show that in SCD, heme, by impairing efferocytosis, leads to hepatic accumulation of secondary necrotic cells and prevents macrophage anti-inflammatory rewiring, thus aggravating tissue damage and sustaining chronic inflammation. Furthermore, efferocytosis failure leaves tissues exposed to the inflammatory and immunogenic contents of dying secondary necrotic cells and excessive leukocyte influx, with potentially increased risk of the development of autoimmunity. Indeed, heme excess in presence of hepatic damage triggers the production of antinuclear autoantibodies, a manifestation of autoimmunity. Autoimmunity and autoimmune liver disease are not uncommon in SCD, although diagnosis is often complicated by the overlapping manifestations between SCD and autoimmune diseases. Thus, the prevalence of autoimmune disease is likely underestimated. Complement abnormalities, chronic inflammation, and increased frequency of infections likely predispose patients with SCD to autoimmune disease.10 This study provides a novel mechanistic explanation of the increasing number of cases of autoimmunity among patients with SCD, identifying heme-driven defective efferocytosis as an additional driver of autoantibody production.
Ultimately, Sharma et al show that macrophage metabolic rewiring achieved through the modulation of the PPARγ/PGC1α pathway results in cell functional reprogramming and provides therapeutic benefit in SCD, ameliorating inflammation and hepatic damage.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal