In this issue of Blood, Andersen et al1 present an elegant structural characterization of the 32-kDa protein lufaxin present in the saliva of the blood-feeding sand fly Lutzomyia longipalpis. They demonstrate that lufaxin can assemble a giant complex containing both a complement proconvertase and the coagulation factor fXa in which activation of the complement C3 proconvertase is prevented and the proteolytic activity of fXa is inhibited.
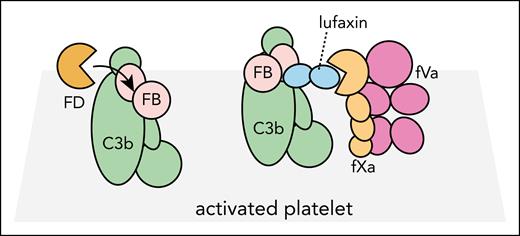
Pattern recognition induces complement activation that triggers cleavage of complement C3. The product C3b is covalently deposited on the surface, thus providing the complement-activating surface.2 C3b associates with factor B (FB), resulting in the alternative pathway (AP) C3 convertase C3bBb that cleaves additional C3. The AP C3 convertase is formed in 2 steps. First, FB binds to C3b to form the proconvertase C3bB. Next, the factor D (FD) cleaves C3b-bound FB that can adopt 2 conformations called open and closed. Only the open FB conformation is a substrate for FD.3 The coagulation cascade may initiate through either the extrinsic or the intrinsic pathways that converge at the assembly of the prothrombinase complex fVa-fXa, catalyzing thrombin formation from prothrombin on activated platelets.2
The physiological relevance of the study conducted by Andersen et al is that there is substantial cross talk between the complement and coagulation cascades, which centers around platelet activation.2,4 The ability of lufaxin to inhibit both the C3 convertase and the prothrombinase individually was already known.5,6 The major novelty is, therefore, that Andersen et al combine information from a crystal structure and 2 cryogenic electron microscopy (cryo-EM) structures to obtain a detailed structure-based model for the dual inhibition activity of lufaxin. Using a cryo-EM structure of the C3bB-lufaxin complex, they show that the N-terminal domain of lufaxin bridges the 2 convertase subunits by contacting 2 domains in FB and 1 domain in C3b. This interaction locks FB in the closed conformation, because lufaxin would severely overlap with the serine protease (SP) domain of FB if the complement factor adopted its open conformation, which is susceptible to FD cleavage. Trapping proconvertase FB in the closed conformation represents a novel mechanism of a complement inhibitor produced by a pathogen.
To address in detail the mechanism whereby lufaxin inhibits coagulation, Andersen et al also determined a cryo-EM structure of the C3bB-lufaxin-fXa complex. This structure reveals that the C-terminal domain of lufaxin interacts tightly with fXa independently of the N-terminal domain and its interaction with the proconvertase. An arginine side chain close to the C-terminus of lufaxin inserts into the S1 specificity pocket of fXa, and a short lufaxin peptide C-terminally of the arginine has been released by proteolysis. The result is a stable lufaxin proteolysis product bound tightly to fXa that renders the protease unable to bind its prothrombin substrate.
In vivo experiments with mice had already confirmed the anti-coagulation activity of lufaxin.5 But, although Andersen et al conclusively show that lufaxin can engage simultaneously with the proconvertase and fXa in vitro, they do not explicitly address whether lufaxin can bridge the complement proconvertase and the coagulation prothrombinase fVa-fXa on platelets. However, a model that combines structures of the complexes C3bB-lufaxin-fXa and fVa-fXa7 suggests that this is feasible.1 Whether such a bridging function of lufaxin is relevant for platelet-bound prothrominase and proconvertase in vivo remains to be determined. If such bridging occurs between 2 platelet-bound complexes (see figure), avidity effects will most likely produce an affinity that is significantly higher than the monovalent lufaxin-proconvertase and lufaxin-fXa affinities, the latter already with a dissociation constant of 3 nM.5 Lufaxin interaction with the proconvertase is independent of the positive regulator properdin,8 but a careful analysis of the interplay with properdin is also required to obtain the full picture of lufaxin function because properdin is likely to be bound to platelet-bound proconvertase.
Lufaxin may interact simultaneously with proconvertase and prothrombinase. On an activated platelet, the open conformation of the proconvertase C3bB will normally be activated by fluid-phase FD. In the presence of lufaxin, the proconvertase is locked in the closed conformation and FB cannot be cleaved by FD. At the same time, lufaxin possibly also bridges the proconvertase with the prothrombinase fVa-fXa, and the latter is unable to cleave prothrombin because lufaxin blocks the active site of fXa.
Lufaxin may interact simultaneously with proconvertase and prothrombinase. On an activated platelet, the open conformation of the proconvertase C3bB will normally be activated by fluid-phase FD. In the presence of lufaxin, the proconvertase is locked in the closed conformation and FB cannot be cleaved by FD. At the same time, lufaxin possibly also bridges the proconvertase with the prothrombinase fVa-fXa, and the latter is unable to cleave prothrombin because lufaxin blocks the active site of fXa.
From a wider perspective, an intriguing aspect of the structural analysis presented by Andersen et al is how efficiently lufaxin uses its 2 domains to neutralize 2 distinct host complexes in an independent manner. A similar modular function of a small 2-domain complement inhibitor was also reported for the pathogen complement evasion protein Staphylococcus aureus protein SSL7 that interacts simultaneously with IgA and complement C5.9 It is also exciting that the more simple interaction between the C-terminal domain of lufaxin and the SP domain was modeled successfully by Alphafold2,10 although with some differences to the experimental structure. This raises the perspective that for other pathogen proteins interfering with host immune system proteins, it may already be possible to predict the intermolecular contacts reliably. From a translational perspective, this functional modularity is also interesting. It suggests that it may be possible to manipulate the 2 activities independently in a program aiming at developing a lufaxin-based reagent for therapeutic intervention in complementopathies accompanied by microvascular thrombosis. Another obvious perspective is that lufaxin may serve as inspiration for development of convertase-fXa linking bifunctional antibodies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal