Key Points
Treatment with abatacept in steroid-refractory cGVHD was associated with a 58% ORR with all responders achieving a PR.
Treatment with abatacept was well tolerated and led to a durable reduction in prednisone dose.
Abstract
Steroid-refractory chronic graft-versus-host disease (cGVHD) after allogeneic transplant remains a significant cause of morbidity and mortality. Abatacept is a selective costimulation modulator, used for the treatment of rheumatologic diseases, and was recently the first drug to be approved by the US Food and Drug Administration for the prophylaxis of acute graft-versus-host disease. We conducted a phase 2 study to evaluate the efficacy of abatacept in steroid-refractory cGVHD. The overall response rate was 58%, seen in 21 out of 36 patients, with all responders achieving a partial response. Abatacept was well tolerated with few serious infectious complications. Immune correlative studies showed a decrease in interleukin -1α (IL-1α), IL-21, and tumor necrosis factor α as well as decreased programmed cell death protein 1 expression by CD4+ T cells in all patients after treatment with abatacept, demonstrating the effect of this drug on the immune microenvironment. The results demonstrate that abatacept is a promising therapeutic strategy for the treatment of cGVHD. This trial was registered at www.clinicaltrials.gov as #NCT01954979.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 3008.
Disclosures
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning Objectives
Upon completion of this activity, participants will:
Assess the efficacy of and clinical response to abatacept in patients with steroid-refractory chronic graft-versus-host disease, based on a phase 2 study
Evaluate the safety, tolerability, and immune effects of abatacept in patients with steroid-refractory chronic graft-versus-host disease, based on a phase 2 study with immune correlation
Determine the clinical implications of the efficacy, safety, and tolerability of abatacept in patients with steroid-refractory chronic graft-versus-host disease, based on a phase 2 study
Release date June 15, 2023; Expiration date: June 15, 2024
Introduction
Chronic graft-versus-host disease (cGVHD) after allogeneic transplant remains a leading cause of morbidity, with a cumulative incidence up to 50%.1 Transplant recipients with cGVHD have decreased quality of life, indicating the substantial burden of this phenomenon despite the curative intent of allogeneic transplant.2 The rates of cGVHD indicate a need for effective and long-lasting therapies for this disease.3 Although systemic corticosteroids are considered as first-line therapy, their use is often associated with incomplete response and significant toxicity. Of patients with cGVHD, <20% maintain a partial response (PR) or complete response (CR) and survive 1 year after initial therapy without additional systemic therapy.4 Currently, there are 3 US Food and Drug Administration (FDA)-approved treatments for cGVHD after failure of prior lines of therapy, namely, ibrutinib, belumosudil, and ruxolitinib, with overall response rates (ORR) of 67%, 73%, and 76%, respectively.5-7 These drugs have meaningfully affected the treatment of steroid-refractory cGVHD and have improved outcomes for patients. Nonetheless, many patients do not respond to currently available treatments, and for the majority of patients, a PR is achieved, demonstrating the need for novel approaches to treating cGVHD. We hypothesized that immunomodulation through costimulatory blockade has the potential to block T-cell activation and mitigate clinical manifestations of cGVHD.
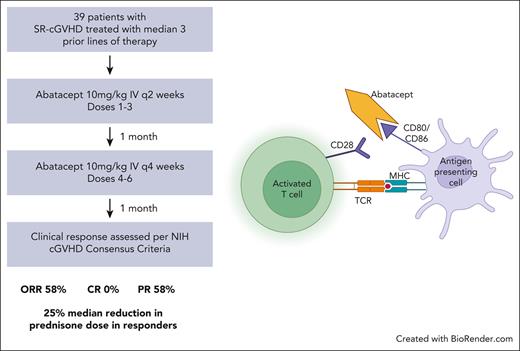
Abatacept is the first of a class of agents called selective costimulation modulators that is used for the treatment of rheumatologic diseases, such as rheumatoid arthritis.8,9 In December 2021, abatacept in combination with a calcineurin inhibitor and methotrexate was approved by the FDA for the prophylaxis of acute GVHD among patients undergoing allogeneic transplant from a matched or 1-allele mismatched unrelated donor.10 Abatacept is a recombinant fusion protein comprised of the extracellular domain of human cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) linked to a fragment of the Fc portion of human immunoglobulin G1 that has been modified to prevent complement fixation and antibody-dependent cellular cytotoxicity.11 Abatacept competes with CD28 on T cells to bind specifically to CD80 and CD86 on antigen-presenting cells, attenuating CD28-mediated T-cell activation. In a murine model, administration of CTLA4Ig was shown to prevent both acute GVHD and cGVHD as well as reverse manifestations of cGVHD.12 Thus, immunomodulation with abatacept may be an innovative and promising therapeutic strategy for the treatment of cGVHD.
We previously reported results from a phase 1 clinical trial that evaluated the safety and clinical efficacy of abatacept among patients with steroid-refractory cGVHD. The study demonstrated safety of the drug and promising clinical results (#NCT01954979).13 Here we report results from the phase 2 study performed to evaluate the overall clinical response rate of abatacept among patients with steroid-refractory cGVHD.
Methods
Study design
This phase 2 trial sought to investigate the clinical efficacy and tolerability of abatacept (Bristol-Myers Squibb, New York, NY) for the treatment of patients with steroid-refractory cGVHD while also studying the effects of the drug on the immune microenvironment. The trial protocol was reviewed and approved by the Dana-Farber Harvard Cancer Center. Informed consent was obtained per the Declaration of Helsinki. A total of 39 patients were treated with abatacept at a dose of 10 mg/kg administered IV for a total of 6 doses. Doses from 1 to 3 were administered every 2 weeks. One month after administration of dose 3, abatacept doses from 4 to 6 were administered every 4 weeks. Patients were examined every 2 weeks after each dose of abatacept and then monthly for 6 months after the last dose of therapy. Patients who completed 6 doses of abatacept and 1 to 3 months of follow-up were eligible to receive extended-duration therapy with monthly doses of abatacept at a dose of 10 mg/kg for up to a total of 12 additional doses. Peripheral blood was drawn before each dose of abatacept and 1 month after completion of therapy to assess the effect of treatment on circulating T cells. The primary end point in the study was the ORR (CR or PR) of abatacept in treating steroid-refractory cGVHD after receiving 6 doses of treatment. Given that the response rate in this patient population was estimated to be between 20% and 30%, for the purpose of statistical design, the null hypothesis was that the response rate would be 25%, and the alternative hypothesis was that the response rate would be 45%.
Eligibility criteria
The participants of this study had progressive steroid-refractory cGVHD despite their current immunosuppressive regimen. Inclusion criteria included recipients of allogeneic bone marrow or stem cell transplant with myeloablative or reduced intensity conditioning, with cGVHD defined by the National Institutes of Health (NIH) consensus criteria. Patient eligibility for the study required steroid-refractory cGVHD, defined as having persistent signs and symptoms of cGVHD despite the use of prednisone at ≥0.25 mg/kg per day for at least 4 weeks without complete resolution of signs and symptoms. Patients were excluded if they had active infection, active cardiac disease, or active malignancy. They could have received neither biologic antibody therapy for cGVHD with rituximab, alemtuzumab, or antithymocyte globulin within 3 months of starting treatment with abatacept nor other experimental drugs within 28 days of starting treatment with abatacept. Patients were also excluded if their ongoing prednisone requirement was >1 mg/kg per day. Participants were allowed to continue taking steroids and any immunosuppressants that they were taking before starting treatment with abatacept. Patients discontinued participation in the study if the addition of new immune suppression was required.
Clinical cGVHD assessment
Patients’ cGVHD was scored per the 2005 NIH consensus development conference on criteria for clinical trials in cGVHD.14 Organ sites considered for scoring included the skin, mouth, eyes, gastrointestinal (GI) tract, liver, lungs, joints and fascia, and the genital tract. Each organ or site was scored based on a 4-point scale (0-3), with 0 representing no involvement, and 3 reflecting severe impairment. Response of cGVHD to abatacept was classified based on the standardized cGVHD assessment per 2014 NIH guidelines.15 CR was defined as resolution of all manifestations of cGVHD in each organ without progression in any other organ, and PR was defined as improvement in at least 1 organ without progression in any other organ. The skin, mouth, GI tract, liver, lung, eye, and joints were considered in evaluation of overall response. Notably, lung cGVHD was scored per the NIH lung symptom score for all patients and also included pulmonary function tests (PFTs) for 11 patients in whom pretreatment and posttreatment PFTs were performed.
Immune correlative studies
Peripheral blood samples were collected from patients before treatment with abatacept and 1 month after receiving 6 doses of the drug. T-cell activation by CD4 and CD8 was evaluated by incubating cells with anti-CD4 phycoerythrin Cy5 (PE-Cy5; clone RPA-T2, BD Pharmingen), anti-CD8 fluorescein isothiocyanate (FITC) (clone HIT8a, BD Pharmingen), anti-CD25 allophycocyanin (APC)-Cy7 (clone M-A2451, BD BioSciences), and anti-PD-1 brilliant violet (clone EH12.1, Fisher Scientific). Appropriate corresponding isotype controls were used. Using multichannel flow analysis, the T cells were selected based on size, and the percentage of CD4+ and CD8+ cells expressing programmed cell death protein 1 (PD-1) and that of CD4+/CD25+/CD69+ T cells were measured and analyzed using Kaluza software (Beckman Coulter, Brea, CA). To evaluate the presence of regulatory T cells, the cells were incubated with PE-conjugated anti-IL10 (clone JES3-9D7, BD Pharmingen), anti-CD4 PE-Cy5 (clone RPA-T2, BD Pharmingen), or anti-CD8 FITC (clone HIT8a, BD Pharmingen). Cells were analyzed via multichannel flow cytometry and the Kaluza software. Interferon gamma (IFN-γ) was measured to assess T- helper 1/T-helper 2 polarization. Peripheral blood mononuclear cells were pulsed with GolgiStop (1 mg/mL; Pharmingen, San Diego, CA) for 4 hours at 37°C. Cells were harvested and labeled with CD4-PE-Cy7 and CD8-FITC, as described earlier. Cells were permeabilized with Cyto-fix/Cytoperm (BD Pharmingen), consisting of formaldehyde and saponin, for 20 minutes at 4°C in the dark. Cells were then washed twice in Perm/Wash solution (BD Pharmingen), and incubated with PE-conjugated IFN-γ (clone B27, Invitrogen) for 30 minutes at 4°C in the dark. Cells in the control sample were incubated with PE mouse antihuman immunoglobulin G isotype control (clone G18-145). Cells were washed in 1× Perm/Wash solution before flow cytometric analysis.
To assess for changes in the cytokine profile, multiplex immunoassay was performed using patient plasma at baseline and 1 month after 6 doses of abatacept, using the ProcartaPlex Immunoassay (Invitrogen, Waltham, MA) per the manufacturer’s instructions. Antibody-coated beads targeting a panel of 16 cytokines (interleukin 1α [IL-1α], IL-2, IL-2R, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17A, IL-21, IL-27, granulocyte-macrophage colony–stimulating factor, macrophage colony–stimulating factor, tumor necrosis factor α [TNF-α], IFN-γ, and B-cell activating factor [BAFF]) and embedded with fluorophore ratios unique to each analyte were incubated with patient sera in duplicates. Fluorophores on each bead were activated using streptavidin-PE and subsequently read using the LuminexMAGPIX system to quantify cytokine concentrations. A standard curve was generated using standard concentrations of each analyte to determine the concentrations of each analyte in the patient plasma.
Statistical analysis
Baseline characteristics were reported descriptively. Univariable logistic regression analysis was performed to investigate clinical factors that were associated with response. Factors considered in logistic regression analysis included age at enrollment, time from transplant to study entry, number of prior therapies, number of sites involved, NIH cGVHD severity score at study enrollment, recipient sex, Eastern Cooperative Oncology Group performance status, HLA typing, primary disease, and conditioning intensity (Table 1). Graft source was not considered because almost all patients had undergone peripheral blood stem cell transplant. Overall survival (OS) was defined as the time from the date of study treatment initiation to death from any cause. Evaluation of patients who were alive was censored at the date they were last known to be alive. Failure-free survival (FFS) was defined as the time from the date of study treatment initiation until the date of documented cGVHD progression, underlying disease relapse, or death from any cause, whichever occurred first. Evaluation of patients who were alive and event-free was censored at the date they were last known to be alive and event-free. The Kaplan-Meier method was used to estimate OS and FFS. Immunologic and cytokine parameters were analyzed primarily descriptively and compared using the Wilcoxon rank-sum test for group comparison and Wilcoxon signed-rank test for paired comparison. Principal component analysis (PCA) was also performed to reduce dimension for highly correlated cytokine parameters. Absolute values were log-transformed before performing PCA. All testing was two-sided, at the significance level of P = .05. Multiple comparisons were not considered. All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC), and R version 3.4 (the CRAN project).
Baseline patient characteristics
| Characteristic . | Number of patients, n = 39 (%) . |
|---|---|
| Age (y), median (range) | 62 (25-77) |
| Female | 21 (53.8) |
| Median time from HCT to study entry (range), mo | 43 (6-173) |
| ECOG performance status | |
| 0 | 1 (2.6) |
| 1 | 27 (69.2) |
| 2 | 11 (28.2) |
| Indication for transplant | |
| AML | 18 (46.2) |
| MDS | 8 (20.5) |
| ALL | 5 (12.8) |
| CML | 2 (5.1) |
| MPD | 2 (5.1) |
| MPN with fibrosis | 2 (5.1) |
| Myelofibrosis | 1 (2.6) |
| NHL | 1 (2.6) |
| Stem cell source | |
| Bone marrow | 4 (10.3) |
| Peripheral blood stem cell | 35 (89.7) |
| Conditioning intensity | |
| Myeloablative | 24 (61.5) |
| Nonmyeloablative | 14 (35.9) |
| Unknown | 1 (2.6) |
| HLA matching (A, B, C, and DRB1) | |
| Matched related | 15 (38.4) |
| Matched unrelated | 22 (56.4) |
| Mismatched related | 1 (2.6) |
| Mismatched unrelated | 1 (2.6) |
| Baseline NIH cGVHD severity score | |
| Mild | 0 (0) |
| Moderate | 18 (46.2) |
| Severe | 21 (53.8) |
| Organs involved | |
| Number of organs involved, median (range) | 3 (2-7) |
| ≥4 organs involved | 19 (48.7) |
| Skin | 33 (84.6) |
| Mouth | 17 (43.5) |
| Eyes | 28 (71.7) |
| GI | 6 (15.3) |
| Liver | 9 (23.1) |
| Lung | 22 (56.4) |
| Joints | 32 (82.1) |
| Prior systemic therapy for cGVHD | |
| Prior lines of therapy, median (range) | 3 (1-8) |
| Corticosteroid (prednisone, methylprednisolone) | 39 (100) |
| Tacrolimus | 24 (61.5) |
| Mycophenolate mofetil | 15 (38.5) |
| Sirolimus | 11 (28.2) |
| Cyclosporine | 2 (5.1) |
| Rituximab | 10 (25.6) |
| Ruxolitinib | 7 (17.9) |
| Ibrutinib | 4 (10.3) |
| Aldesleukin | 7 (17.9) |
| Characteristic . | Number of patients, n = 39 (%) . |
|---|---|
| Age (y), median (range) | 62 (25-77) |
| Female | 21 (53.8) |
| Median time from HCT to study entry (range), mo | 43 (6-173) |
| ECOG performance status | |
| 0 | 1 (2.6) |
| 1 | 27 (69.2) |
| 2 | 11 (28.2) |
| Indication for transplant | |
| AML | 18 (46.2) |
| MDS | 8 (20.5) |
| ALL | 5 (12.8) |
| CML | 2 (5.1) |
| MPD | 2 (5.1) |
| MPN with fibrosis | 2 (5.1) |
| Myelofibrosis | 1 (2.6) |
| NHL | 1 (2.6) |
| Stem cell source | |
| Bone marrow | 4 (10.3) |
| Peripheral blood stem cell | 35 (89.7) |
| Conditioning intensity | |
| Myeloablative | 24 (61.5) |
| Nonmyeloablative | 14 (35.9) |
| Unknown | 1 (2.6) |
| HLA matching (A, B, C, and DRB1) | |
| Matched related | 15 (38.4) |
| Matched unrelated | 22 (56.4) |
| Mismatched related | 1 (2.6) |
| Mismatched unrelated | 1 (2.6) |
| Baseline NIH cGVHD severity score | |
| Mild | 0 (0) |
| Moderate | 18 (46.2) |
| Severe | 21 (53.8) |
| Organs involved | |
| Number of organs involved, median (range) | 3 (2-7) |
| ≥4 organs involved | 19 (48.7) |
| Skin | 33 (84.6) |
| Mouth | 17 (43.5) |
| Eyes | 28 (71.7) |
| GI | 6 (15.3) |
| Liver | 9 (23.1) |
| Lung | 22 (56.4) |
| Joints | 32 (82.1) |
| Prior systemic therapy for cGVHD | |
| Prior lines of therapy, median (range) | 3 (1-8) |
| Corticosteroid (prednisone, methylprednisolone) | 39 (100) |
| Tacrolimus | 24 (61.5) |
| Mycophenolate mofetil | 15 (38.5) |
| Sirolimus | 11 (28.2) |
| Cyclosporine | 2 (5.1) |
| Rituximab | 10 (25.6) |
| Ruxolitinib | 7 (17.9) |
| Ibrutinib | 4 (10.3) |
| Aldesleukin | 7 (17.9) |
Baseline patient characteristics of the 39 patients enrolled in the trial including key transplant, cGVHD, and immunosuppressant management features.
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; ECOG, Eastern Cooperative Oncology Group; HCT, hematopoietic cell transplant; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorder; MPN, myeloproliferative neoplasm; NHL, non-Hodgkin lymphoma.
Results
Baseline characteristics and cGVHD scoring
Table 1 shows the baseline characteristics of the 39 subjects enrolled in the phase 2 trial including their baseline cGVHD score. The average age of patients was 62 years (range, 25-77 years), and 54% of the patients were female. The majority of patients (97.4%) had an Eastern Cooperative Oncology Group performance status of 1 or 2. Primary disease was acute myeloid leukemia among 46% of patients, myelodysplastic syndrome among 20% of patients, and acute lymphoblastic leukemia, chronic myeloid leukemia, non-Hodgkin lymphoma, myeloproliferative neoplasm, and myelofibrosis among smaller proportions of patients. Notable transplant characteristics included that the stem cell source was peripheral blood stem cells for 90% of patients and bone marrow for 10% of patients. In addition, 62% of patients had undergone myeloablative conditioning, whereas 36% had undergone nonmyeloablative conditioning. A matched unrelated donor was used for 56% patients, and a matched related donor for 38% of patients. At baseline, 18 patients had moderate cGVHD, and 21 patients had severe cGVHD per the 2014 NIH consensus criteria, with 19 patients (48.7%) having at least 4 organs involved. Baseline cGVHD assessment of the 39 evaluable patients showed involvement of the skin in 85% (n = 33), mouth in 44% (n = 17), eyes in 72% (n = 28), GI tract in 15% (n = 6), liver in 23% (n = 9), lung in 56% (n = 22), and joints in 82% (n = 32). In the 23 patients for whom descriptive data regarding skin cGVHD findings were available, 16 patients had sclerotic features, 10 had maculopapular rash/erythema, 8 had lichen planus–like features, 6 had papulosquamous lesions or ichthyosis, and 2 had keratosis pilaris–like cGVHD. The median number of prior lines of systemic therapy for cGVHD was 3, with a range from 1 to 8. At baseline, before receiving abatacept, patients were on a median of 2 other systemic immunosuppressive agents; these included corticosteroids among 97% of patients (n = 38), a calcineurin inhibitor among 44% (n = 17), mycophenolate mofetil among 18% (n = 7), ruxolitinib among 10% (n = 4), and aldesleukin among 3% (n = 1). No participant had received ibrutinib or belumosudil. Ongoing systemic immunosuppressants for cGVHD were prednisone (n = 38), tacrolimus (n = 12), mycophenolate (n = 7), sirolimus (n = 6), cyclosporine (n = 1), aldesleukin (n = 1), and ruxolitinib (n = 4).
Clinical response
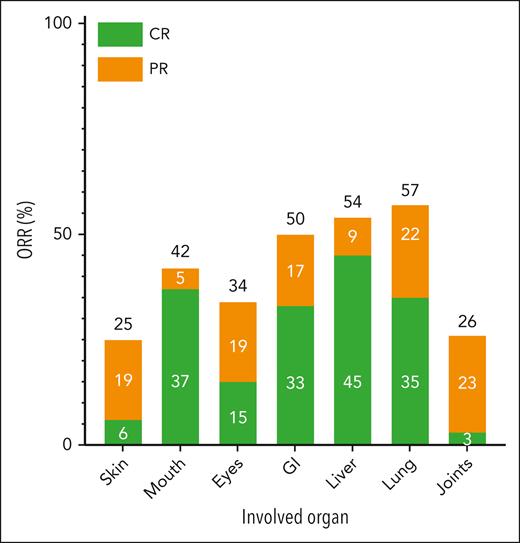
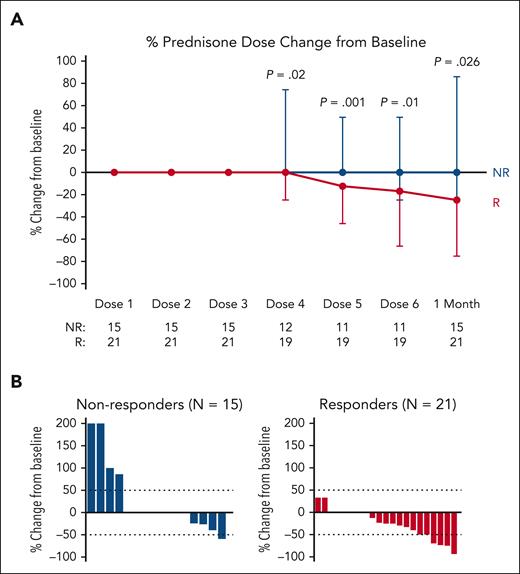
In total, 36 patients were included in the analysis of cGVHD response at 1 month after 6 doses of abatacept. Of the 3 patients who could not be analyzed for cGVHD response at 1 month after 6 doses of abatacept, 1 patient was lost to follow-up, 1 patient was admitted to the hospital because of an unrelated adverse event at the 1-month follow-up time point, and 1 patient died before the 1-month follow-up because of infection. Of the 36 evaluable patients, 58% (n = 21), achieved an overall PR. The sites with greatest improvement (either CR or PR) in all-comers included lung (57%), liver (54%), GI tract (50%), and mouth (42%). In the 13 patients with a partial lung response, 8 patients had an improvement in the NIH lung symptom score from 1 to 0, 4 patients had an improvement in the score from 2 to 1, and 1 patient had an improvement in the score from 3 to 2. In the 11 patients with complete PFT data (included in supplemental Table 1, which is available on the Blood website), there was no change in the pretreatment and posttreatment PFTs; of these 11 patients, 5 patients had a complete lung response, 3 patients had a partial lung response, and 3 patients had no change per their NIH lung symptom scores. Progression of cGVHD was noted in 33% (n = 12) of patients, with sites of involvement including skin (6%), mouth (26%), eyes (4%), liver (9%), lung (13%), and joints (7%). Table 2 shows the site-specific baseline and final cGVHD disease scores in the 36 evaluable patients. The organ-specific response rates are shown in Figure 1. To investigate potential factors that are associated with the overall response, univariable logistic regression analysis was performed, and none of the factors were found to be associated with response. Treatment with abatacept also led to a durable reduction in prednisone dose as shown in Figure 2A in responders compared with nonresponders as early as after 3 doses of abatacept and continuing until the 1-month follow-up after receiving 6 doses of abatacept. The waterfall plot in Figure 2B shows, in greater detail, the percent change from baseline in prednisone dose in responders and nonresponders, with the majority of responders having a reduction in prednisone dose.
Site-specific baseline and final cGVHD scores based on the organ system
| Patient . | Time point . | Skin . | Mouth . | Eyes . | GI tract . | Liver . | Lung . | Joints . | Genital . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Baseline | 2 | 0 | 1 | 0 | 0 | 1 | 2 | 0 |
| Final | 2 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | |
| 2∗ | Baseline | 2 | 1 | 1 | 3† | 1‡ | 2† | 2 | 0 |
| Final | 2 | 1 | 1 | 2† | 0‡ | 1† | 2 | 0 | |
| 3 | Baseline | 3 | 0§ | 2 | 2‡ | 1‡ | 1‡ | 2 | 0 |
| Final | 3 | 1§ | 2 | 0‡ | 0‡ | 0‡ | 2 | 0 | |
| 4∗ | Baseline | 2† | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
| Final | 1† | 0 | 1 | 0 | 0 | 1 | 2 | 0 | |
| 5∗ | Baseline | 2 | 1‡ | 2 | 1 | 1‡ | 1‡ | 2† | 0 |
| Final | 2 | 0‡ | 2 | 1 | 0‡ | 0‡ | 1† | 0 | |
| 6 | Baseline | 3 | 0§ | 0 | 0 | 0 | 0 | 2 | 0 |
| Final | 3 | 1§ | 0 | 0 | 0 | 0 | 2 | 0 | |
| 7 | Baseline | 2 | 1 | 2 | 0 | 0 | 1 | 2 | 0 |
| Final | 2 | 1 | 2 | 0 | 0 | 1 | 2 | 0 | |
| 8 | Baseline | 0 | 1 | 2 | 0 | 0 | 1§ | 1 | 0 |
| Final | 0 | 1 | 2 | 0 | 0 | 2§ | 1 | 0 | |
| 9∗ | Baseline | 2 | 0 | 0 | 0 | 1‡ | 1‡ | 2† | 0 |
| Final | 2 | 0 | 0 | 0 | 0‡ | 0‡ | 1† | 0 | |
| 10∗ | Baseline | 0 | 1 | 3† | 0 | 3† | 0 | 2† | 0 |
| Final | 1 | 1 | 2† | 0 | 1† | 0 | 1† | 0 | |
| 11 | Baseline | 2§ | 2† | 1 | 0 | 0 | 0 | 0§ | 0 |
| Final | 3§ | 1† | 1 | 0 | 0 | 1 | 1§ | 0 | |
| 12 | Baseline | 3 | 1§ | 3 | 1‡ | 0 | 2 | 3† | 0 |
| Final | 3 | 2§ | 3 | 0‡ | 0 | 2 | 2† | 0 | |
| 13∗ | Baseline | 1‡ | 1‡ | 0 | 0 | 0 | 0 | 2† | 2 |
| Final | 0‡ | 0‡ | 0 | 0 | 0 | 0 | 1† | 2 | |
| 14∗ | Baseline | 2† | 0 | 2† | 0 | 0 | 0 | 0 | 0 |
| Final | 1† | 0 | 1† | 0 | 0 | 0 | 0 | 0 | |
| 15∗ | Baseline | 2 | 0 | 1 | 0 | 0 | 0 | 2† | 0 |
| Final | 2 | 0 | 1 | 0 | 0 | 0 | 1† | 0 | |
| 16∗ | Baseline | 2 | 1‡ | 3† | 0 | 1 | 0 | 2 | 0 |
| Final | 2 | 0‡ | 2† | 0 | 1 | 0 | 2 | 1 | |
| 17∗ | Baseline | 3 | 0 | 2 | 0 | 0 | 3† | 2 | 0 |
| Final | 3 | 0 | 2 | 0 | 1 | 2† | 2 | 0 | |
| 18∗ | Baseline | 0 | 1‡ | 1‡ | 0 | 0 | 0 | 1 | 1 |
| Final | 0 | 0‡ | 0‡ | 0 | 0 | 0 | 1 | 0 | |
| 19∗ | Baseline | 0 | 2‡ | 2† | 0 | 0 | 0 | 0 | 0 |
| Final | 0 | 0‡ | 1† | 0 | 0 | 0 | 0 | 0 | |
| 20∗ | Baseline | 3† | 0 | 0 | 0 | 0 | 1‡ | 2 | 0 |
| Final | 2† | 0 | 0 | 0 | 0 | 0‡ | 2 | 0 | |
| 21 | Baseline | 2† | 0 | 0 | 0 | 0 | 1§ | 2 | 0 |
| Final | 1† | 0 | 0 | 0 | 1 | 2§ | 2 | 0 | |
| 22 | Baseline | 1 | 0§ | 2† | 0 | 0 | 2† | 0 | 0 |
| Final | 1 | 1§ | 1† | 0 | 1 | 1† | 0 | 0 | |
| 23 | Baseline | 2 | 0§ | 1§ | 0 | 0 | 1‡ | 1 | 1 |
| Final | 2 | 1§ | 3§ | 0 | 0 | 0‡ | 1 | 1 | |
| 24∗ | Baseline | 0 | 1‡ | 2‡ | 0 | 0 | 2† | 0 | 0 |
| Final | 0 | 0‡ | 0‡ | 0 | 0 | 1† | 0 | 0 | |
| 25∗ | Baseline | 2† | 0 | 0 | 0 | 1‡ | 0 | 1‡ | 0 |
| Final | 1† | 0 | 0 | 0 | 0‡ | 0 | 0‡ | 0 | |
| 26∗ | Baseline | 2 | 1 | 3 | 0 | 0 | 1‡ | 1 | 0 |
| Final | 2 | 1 | 3 | 0 | 0 | 0‡ | 1 | 0 | |
| 27∗ | Baseline | 3 | 0 | 1‡ | 0 | 0 | 0 | 2 | 0 |
| Final | 3 | 0 | 0‡ | 0 | 0 | 0 | 2 | 0 | |
| 28 | Baseline | 1‡ | 0 | 0 | 0 | 2§ | 0 | 0 | 0 |
| Final | 0‡ | 0 | 0 | 0 | 3§ | 0 | 0 | 0 | |
| 29 | Baseline | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Final | 3 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | |
| 30 | Baseline | 3 | 1 | 1 | 0 | 0 | 1‡ | 2§ | 0 |
| Final | 3 | 1 | 1 | 0 | 0 | 0‡ | 3§ | 0 | |
| 31∗ | Baseline | 3 | 1‡ | 0 | 0 | 0 | 0 | 2 | 0 |
| Final | 3 | 0‡ | 0 | 0 | 0 | 0 | 2 | 0 | |
| 32∗ | Baseline | 2† | 0 | 0 | 0 | 0 | 3 | 2† | 0 |
| Final | 1† | 0 | 1 | 0 | 0 | 3 | 1† | 0 | |
| 33∗ | Baseline | 0 | 0 | 2 | 1 | 0 | 2† | 3 | 0 |
| Final | 0 | 0 | 2 | 1 | 0 | 1† | 3 | 0 | |
| 34∗ | Baseline | 2 | 0 | 1‡ | 0 | 0 | 1‡ | 2 | 0 |
| Final | 2 | 0 | 0‡ | 0 | 0 | 0‡ | 2 | 0 | |
| 35 | Baseline | 2§ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Final | 3§ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 36 | Baseline | 3 | 0 | 1 | 1 | 0 | 1§ | 2 | 0 |
| Final | 3 | 0 | 1 | 1 | 0 | 2§ | 2 | 0 |
| Patient . | Time point . | Skin . | Mouth . | Eyes . | GI tract . | Liver . | Lung . | Joints . | Genital . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Baseline | 2 | 0 | 1 | 0 | 0 | 1 | 2 | 0 |
| Final | 2 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | |
| 2∗ | Baseline | 2 | 1 | 1 | 3† | 1‡ | 2† | 2 | 0 |
| Final | 2 | 1 | 1 | 2† | 0‡ | 1† | 2 | 0 | |
| 3 | Baseline | 3 | 0§ | 2 | 2‡ | 1‡ | 1‡ | 2 | 0 |
| Final | 3 | 1§ | 2 | 0‡ | 0‡ | 0‡ | 2 | 0 | |
| 4∗ | Baseline | 2† | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
| Final | 1† | 0 | 1 | 0 | 0 | 1 | 2 | 0 | |
| 5∗ | Baseline | 2 | 1‡ | 2 | 1 | 1‡ | 1‡ | 2† | 0 |
| Final | 2 | 0‡ | 2 | 1 | 0‡ | 0‡ | 1† | 0 | |
| 6 | Baseline | 3 | 0§ | 0 | 0 | 0 | 0 | 2 | 0 |
| Final | 3 | 1§ | 0 | 0 | 0 | 0 | 2 | 0 | |
| 7 | Baseline | 2 | 1 | 2 | 0 | 0 | 1 | 2 | 0 |
| Final | 2 | 1 | 2 | 0 | 0 | 1 | 2 | 0 | |
| 8 | Baseline | 0 | 1 | 2 | 0 | 0 | 1§ | 1 | 0 |
| Final | 0 | 1 | 2 | 0 | 0 | 2§ | 1 | 0 | |
| 9∗ | Baseline | 2 | 0 | 0 | 0 | 1‡ | 1‡ | 2† | 0 |
| Final | 2 | 0 | 0 | 0 | 0‡ | 0‡ | 1† | 0 | |
| 10∗ | Baseline | 0 | 1 | 3† | 0 | 3† | 0 | 2† | 0 |
| Final | 1 | 1 | 2† | 0 | 1† | 0 | 1† | 0 | |
| 11 | Baseline | 2§ | 2† | 1 | 0 | 0 | 0 | 0§ | 0 |
| Final | 3§ | 1† | 1 | 0 | 0 | 1 | 1§ | 0 | |
| 12 | Baseline | 3 | 1§ | 3 | 1‡ | 0 | 2 | 3† | 0 |
| Final | 3 | 2§ | 3 | 0‡ | 0 | 2 | 2† | 0 | |
| 13∗ | Baseline | 1‡ | 1‡ | 0 | 0 | 0 | 0 | 2† | 2 |
| Final | 0‡ | 0‡ | 0 | 0 | 0 | 0 | 1† | 2 | |
| 14∗ | Baseline | 2† | 0 | 2† | 0 | 0 | 0 | 0 | 0 |
| Final | 1† | 0 | 1† | 0 | 0 | 0 | 0 | 0 | |
| 15∗ | Baseline | 2 | 0 | 1 | 0 | 0 | 0 | 2† | 0 |
| Final | 2 | 0 | 1 | 0 | 0 | 0 | 1† | 0 | |
| 16∗ | Baseline | 2 | 1‡ | 3† | 0 | 1 | 0 | 2 | 0 |
| Final | 2 | 0‡ | 2† | 0 | 1 | 0 | 2 | 1 | |
| 17∗ | Baseline | 3 | 0 | 2 | 0 | 0 | 3† | 2 | 0 |
| Final | 3 | 0 | 2 | 0 | 1 | 2† | 2 | 0 | |
| 18∗ | Baseline | 0 | 1‡ | 1‡ | 0 | 0 | 0 | 1 | 1 |
| Final | 0 | 0‡ | 0‡ | 0 | 0 | 0 | 1 | 0 | |
| 19∗ | Baseline | 0 | 2‡ | 2† | 0 | 0 | 0 | 0 | 0 |
| Final | 0 | 0‡ | 1† | 0 | 0 | 0 | 0 | 0 | |
| 20∗ | Baseline | 3† | 0 | 0 | 0 | 0 | 1‡ | 2 | 0 |
| Final | 2† | 0 | 0 | 0 | 0 | 0‡ | 2 | 0 | |
| 21 | Baseline | 2† | 0 | 0 | 0 | 0 | 1§ | 2 | 0 |
| Final | 1† | 0 | 0 | 0 | 1 | 2§ | 2 | 0 | |
| 22 | Baseline | 1 | 0§ | 2† | 0 | 0 | 2† | 0 | 0 |
| Final | 1 | 1§ | 1† | 0 | 1 | 1† | 0 | 0 | |
| 23 | Baseline | 2 | 0§ | 1§ | 0 | 0 | 1‡ | 1 | 1 |
| Final | 2 | 1§ | 3§ | 0 | 0 | 0‡ | 1 | 1 | |
| 24∗ | Baseline | 0 | 1‡ | 2‡ | 0 | 0 | 2† | 0 | 0 |
| Final | 0 | 0‡ | 0‡ | 0 | 0 | 1† | 0 | 0 | |
| 25∗ | Baseline | 2† | 0 | 0 | 0 | 1‡ | 0 | 1‡ | 0 |
| Final | 1† | 0 | 0 | 0 | 0‡ | 0 | 0‡ | 0 | |
| 26∗ | Baseline | 2 | 1 | 3 | 0 | 0 | 1‡ | 1 | 0 |
| Final | 2 | 1 | 3 | 0 | 0 | 0‡ | 1 | 0 | |
| 27∗ | Baseline | 3 | 0 | 1‡ | 0 | 0 | 0 | 2 | 0 |
| Final | 3 | 0 | 0‡ | 0 | 0 | 0 | 2 | 0 | |
| 28 | Baseline | 1‡ | 0 | 0 | 0 | 2§ | 0 | 0 | 0 |
| Final | 0‡ | 0 | 0 | 0 | 3§ | 0 | 0 | 0 | |
| 29 | Baseline | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Final | 3 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | |
| 30 | Baseline | 3 | 1 | 1 | 0 | 0 | 1‡ | 2§ | 0 |
| Final | 3 | 1 | 1 | 0 | 0 | 0‡ | 3§ | 0 | |
| 31∗ | Baseline | 3 | 1‡ | 0 | 0 | 0 | 0 | 2 | 0 |
| Final | 3 | 0‡ | 0 | 0 | 0 | 0 | 2 | 0 | |
| 32∗ | Baseline | 2† | 0 | 0 | 0 | 0 | 3 | 2† | 0 |
| Final | 1† | 0 | 1 | 0 | 0 | 3 | 1† | 0 | |
| 33∗ | Baseline | 0 | 0 | 2 | 1 | 0 | 2† | 3 | 0 |
| Final | 0 | 0 | 2 | 1 | 0 | 1† | 3 | 0 | |
| 34∗ | Baseline | 2 | 0 | 1‡ | 0 | 0 | 1‡ | 2 | 0 |
| Final | 2 | 0 | 0‡ | 0 | 0 | 0‡ | 2 | 0 | |
| 35 | Baseline | 2§ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Final | 3§ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 36 | Baseline | 3 | 0 | 1 | 1 | 0 | 1§ | 2 | 0 |
| Final | 3 | 0 | 1 | 1 | 0 | 2§ | 2 | 0 |
Lung cGVHD was scored based on the NIH lung symptom score, as assessed by clinicians.
Patients who had a partial overall response.
PR.
CR.
Progression.
Organ-specific response rate in the 36 evaluable patients treated with abatacept. The response by organ system is depicted as either a CR (green) or a PR (orange) in the 36 evaluable patients.
Organ-specific response rate in the 36 evaluable patients treated with abatacept. The response by organ system is depicted as either a CR (green) or a PR (orange) in the 36 evaluable patients.
Change in prednisone dose over time. (A) Percent change from baseline prednisone dose in clinical responders and clinical nonresponders over time. Points are medians with interquartile ranges. Wilcoxon rank-sum test was used for the group comparison. (B) Percent change in prednisone dose in nonresponders and responders from before treatment to 1 month after receiving 6 doses of abatacept.
Change in prednisone dose over time. (A) Percent change from baseline prednisone dose in clinical responders and clinical nonresponders over time. Points are medians with interquartile ranges. Wilcoxon rank-sum test was used for the group comparison. (B) Percent change in prednisone dose in nonresponders and responders from before treatment to 1 month after receiving 6 doses of abatacept.
The median follow-up time among survivors was 43.1 months (range, 8.1-66.3 months). The 3-year OS was 72% (95% confidence interval, 55%-84%), and 3-year FFS was 66% (95% confidence interval, 48%-79%; supplemental Figure 1).
Safety
Tables 3 and 4 show the significant adverse events that were possibly related to abatacept. The most common adverse events were neutropenia, fatigue, headache, and upper respiratory infection. Seven events of neutropenia (5 grade 2 events, 1 grade 3 event, and 1 grade 4 event) were reported in a total of 4 patients. There were 3 events of grade 1 fatigue and 6 events of grade 2 fatigue. Grade 2 headache was observed among 4 patients. Grade 2 upper respiratory infection was noted among 3 patients. Infections of grades from 2 to 4 were observed among 6 participants. Four patients died while on the study. One patient developed grade 4 hepatic and respiratory failure secondary to concurrent herpes simplex virus hepatitis, which was deemed to be related to abatacept. This patient had discontinued acyclovir ∼2 weeks before the event. Another patient died from cardiac arrest, unrelated to abatacept, after the 4-month follow-up visit after completion of abatacept therapy. The third patient discontinued treatment per physician decision because of a lack of response after receiving 2 doses of abatacept and died from respiratory failure related to pulmonary GVHD, unrelated to abatacept. The fourth patient discontinued treatment because of progression of cGVHD and died from mesenteric ischemia, unrelated to abatacept.
Adverse events possibly related to abatacept
| . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| Decreased neutrophil count∗ | — | 5 | 1 | 1 |
| Fatigue | 3 | 6 | — | — |
| Headache | — | 4 | — | — |
| URI | — | 3 | — | — |
| AST increased | 2† | 1 | — | — |
| ALT increased | 1 | 2 | — | — |
| Lung infection | — | 2 | — | — |
| UTI | — | 1 | — | — |
| Viral URI | 1 | — | — | — |
| Eye infection | — | 1 | — | — |
| Nausea | 1 | — | — | — |
| Diarrhea | 1 | — | — | — |
| Pulmonary edema | — | — | 1 | — |
| Cough | 1 | 2 | 1 | — |
| Flu-like symptoms | 1 | — | — | — |
| Myalgia | 2 | — | — | — |
| Oral pain | — | 1 | — | — |
| Dyspnea | — | 1 | — | — |
| Cystitis, noninfective | — | 1 | — | — |
| Muscle weakness | 1 | 1 | — | — |
| . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| Decreased neutrophil count∗ | — | 5 | 1 | 1 |
| Fatigue | 3 | 6 | — | — |
| Headache | — | 4 | — | — |
| URI | — | 3 | — | — |
| AST increased | 2† | 1 | — | — |
| ALT increased | 1 | 2 | — | — |
| Lung infection | — | 2 | — | — |
| UTI | — | 1 | — | — |
| Viral URI | 1 | — | — | — |
| Eye infection | — | 1 | — | — |
| Nausea | 1 | — | — | — |
| Diarrhea | 1 | — | — | — |
| Pulmonary edema | — | — | 1 | — |
| Cough | 1 | 2 | 1 | — |
| Flu-like symptoms | 1 | — | — | — |
| Myalgia | 2 | — | — | — |
| Oral pain | — | 1 | — | — |
| Dyspnea | — | 1 | — | — |
| Cystitis, noninfective | — | 1 | — | — |
| Muscle weakness | 1 | 1 | — | — |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; URI, upper respiratory infection; UTI, urinary tract infection.
Events occurred in total of 4 patients.
Events occurred in 1 patient.
Serious adverse events possibly related to abatacept
| . | Grade . | Number of events . |
|---|---|---|
| Elevated AST | 3 | 1 |
| Upper respiratory infection | 3 | 1 |
| Lung infection | 3 | 1 |
| 4 | 1 | |
| Hemolysis | 4 | 1∗ |
| Respiratory failure | 4 | 1∗ |
| Hepatic infection | 4 | 1∗ |
| Hepatic failure | 4 | 1∗ |
| Death | 5 | 1∗ (concurrent HSV hepatitis) |
| . | Grade . | Number of events . |
|---|---|---|
| Elevated AST | 3 | 1 |
| Upper respiratory infection | 3 | 1 |
| Lung infection | 3 | 1 |
| 4 | 1 | |
| Hemolysis | 4 | 1∗ |
| Respiratory failure | 4 | 1∗ |
| Hepatic infection | 4 | 1∗ |
| Hepatic failure | 4 | 1∗ |
| Death | 5 | 1∗ (concurrent HSV hepatitis) |
AST, aspartate aminotransferase; HSV, herpes simplex virus.
Events that occurred in the same patient.
Immune correlative studies
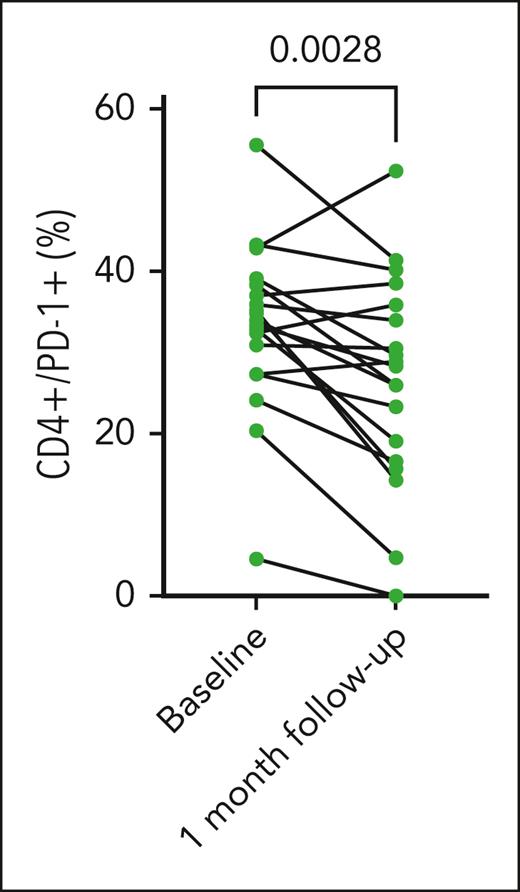
T-cell phenotype was assessed before and after treatment with abatacept. PD-1 expression on circulating CD4+ T cells had decreased after treatment with abatacept (Figure 3). No significant difference was seen in the presence of activated T-cell populations, as assessed via flow cytometric staining for IFN-γ expression on CD4+ and CD8+ T cells, PD-1 expression on CD8+ and CD4+/CD25+/CD69+ T cells (supplemental Figure 2A-D). Similarly, expression of IL-10 by CD8+ and CD4+ T cells was unchanged from baseline to 1 month after the sixth dose of abatacept (supplemental Figure 2E-F). No significant change in BAFF expression on CD19+ B cells was observed after treatment with abatacept (supplemental Figure 2G).
PD-1 expression on CD4+ T cells before and after treatment with abatacept. PD-1 expression on CD4+ T cells was assessed via flow cytometry at baseline and after 6 doses of abatacept treatment. There was a decrease in PD-1 expression by CD4+ T cells after treatment with abatacept (P = .0028).
PD-1 expression on CD4+ T cells before and after treatment with abatacept. PD-1 expression on CD4+ T cells was assessed via flow cytometry at baseline and after 6 doses of abatacept treatment. There was a decrease in PD-1 expression by CD4+ T cells after treatment with abatacept (P = .0028).
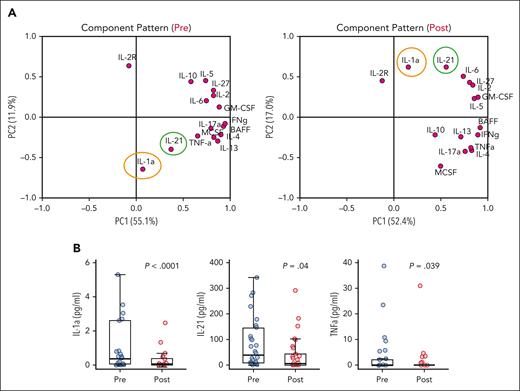
Multiplex immunoassay was used to detect multiple cytokines in patient plasma. As shown in the correlation table and the corresponding heatmap (supplemental Figures 3 and 4), IL-1α and IL-21 were highly correlated both before (r = 0.82) and after (r = 0.78) treatment. Consistent findings were observed using PCA, as shown in the component pattern plot (Figure 4A). When the pretreatment and posttreatment concentrations were compared, there was a significant difference in plasma concentrations of IL-1α, IL-2, and TNF-α (Figure 4B). To assess whether these cytokines could be used as a biomarker of response to treatment, we also compared the baseline cytokine levels of responders and nonresponders. No statistically significant difference was observed either in pretreatment or posttreatment levels of these cytokines when responders were compared with nonresponders.
Cytokine profile analysis before and after treatment with abatacept. (A) Cytokine profile component pattern. PC1 denotes principal component 1 score. PC2 denotes principal component 2 score. (B) Cytokine profile of patient plasma before treatment with abatacept and 1 month after receipt of 6 doses of abatacept. Plasma concentrations of IL-1α, IL-21, and TNF-α were measured using multiplex immunoassay at baseline before treatment with abatacept and 1 month after receipt of 6 doses of abatacept.
Cytokine profile analysis before and after treatment with abatacept. (A) Cytokine profile component pattern. PC1 denotes principal component 1 score. PC2 denotes principal component 2 score. (B) Cytokine profile of patient plasma before treatment with abatacept and 1 month after receipt of 6 doses of abatacept. Plasma concentrations of IL-1α, IL-21, and TNF-α were measured using multiplex immunoassay at baseline before treatment with abatacept and 1 month after receipt of 6 doses of abatacept.
Discussion
In this phase 2 study, we sought to investigate the clinical efficacy and safety profile of abatacept in the treatment of steroid-refractory cGVHD. The ORR was 58%, with a PR of 58% and CR of 0%. Greatest improvement was observed in the lungs, liver, GI tract, and mouth. In addition, patients who had progression of GVHD in some organs still had a response to treatment in other organs after treatment with abatacept. Furthermore, treatment with abatacept facilitated reduction in corticosteroid dose, thus mitigating the untoward effects of steroids.
Laboratory correlates were drawn to understand the effect of abatacept on the immune microenvironment. We would expect that treatment with abatacept would lead to a decrease in activated T cells as well as a decrease in proinflammatory cytokines and cytokines associated with B-cell activation and maturation. Our data show that there was a decrease in the frequency of PD-1 expression on CD4+ T cells after treatment with abatacept. Given that abatacept blocks CD28 costimulation, it is more likely to have a greater effect on CD4+ T cells because it relies more on this pathway for activation compared with CD8+ T cells.16 In studies of rheumatoid arthritis, abatacept has been shown to decrease the number of activated T cells and increase the inhibitory capacity of regulatory T cells.17-19 Blockade of costimulation with abatacept has also been shown to lead to a loss in plasma cells and subsequent reduction in antibody production.20,21 T-follicular helper cells are reportedly implicated as an important factor in sustaining B-cell activation and coordinating T- and B-cell responses in cGVHD.22 Phenotypic and functional analysis of circulating T-follicular helper cells has shown that patients with active cGVHD had decreased circulating T-follicular helper cells because of increased homing to secondary lymphoid organs. In addition, circulating T-follicular helper cells demonstrated a highly activated profile, with a predominance of T-helper 2/T-helper 17 subtypes. In cGVHD, T-follicular helper cells interact with B cells via CD40L, inducible T-cell costimulator, and IL-21 secretion, which in turn promotes germinal center formation and production of high-affinity class-switched antibodies.23,24 CD28 signaling is critical to T-follicular helper cell development, because blockade of CD28 limits germinal center formation and reduces the number of T-follicular helper cells.25,26 Transcriptional profiling of T-cell populations in the peripheral blood taken from patients with multiple sclerosis treated with abatacept showed decrease in the relative abundance of CD4+ T-follicular helper cells and regulatory T cells.27 Thus, abatacept not only suppresses T-cell–mediated effector functions, but, through its effects on T-follicular helper cells, B-cell activation is also curbed. In correlative studies, we saw that there was a significant reduction in IL-1α, IL-21, and TNF-α in all patients before and after treatment with abatacept. These cytokines are implicated in B-cell activation and function, which may suggest modulation of the B-cell by abatacept through T-follicular helper cells, supporting the notion that abatacept modulates cGVHD through both B- and T-cell interactions.
These promising results should be interpreted in the context that this was a study of 39 patients. In addition, patients were also still taking other immunosuppressive agents, including calcineurin inhibitors, corticosteroids, and ruxolitinib, which might also have had an effect on the modulation of cGVHD response. Nonetheless, no new medications were added at the time of abatacept treatment, and as such, the GVHD responses observed in this study after treatment with abatacept are promising.
Although the response rate of 58% of abatacept in steroid-refractory cGVHD is clinically meaningful, it is important to consider these data in the context of the response rates observed after therapy with each of the 3 currently approved FDA drugs for cGVHD, namely, ibrutinib, ruxolitinib, and belumosudil. Ibrutinib, a selective and irreversible Bruton tyrosine kinase inhibitor, was the first drug to be approved for this indication based on results of a phase 1b/2, open-label multicenter trial, which showed an ORR of 67%, with a CR of 21% and a PR of 45%. The median steroid dose among responders decreased from 0.29 mg/kg per day at baseline to 0.12 mg/kg per day at 49 weeks, with 5 responders completely discontinuing steroids.5 The REACH3 trial evaluated the efficacy of ruxolitinib in the treatment of cGVHD in a phase 3 open-label, randomized, multicenter trial. The best ORR was 76.4%, with a CR of 12.1% and a PR of 64.2%.7 The ROCKstar study was a phase 2 randomized multicenter study that investigated the use of belumosudil, a selective inhibitor of Rho-associated coiled-coil–containing protein kinase 2 (ROCK2), for the treatment of cGVHD after failure of ≥2 prior lines of therapy. The best ORR in patients treated with belumosudil 200 mg daily was 74%, with a CR of 6% and a PR of 68%. Responses were observed in all organs, with CR and PR achieved in organs considered difficult to treat, such as the liver and lung. In addition, 65% of patients decreased their steroid dose during treatment with belumosudil, with a mean reduction of steroid dose of 54% among responders.6 These 3 recently approved agents have improved outcomes for patients with chronic GVHD and offer important new options for treatment of this debilitating disease. Nonetheless, only a minority of patients achieved a CR, underscoring the difficulty in treating this disease, and highlighting the unmet need for novel agents. In addition, it is critical to identify biomarkers that are predictive of response to each agent to select treatment more appropriately and to inform novel combinatorial approaches.
The immunomodulatory role of abatacept has already led to its approval in combination with a calcineurin inhibitor and methotrexate for prophylaxis of acute GVHD, and extended use of abatacept in the early posttransplant period for prevention of cGVHD is currently being studied (NCT04380740). In our study, abatacept was well tolerated and resulted in improvement in cGVHD in 58% of patients, with a concomitant reduction in steroid use. Future studies aimed at exploring the subpopulations of patients that may benefit the most from abatacept therapy are needed. Abatacept may be a promising alternative agent in settings in which the currently approved drugs are not tolerated because of their side effect profile or lack of efficacy. Future studies exploring combination strategies with abatacept and the other currently approved drugs for cGVHD have the potential to capitalize on the different mechanisms of actions of the 3 currently approved kinase inhibitors and the immunomodulatory effects of abatacept. Furthermore, research efforts focused on biomarkers of response and mechanisms of resistance to currently approved cGVHD drugs as well as clinical trials investigating combinatorial strategies are needed to evolve future treatment paradigms for this complex disease.
Acknowledgments
The visual abstract was created with BioRender.com.
This study was supported by research funding from Bristol-Myers Squibb (J.R.).
Authorship
Contribution: A.G.K., H.T.K., J.R., and R.J.S. wrote the manuscript; D.S., A.G.K., and Z.M.A. performed the laboratory correlative studies; H.T.K., A.G.K., and J.R. performed data analysis and statistical analysis as described in the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: D.S. is a consultant for The Blackstone Group. J.A. receives honoraria from Juno/BMS. C.C. is a consultant for Cimeio, Editas, Kadmon, Pfizer, Mallinckrodt, CareDx, Incyte, Omeros, Syndax, Mesoblast, Deciphera, and Jazz. S.N. is a member of the ad hoc advisory board for Kite/Gilead, Iovance, and GlaxoSmithKline. D.E.A. is a member of the advisory board for Celgene, Juno, Partner Tx, Bristol Myers Squibb, Karyopharm, Aviv MedTech Ltd, Takeda, Legend Biotech, and Chugai; receives research funding from Celgene, Juno, Pharmacyclics, and Kite Pharma; and is a consultant for Kite Pharma, Janssen, Parexcel, Takeda, and Sanofi. R.J.S. is a consultant for Vor Biopharma, Smart Immune, Neovii, CLS Behring, Bluesphere Bio, Cugene, and Jasper; is on the data safety monitoring board for Juno Therapeutics, BMS, and Celgene USA; and is on the board of directors of NMDP Be The Match. J.R. received funding from Bristol Myers Squibb to conduct this study and is a consultant for Attivare Therapeutics, Parexel, Bioclinica, Karyopharm, Imaging Endpoints, and Wolters Kluwer Health. The remaining authors declare no competing financial interests.
Correspondence: Jacalyn Rosenblatt, Division of Hematology and Hematologic Malignancies, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215KS 115; e-mail: jrosenb1@bidmc.harvard.edu.
References
Author notes
∗R.J.S. and J.R. contributed equally to this study.
Data are available on request from the corresponding author, Jacalyn Rosenblatt (jrosenb1@bidmc.harvard.edu).
The clinical protocol is available in a data supplement in the online version of the article.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal