Abstract
Erythropoietic protoporphyria (EPP) is an inherited cutaneous porphyria caused by reduced expression of ferrochelatase, the enzyme that catalyzes the final step in heme biosynthesis. The resultant accumulation of protoporphyrin IX leads to severe, painful cutaneous photosensitivity, as well as potentially life-threatening liver disease in a small percentage of patients. X-linked protoporphyria (XLP) is clinically similar to EPP but results from increased activity of δ-aminolevulinic acid synthase 2, the first step in heme biosynthesis in the bone marrow, and also causes protoporphyrin accumulation. Although historically the management of EPP and XLP (collectively termed protoporphyria) centered around avoidance of sunlight, novel therapies have recently been approved or are in development, which will alter the therapeutic landscape for these conditions. We present 3 patient cases, highlighting key treatment considerations in patients with protoporphyria, including (1) approach to photosensitivity, (2) managing iron deficiency in protoporphyria, and (3) understanding hepatic failure in protoporphyria.
Introduction
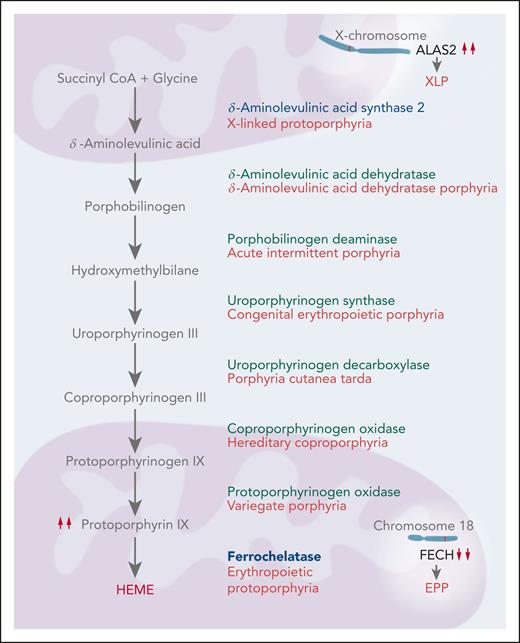
The porphyrias are a diverse group of metabolic disorders, each characterized by alterations in 1 of the 8 enzymatic steps in heme biosynthesis (Figure 1). Porphyrias can be classified as hepatic or erythropoietic, reflecting whether porphyrin precursors or porphyrins accumulate initially in the liver or bone marrow. A clinical classification of acute, blistering cutaneous, and nonblistering cutaneous is also useful because it is based on 3 very distinct presentations that should prompt different first-line diagnostic testing. The 3 most common porphyrias, acute intermittent porphyria, porphyria cutanea tarda, and erythropoietic protoporphyria (EPP) are examples of these 3 clinical categories.1 This How I Treat article focuses on the 2 nonblistering cutaneous porphyrias, EPP and X-linked protoporphyria (XLP), collectively called protoporphyria.
The heme biosynthetic pathway. Heme biosynthesis occurs by 8 sequential enzymatic steps. Decreased activity of ferrochelatase, the final enzyme in the pathway, leads to EPP. XLP is caused by gain-of-function mutations in ALAS2, the enzyme that catalyzes the initial step in this pathway within the erythroblast.
The heme biosynthetic pathway. Heme biosynthesis occurs by 8 sequential enzymatic steps. Decreased activity of ferrochelatase, the final enzyme in the pathway, leads to EPP. XLP is caused by gain-of-function mutations in ALAS2, the enzyme that catalyzes the initial step in this pathway within the erythroblast.
EPP
EPP (Online Mendelian Inheritance in Man, 177000) is a cutaneous porphyria characterized by cutaneous burning, pruritus, edema, and pain after exposure to sunlight (Figure 2).2 It is the third most commonly diagnosed porphyria, with a prevalence previously thought to be ∼1:100 000, likely because of underdiagnosis; however, recent genetic evidence has revealed that its true prevalence is ∼1:17 000.3-5 EPP results from genetic variants in ferrochelatase (FECH), the enzyme that catalyzes the insertion of a molecule of iron into the protoporphyrin IX macrocycle to make heme (Figure 3A). Of patients with EPP, ∼96% have a rare loss-of-function variant in 1 FECH allele (chromosome 18) and a common hypomorphic FECH variant c.315-48T>C on the other allele.6,7 Residual FECH activity in patients with EPP is ∼35% of normal,7 and 2 copies of FECH c.315-48T>C do not result in EPP.8-10 The prevalence of the low expression allele is as high as 50% in some populations, such as in eastern Asia, in which EPP can have a “pseudo autosomal dominant” inheritance pattern with multiple successive generations being affected.3,5,11 The median age at symptom onset is ∼2 years, although there is often a delay in diagnosis, likely because of poor familiarity with the disease among physicians, few objective signs, and young children’s difficulty expressing their symptoms, among other reasons.12-14 Furthermore, there are reported cases of acquired EPP arising in the context of myelodysplastic syndrome.15
Cutaneous manifestations of phototoxic reactions in protoporphyria. (A) Mild cutaneous edema during a phototoxic reaction. (B) Severe cutaneous edema with petechiae during a phototoxic reaction. Note: in protoporphyria, visible cutaneous changes may not be present, even during severely painful phototoxic reactions.
Cutaneous manifestations of phototoxic reactions in protoporphyria. (A) Mild cutaneous edema during a phototoxic reaction. (B) Severe cutaneous edema with petechiae during a phototoxic reaction. Note: in protoporphyria, visible cutaneous changes may not be present, even during severely painful phototoxic reactions.
Production of protoporphyrin and chemical structure. (A) Protoporphyrin is primarily produced within the erythroblast in the bone marrow. Iron enters the cell through the transferrin receptor (TFR) and is converted from the ferric (Fe3+) to the ferrous (Fe2+) form. The formation of heme requires insertion of Fe2+ into protoporphyrin, which is catalyzed by FECH. XLP is caused by gain-of-function variants in ALAS2, and EPP by loss-of-function variants in FECH. (B) Protoporphyrin (depicted in purple) is photoreactive and can cause burning, itching, and pain of the skin. (C) The chemical structure of protoporphyrin (left) and chlorophyl (right) are similar. (D) Plasma protoporphyrin (depicted in purple) enters the liver from plasma and can cause liver damage and protoporphyrin-containing gallstones. PPIX, protoporphyrin IX.
Production of protoporphyrin and chemical structure. (A) Protoporphyrin is primarily produced within the erythroblast in the bone marrow. Iron enters the cell through the transferrin receptor (TFR) and is converted from the ferric (Fe3+) to the ferrous (Fe2+) form. The formation of heme requires insertion of Fe2+ into protoporphyrin, which is catalyzed by FECH. XLP is caused by gain-of-function variants in ALAS2, and EPP by loss-of-function variants in FECH. (B) Protoporphyrin (depicted in purple) is photoreactive and can cause burning, itching, and pain of the skin. (C) The chemical structure of protoporphyrin (left) and chlorophyl (right) are similar. (D) Plasma protoporphyrin (depicted in purple) enters the liver from plasma and can cause liver damage and protoporphyrin-containing gallstones. PPIX, protoporphyrin IX.
A biochemical diagnosis of EPP is made by demonstrating elevation of total erythrocyte protoporphyrin (>3-4 times the upper limit of normal), which is predominantly (>85%) metal-free protoporphyrin, because decreased FECH activity also impairs chelation of protoporphyrin with zinc.16 Protoporphyrin is a photoreactive chemical, with a porphyrin structure similar to chlorophyll. Light-mediated protoporphyrin activation results in the production of reactive oxygen species, complement activation, and endothelial damage (Figure 3B-C).17-19 The degree of light sensitivity varies significantly among patients with EPP, variability that is somewhat associated with differences in protoporphyrin levels and skin pigmentation but is otherwise incompletely understood.12 Day-to-day variability also exists, and many patients experience a “priming” effect manifested by shorter than usual length of sunlight exposure to symptom onset if there was significant sunlight exposure the day before.20-22
All tissues synthesize heme for a variety of hemoproteins.23 Approximately 80% of total heme synthesis occurs in the bone marrow primarily for hemoglobin, with the remaining 20% occurring mainly in the liver for cytochrome P450 enzymes. Excess protoporphyrin in protoporphyria accumulates primarily in bone marrow erythroid cells and persists in circulating erythrocytes. Erythrocyte protoporphyrin then gradually decreases throughout the red blood cell (RBC) lifespan and exists the RBC through a light-induced process and the ABCG2 transporter, becoming bound to plasma proteins such as albumin.24,25
Metal-free protoporphyrin in the plasma is lipophilic. Therefore, rather than being excreted in the urine, it is taken up by the liver and then undergoes biliary excretion, enterohepatic circulation, and elimination in the feces. Approximately 20% of patients with EPP develop cholelithiasis, and between 1% and 4% of patients develop a life-threatening form of liver disease because of the toxic effects of protoporphyrin on the liver, particularly the cholangiocytes (Figure 3D).26,27
XLP
XLP (Online Mendelian Inheritance in Man, 300752) is a rare disorder that is phenotypically similar to EPP. It results from hypermorphic C-terminal truncation variants of the gene that encodes δ-aminolevulinic acid synthase 2 (ALAS2), leading to enzymatic gain of function.28 As a result, heme intermediates are overproduced and protoporphyrin production exceeds the capacity of normal FECH activity, the second rate-limiting step in heme biosynthesis. In contrast to EPP, patients with XLP have elevated levels of both metal-free protoporphyrin and zinc-chelated protoporphyrin, with the latter comprising 15% to 50% of total erythrocyte protoporphyrin, a characteristic that may raise suspicion for the diagnosis of XLP before genetic confirmation.12 Up to 10% of patients with the EPP phenotype will have XLP upon molecular testing.12,29
Inheritance of XLP is X-linked, and females may be equally symptomatic because of varying degrees of lyonization.12 In addition, a pathogenic variant in the mitochondrial unfoldase gene ClpX responsible for ALAS posttranslational stability coupled with a second variant in the iron-responsive element of the ALAS2 gene has been described to cause protoporphyria in 1 proband.30,31
Pitfalls of testing
The biochemical diagnosis of protoporphyrias is based on the measurement of erythrocyte total and metal-free protoporphyrin. The latter comprises 85% to 100% of total erythrocyte protoporphyrin in EPP, and 50% to 85% in XLP.12,16 Zinc protoporphyrin predominates in other conditions that increase erythrocyte protoporphyrin. Plasma and fecal porphyrin levels can be variably increased, and urine porphyrin levels are normal. Genetic testing is recommended to distinguish between EPP and XLP and to confirm the diagnosis.12
Measurement of erythrocyte protoporphyrin levels is fraught with a lack of standardized nomenclature and methodology.32 Some large commercial laboratories measure protoporphyrin by hematofluorometry, a method that only measures zinc protoporphyrin and that was developed to screen for lead poisoning and iron deficiency. Hematofluorometers report the molar ratio of zinc protoporphyrin to heme by fluorescence but do not measure total or metal-free protoporphyrin.16 Nevertheless, these major laboratories report “free erythrocyte protoporphyrin” (FEP) levels that, in actuality, reflect only zinc protoporphyrin, which may not be increased in EPP. This inappropriate method and inaccurate terminology propagates confusion that can lead to missed diagnoses of protoporphyria.32 Accurate testing reports should only refer to erythrocyte total protoporphyrin, zinc protoporphyrin, and metal-free protoporphyrin, and comment whether the results are consistent with protoporphyria. The term FEP became unclear after the discovery of zinc protoporphyrin in erythrocytes in the 1970s and should no longer be used.
High performance liquid chromatography or extraction methods that measure total, metal-free, and zinc protoporphyrin are recommended for the diagnosis of protoporphyria.16 Current lists of laboratories that perform such testing can be found on websites for the United Porphyrias Association, the European Porphyria Network, and the American Porphyria Foundation.
Case 1: routine management of protoporphyria
A 49-year-old nurse reported that, beginning in early childhood, she experienced cutaneous tingling followed by burning and severe pain within 3 minutes of exposure to sunlight and bright indoor light. Symptoms take up to 72 hours to resolve, and the patient notes a heightened sensitivity to light a day after increased light exposure. She stopped working to avoid workplace indoor lights and sunlight exposure during her commute. Laboratory tests revealed a total erythrocyte protoporphyrin level of 828 μg/dL (reference, <80 μg/dL), zinc protoporphyrin 45 μg/dL (reference, <60 μg/dL), metal-free protoporphyrin 738 μg/dL (reference, <20 μg/dL), and plasma protoporphyrin level of 17.9 μg/dL (reference, <1.0 μg/dL). Genetic testing was positive for both a rare pathogenic variant affecting 1 FECH allele (c.194+2T>G) and the common hypomorphic FECH variant (c.315-48T>C).
How is protoporphyria routinely managed?
Historically, management of the cutaneous manifestations of protoporphyria has primarily been sunlight avoidance and use of sun-protective clothing. Patients usually learn these self-protective behaviors at an early age. Traditional sunscreens only block UVA and UVB light and not the blue spectrum of visible light that is responsible for symptoms in EPP and XLP (peak ∼405 nm); however, some patients note mild improvement with opaque zinc-based sunscreen formulations. Narrow-band UVB phototherapy may increase sunlight tolerance in some patients but is not well studied.33 Various oral treatments have been tested without convincing evidence for efficacy, including beta carotene,34 vitamin C,35 and N-acetylcysteine.36,37 Analgesics such as acetaminophen, nonsteroidal anti-inflammatory drugs, and opiates do not relieve the phototoxic symptoms of protoporphyria.32
Afamelanotide is an α-melanocyte stimulating hormone analog approved by the US Food and Drug Administration in 2019 for the treatment of EPP, based on the results of 2 phase 3 randomized placebo-controlled multicenter trials that included 168 patients in Europe and the United States.38 Patients received either afamelanotide (16 mg) via subcutaneous implant, or placebo, and the primary end point was hours of pain-free light exposure. Secondary end points included frequency and duration of phototoxic reactions, quality of life, and safety. In both studies, pain-free light exposure times were longer in patients receiving afamelanotide, and all patients receiving afamelanotide experienced improvement in quality of life. No concerning safety signals were observed.38 A follow-up study of 115 patients in Europe demonstrated high patient continuation and sustained improvement in quality of life over a period of 8 years.39
Afamelanotide acts by stimulating the melanocortin-1 receptor, thereby increasing the production of eumelanin in the skin and causing a tanning effect.40 Elevated dermal levels of eumelanin provide photoprotection by absorbing and scattering UV and visible light and scavenging damaging free radicals.40-42 Afamelanotide is administered as a biodegradable subcutaneous implant, usually inserted 3 to 4 inches above the anterior iliac crest by a trained provider every 8 weeks. Afamelanotide is generally well tolerated, with some patients reporting mild headache and nausea.38
Patients receiving afamelanotide must undergo a baseline dermatologic exam, with subsequent skin exams every 6 months. Because of the high cost of the medication and the restricted nature of its use for protoporphyria, patients must obtain insurance before authorization and register with the pharmaceutical company before initiation of treatment.
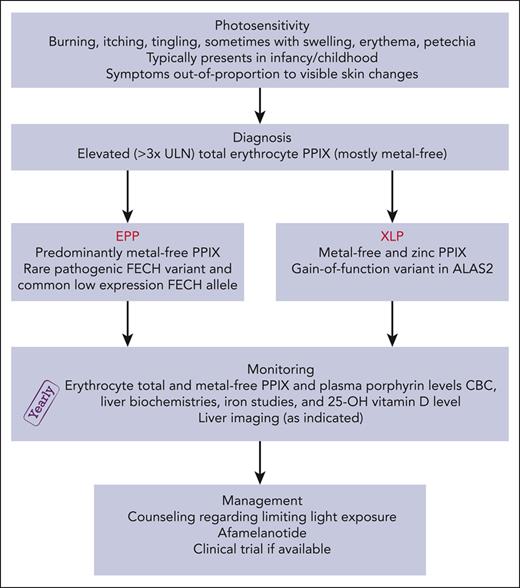
Patients with protoporphyria are at risk for vitamin D deficiency due to sun avoidance behavior, and 25-hydroxyvitamin D levels should be monitored yearly.43,44 We also monitor complete blood count, liver chemistries, iron studies, erythrocyte protoporphyrin (including the proportions of metal-free and zinc protoporphyrin), and plasma porphyrin levels every year (Figure 4).32
Evaluation and treatment algorithm for patients with protoporphyria. CBC, complete blood count; HAV, hepatitis A virus; HBV, hepatitis B virus; PPIX, protoporphyrin IX; ULN, upper limit of normal.
Evaluation and treatment algorithm for patients with protoporphyria. CBC, complete blood count; HAV, hepatitis A virus; HBV, hepatitis B virus; PPIX, protoporphyrin IX; ULN, upper limit of normal.
Within 10 days after her first afamelanotide implant, our patient reported increased skin pigmentation and decreased sunlight sensitivity. After her second implant, she was able to spend several hours outdoors with her family and was considering resuming work as a nurse.
Case 2: iron supplementation in protoporphyria
A 50-year-old woman with EPP presented for routine follow-up care at our porphyria center. Laboratory studies revealed a hemoglobin level of 9.9 g/dL (reference range, 12.0-15.5 g/dL), mean cell volume of 77 fL (reference range, 81.6-98.3 fL), ferritin of 8 μg/L (reference range, 10-200 μg/L), iron of 43 μg/dL (reference range, 30-160 μg/dL), total iron binding capacity of 450 μg/dL (reference range, 230-404 μg/dL), and transferrin saturation of 10% (reference range, 13%-45%). The patient reported heavy menstrual bleeding, fatigue, and pagophagia. Upper and lower endoscopies did not reveal a source of blood loss. She had avoided iron supplements because she was told they might exacerbate her photosensitivity.
What is known about hematologic parameters in EPP?
Mild anemia with microcytic indices has been described in EPP45,46; however, the exact mechanism for these findings is not known. Among a cohort of 52 patients with EPP, Mathews-Roth found that the vast majority had microcytes and other abnormal RBC morphology on peripheral blood smear review, despite only 19 patients meeting criteria for anemia.45 Holme et al47 evaluated 178 individuals with EPP and found that 48% of women and 33% of men were anemic based on World Health Organization criteria. Within a cohort of 147 North American patients with EPP, Balwani et al12 reported anemia in 37% of individuals.
What is known about the iron/hepcidin axis in EPP?
In a mouse model of EPP (Fechm1Pas/m1Pas) with severe FECH deficiency (<5% activity), hypochromic microcytic anemia with minimal hemolysis and without ringed sideroblasts is observed.48,49 Lyoumi et al48 demonstrated that, compared with controls, mice with EPP had similar levels of serum iron, ferritin, and total body iron stores; however, iron was preferentially redistributed from the liver to the spleen in the FECH-deficient mice. Furthermore, in comparison with control mice, mice with EPP had a 2-fold increase in serum transferrin levels that strongly correlated with erythrocyte protoporphyrin levels, a 2-fold reduction in transferrin saturation, and no difference in hepcidin messenger RNA or transferrin receptor 1 expression in bone marrow erythroid cells. These murine studies suggest that severe FECH deficiency is not characterized by overtly low iron stores but rather by alterations in whole-body iron distribution, data that are consistent with some, but not all, studies in humans.
Various clinical studies have investigated whether erythropoiesis is iron restricted in protoporphyria. In a cohort of 55 patients with EPP, Delaby et al50 reported normal serum iron and soluble transferrin receptor (sTfR) levels and, similar to the aforementioned EPP mouse model, elevated serum transferrin levels that correlated with erythrocyte protoporphyrin levels. These authors postulate a protoporphyrin-liver feedback mechanism that induces hepatic transferrin production and mobilization of tissue iron stores but without iron-mediated restriction of heme production.50,51 Likewise, Holme et al47 found normal sTfR levels and serum iron concentrations in their EPP cohort despite a trend toward lower serum ferritin values; however, unlike the former study, there was no clear association between protoporphyrin levels and transferrin. Conversely, Barman-Aksoezen et al52 describe 67 patients with EPP in whom sTfR levels were significantly increased compared with controls. Therefore, although some data point to hematopoiesis as being iron restricted in EPP, most data do not; additional investigations are needed in this area.
Variation in the expression of hepcidin does not explain the anemia observed in patients with protoporphyria. In a study of 8 patients with EPP, Bossi et al53 found that serum and urine hepcidin levels were appropriately low and enteral iron absorption occurred normally. Graziadei et al54 showed no overall difference between hepcidin levels among 20 patients with EPP vs controls; however, when stratified by sex and microcytosis, patients who were normocytic had higher hepcidin levels and females with microcytic anemia had expectedly lower levels. Barman-Aksoezen et al52 reported low levels of hepcidin in their cohort, although without an anticipated increase in iron absorption or total body iron stores. Finally, in the Fechm1Pas/m1Pas mouse model in which hepcidin was induced to provoke an iron-deficient state, Schmidt et al55 observed no improvement in erythrocyte protoporphyrin levels or hepatic protoporphyrin accumulation. Taken together, these studies support the notion that hepcidin in EPP is appropriately regulated and that that there are mechanisms independent of hepcidin to explain the iron-deficient phenotype observed in this disorder.51,52
How might iron modulate disease expression in EPP?
The alterations in iron homeostasis in EPP are incompletely understood although various mechanisms have been proposed. First, iron deficiency should limit ALAS2 expression through its iron-responsive element by decreasing ALAS2 translation, which should decrease heme biosynthetic drive and therefore protoporphyrin levels.56-61 Second, iron deficiency augments alternative FECH splicing, leading to a reduction in FECH activity, which should increase protoporphyrin levels.58,62 Third, FECH is an iron-sulfur cluster protein and reliant on iron both as a substrate and for the formation of its iron-sulfur moieties.58,63 The interplay between these mechanisms in vivo requires further study; however, the net effect of iron restriction in EPP is thought to be protective, limiting aberrant protoporphyrin accumulation through a predominant effect on ALAS2 expression.57,58
How do we approach iron supplementation in EPP?
Although contradictory reports outline both the potential benefits and harms of iron supplementation in patients with EPP, the majority of published literature suggests worsened photosensitivity, possibly resulting from increased erythrocyte protoporphyrin levels with iron therapy.64-69 However, although erythropoiesis in most patients with EPP does not seem to be limited by a mild reduction in iron stores, a subset of patients present with more severe iron deficiency due to causes such as menstruation, dietary restriction, or gastrointestinal bleeding. Notably, a decrease in protoporphyrin and improvement in EPP symptoms has been described in pregnancy, which is frequently an iron-deficient state.70,71
In all patients with EPP, we monitor hemoglobin values, RBC indices, and iron stores at least yearly. Evaluation of sTfR levels may also be useful in detecting true iron deficiency.72 In our practice, we reserve treatment of iron deficiency to patients with EPP who are symptomatic and/or have a hemoglobin level of <10 g/dL and ferritin of <10 μg/L, supplementing iron to achieve a goal ferritin in the low-to-normal range.32 We pay particular attention to women of childbearing potential, who may have increased iron demands. IV iron should be used with caution, and we suggest follow-up of erythrocyte protoporphyrin levels after each dose.
How do we manage iron deficiency in XLP?
Treatment of iron deficiency in XLP is thought to be beneficial because supplemental iron in the setting of normal FECH activity should potentiate heme formation, thus lowering protoporphyrin production.68,73 However, iron supplementation will not decrease protoporphyrin levels in patients with XLP who have replete iron stores. A reduction in erythrocyte protoporphyrin levels after iron supplementation was recently demonstrated in a study of 2 patients with XLP who had baseline ferritin levels of ≤30 ng/mL.69 We, and others, recommend correction of iron deficiency in patients with XLP, with either oral or IV iron formulations, targeting a normal ferritin.32
The patient in case 2 was intolerant of oral iron and, as such, she received 2 doses of IV ferumoxytol, 510 mg. Within 4 weeks she reported an improvement in her energy, and her pagophagia resolved. The patient noticed no change in her symptoms of photosensitivity; that said, because it was wintertime in New England, she had spent little time outdoors. A total erythrocyte protoporphyrin level that was checked before receipt of IV iron was 1455 μg/L (of which 96% was metal free). This value increased to 1838 μg/dL (98% metal free) 1 week after the first dose of iron, then further increased to 3047 μg/dL (99% metal free) 4 months after her second infusion, possibly related to a transient increase in erythropoiesis. One year after receipt of IV iron, the patient’s metal-free protoporphyrin level was 2266 μg/dL (98% metal free), and liver enzymes remained within normal limits. Interpretation of these changes in erythrocyte protoporphyrin levels is uncertain because protoporphyrin is known to vary by ∼25% in patients with EPP over time.74
Case 3: how we approach hepatic dysfunction in protoporphyria
A 54-year-old man with EPP presented with jaundice, right upper quadrant pain, and elevated liver biochemistries.75,76 A liver biopsy revealed cirrhosis and extensive protoporphyrin accumulation. After other etiologies of liver disease were excluded, he was diagnosed with acute and chronic liver disease due to EPP. The patient’s clinical condition worsened, and he underwent orthotopic liver transplantation (OLT). His health stabilized for 2 years after transplant, but he then again developed elevated liver biochemistries. Biopsy of the liver allograft was negative for rejection and revealed widespread protoporphyrin deposition in the hepatocytes, canaliculi, and intrahepatic ducts. He died of liver failure soon thereafter.
Protoporphyria and liver disease
Protoporphyric hepatopathy results from the accumulation of protoporphyrin in hepatocytes and bile canaliculi, with toxic effects on cholangiocytes and Kupffer cells leading to cholestasis.26,27 We use the term protoporphyric hepatopathy to refer to all liver disease associated with protoporphyria, which can be chronic and subclinical or rapidly progressive and life threatening.77-79 Diagnosis requires a liver biopsy revealing protoporphyrin deposition, particularly characteristic Maltese-cross inclusions visible under polarizing microscopy.80,81 Current data are insufficient to predict which patients with protoporphyria will go on to develop hepatic complications, although it is likely that both environmental and genetic factors contribute.
Mild elevations in transaminases are thought to be common in patients with protoporphyria.12 For example, in a US cohort, 33% of patients with EPP and 40% of patients with XLP were found to have elevations in aminotransferase levels or a medical history of liver disease.12 However, in a prospective cohort study of 114 adult patients with EPP in The Netherlands, 6.2% of patients had abnormal liver enzymes, 29% had liver steatosis, and 9.6% had significant fibrosis detected on transient elastography. These changes were not more common than expected in the general population; yet, 8% of patients were thrombocytopenic and 22% had a spleen size of >12 cm.79
Liver failure occurs in between 1% and 4% of patients with protoporphyria, with impaired clearance of protoporphyrin potentiating elevations in plasma and erythrocyte protoporphyrin levels and a resultant vicious cycle of further hepatic damage.26,78 Liver failure may also lead to a Coombs-negative hemolytic anemia, which, in turn, upregulates the synthesis of heme in the bone marrow, further increasing circulating levels of protoporphyrin.26,82
What is our overall approach to liver health in patients with protoporphyria?
We recommend that patients with EPP maintain a healthy weight, abstain from alcohol or drink sparingly,83 undergo vaccination for hepatitis A and B, avoid hepatotoxic medications or supplements, and have liver biochemistries checked at least yearly. Detailed guidance for the surveillance and management of protoporphyric liver disease is outside the scope of this review, but we will highlight key topics relevant to the practicing hematologist.
What should the hematologist know about management of liver disease in protoporphyria?
Various interventions have been investigated for treatment of advanced protoporphyric hepatopathy, with the aim of removing excess protoporphyrin from the circulation and suppressing protoporphyrin synthesis by the bone marrow, thus bridging patients to OLT.26,84 The treatment methods considered to have the highest efficacy in bridging patients to OLT who have acute liver failure due to protoporphyric hepatopathy include therapeutic plasma exchange (TPE), which reduces the amount of protoporphyrin reaching the liver, and RBC transfusion to correct anemia, with the aim of preventing augmented erythropoiesis and synthesis of protoporphyrin.84,85 Hepatocytes are only exposed to protoporphyrin in the plasma compartment, and, as such, TPE should provide immediate hepato-protection. However, the effect of TPE is transient, and patients may require daily exchanges for weeks until OLT is performed.85,86 Plasma and erythrocyte porphyrin levels should be measured before and after each TPE session to assess when this and other interventions should be repeated.85
RBC exchange, rather than simple transfusion, reduces protoporphyrin in the erythrocyte compartment, thus lowering total circulating protoporphyrin levels.75,85 Large amounts of protoporphyrin can be removed by RBC exchange, because protoporphyrin levels are generally higher in RBCs than in the plasma. However, although RBC transfusion or exchange may be useful in the short term, risks of infection, allo-antibody formation, and iron overload should be considered. Other potential therapies described in case reports and small case series include ursodeoxycholic acid and cholestyramine,87,88 IV hemin,76,86,89 high-dose vitamin E,90 cimetidine,91 and activated charcoal.92
Patients with protoporphyria-related liver failure should be managed by a multidisciplinary team at a center with expertise in the treatment of protoporphyric hepatopathy and access to liver transplantation. Care often requires a combination of treatment modalities, with plasma and erythrocyte protoporphyrin levels measured frequently to determine response. Medical interventions will sometimes lead to improvement but eventual progression and the need for OLT is expected.93
Consideration of HSCT in protoporphyria
Hematopoietic stem cell transplant (HSCT) corrects the defect in heme biosynthesis in the bone marrow but should only be considered in patients with significant liver complications; particularly in young patients after successful OLT in order to preserve graft function, in patients with progressive liver disease who do not yet meet criteria for OLT, and after improvement of liver failure without advanced liver fibrosis.94-100 Most published cases of HSCT for protoporphyria are from matched-unrelated donors with a nonmyeloablative, reduced-intensity conditioning regimen, although reports of haploidentical transplants are emerging.100 Similar to HSCT approaches in other nonmalignant diseases, in patients with protoporphyria, we recommend reduced-intensity conditioning, use of bone marrow as the stem cell source, and either ex-vivo graft manipulation (such as αβ T-cell receptor and CD19+ depletion) or posttransplant cyclophosphamide for prevention of graft-versus-host disease, especially if a haploidentical donor is selected.101,102 Reported cases describe both successful HSCT as well as engraftment failure; however, when successful, HSCT is curative.84,103
Living with a rare genetic disease
Individuals with protoporphyria can experience severe pain when exposed to sunlight, and efforts to avoid symptoms markedly impair quality of life.104,105 The pain experienced is out of proportion to the degree of visible skin damage, which is often minimal or absent. Therefore, patients, especially children and young adults, find explaining this “invisible” condition to others challenging.13,104-106 In managing patients with protoporphyria, addressing and validating psychosocial concerns and encouraging access to online resources, patient support groups, expert centers, clinical trials, and specialized programs for affected children is critical.
What does the future hold for the treatment of protoporphyria?
The recent introduction of afamelanotide has been an important advance in the management of protoporphyria, and various other innovative treatments are under investigation (Table 1). A phase 2 trial of a selective melanocortin-1 receptor agonist, dersimelagon (Mitsubishi Tanabe), a small molecule given orally, demonstrated a significant increase in the duration of symptom-free sunlight exposure after 16 weeks of therapy.107 A phase 3 clinical trial of dersimelagon has been completed, and an extension study is ongoing (ClinicalTrials.gov NCT04402489, NCT05005975).
Novel and repurposed treatments for EPP and XLP
| Medication . | Mechanism of action . | Manufacturer . | Development stage . |
|---|---|---|---|
| Afamelanotide | α-MSH analog | Clinuvel | FDA approved |
| Dersimelagon | Selective melanocortin-1 receptor agonist | Mitsubishi Tanabe | Phase 3 clinical trial |
| Cimetidine | Inhibition of ALAS | Generic | Phase 2 clinical trial |
| Bitopertin | Selective glycine transport inhibitor | Disc Medicine | Phase 2 clinical trial |
| — | ABCG2 transporter inhibitor | — | Animal models |
| — | Antisense oligonucleotide therapy | — | Animal models |
| Medication . | Mechanism of action . | Manufacturer . | Development stage . |
|---|---|---|---|
| Afamelanotide | α-MSH analog | Clinuvel | FDA approved |
| Dersimelagon | Selective melanocortin-1 receptor agonist | Mitsubishi Tanabe | Phase 3 clinical trial |
| Cimetidine | Inhibition of ALAS | Generic | Phase 2 clinical trial |
| Bitopertin | Selective glycine transport inhibitor | Disc Medicine | Phase 2 clinical trial |
| — | ABCG2 transporter inhibitor | — | Animal models |
| — | Antisense oligonucleotide therapy | — | Animal models |
α-MSH, α-melanocyte stimulating hormone; FDA, US Food and Drug Administration.
Other novel treatment approaches include strategies to lower erythrocyte protoporphyrin levels by decreasing its production, which may reduce photosensitivity and could reduce the risk for hepatobiliary complications. Bitopertin (Disc Medicine) is an oral selective inhibitor of the glycine transporter, which limits erythroid cells’ uptake of glycine, an initial substrate for heme synthesis. A phase 2 clinical trial of bitopertin in EPP is ongoing (ClinicalTrials.gov NCT05308472).108 Cimetidine, which may have off-target effects on ALAS, has been proposed as a possible treatment for patients with EPP or XLP.109 A clinical trial of cimetidine in protoporphyria is underway (ClinicalTrials.gov NCT05020184).
Other new potential therapeutic targets include the ABCG2 transporter, which regulates protoporphyrin efflux out of erythrocytes and other cells and, if inhibited, would cause protoporphyrin to remain sequestered within circulating erythrocytes and hepatocytes.110 Finally, antisense oligonucleotides to inhibit aberrant FECH splicing are undergoing preclinical investigation.111,112
Conclusions
EPP and XLP are nonblistering cutaneous porphyrias characterized by excess protoporphyrin in the bone marrow and high levels of circulating protoporphyrin, resulting in photosensitivity and an increased risk of biliary stones and liver damage. Recommended management includes monitoring for liver damage and other disease-related complications. Although the understanding of the molecular basis and clinical presentation of protoporphyrias has advanced, the development of novel and promising new therapies has occurred only recently. Therefore, it is becoming even more important for physicians to be aware of the diagnosis and management of these rare, but often undiagnosed, conditions.
Acknowledgments
The authors thank Karl Anderson of the University of Texas Medical Branch and Mark Fleming and Sarah Ducamp of Boston Children’s Hospital for their invaluable expertise in critically appraising the manuscript. The authors thank the Porphyria Consortium EPP guidelines writing committees for their insightful discussions related to the diagnosis and management of protoporphyria.
R.K.L. and A.K.D. are members of the Porphyrias Consortium (PC). The PC is part of the Rare Diseases Clinical Research Network, which is funded by the National Institutes of Health and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation. PC is funded under grant number U54DK083909 as a collaboration between NCATS and the National Institute of Diabetes and Digestive and Kidney Diseases. A.K.D. receives funding from a National Institutes of Health NIAMS K23 grant 1K23AR079586-01.
Authorship
Contribution: R.K.L. performed research, designed the figures, and wrote the manuscript; and A.K.D. performed research, critically appraised the figures, and wrote the manuscript.
Conflict-of-interest disclosure: R.K.L. serves as a consultant for Mitsubishi Tanabe, Alnylam Pharmaceuticals, and Recordati Pharmaceuticals. A.K.D. has received research funding from Disc Medicine for other research unrelated to this publication, and serves as a consultant for Alnylam Pharmaceuticals and Recordati Pharmaceuticals.
Correspondence: Rebecca Karp Leaf, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St, Boston, MA 02114; e-mail: rkarp-leaf@mgh.harvard.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal