In this issue of Blood, Halkidis et al1 present an article that is a must-read for all hematologists, not just those specializing in autoimmune thrombotic thrombocytopenic purpura (iTTP). Every researcher and clinician will benefit from studying this article because it highlights that a scientific topic, no matter how well studied, may benefit from critical reexamination, performance of additional studies, and adjusting the conclusions as indicated by this process.
iTTP is mediated by severe ADAMTS13 deficiency due to autoantibodies leading to unusually large and extremely adhesive von Willebrand factor (VWF) multimers that mediate systemic platelet clumping in the microcirculation with resulting ischemic organ damage, thrombocytopenia, and microangiopathic hemolytic anemia.2 The polyclonal anti-ADAMTS13 autoantibodies, mostly immunoglobulin G (IgG) but also IgA and IgM,3 are directed against various epitopes of the ADAMTS13 multidomain protein.4 Most patients have autoantibodies capable of inhibiting the ADAMTS13 activity in vivo as well as in vitro,2 but the antibodies that increase the clearance of ADAMTS13 from the circulation are primarily involved in many instances.5 All patients with ADAMTS13 inhibitors have autoantibodies directed against the spacer domain, and fine-mapping of those showed 16 different antispacer epitope profiles with 3 hot-spot regions shared by almost all patients with iTTP.6
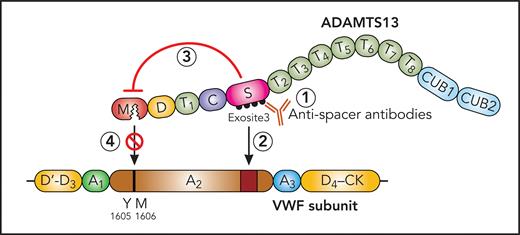
In 2015, Zheng’s group studied the mechanism of 3 ADAMTS13 inhibiting monoclonal antibody fragments derived from patients with iTTP, single-chain fragments of the variable regions (scFv) 4-20, 4-16, and 3-1, using hydrogen-deuterium exchange mass spectrometry and convincingly showed that all 3 bound to the same discontinuous 5-loop structure in the spacer domain that was also targeted by the polyclonal IgGs from 20 of 23 patients with iTTP.7 Deletion of any 1 of the 5 epitopic loops in the spacer domain abolished VWF binding and its proteolytic cleavage. The conclusion was that ADAMTS13 inhibition by the scFvs and iTTP patient antibodies was caused by disrupting the spacer to VWF binding7 (see figure).
Proposed mechanism of ADAMTS13 inhibition by antispacer domain autoantibodies. ADAMTS13 with metalloprotease (M), disintegrin (D), 8 thrombospondin-type 1 (T1-8), cysteine-rich (C), spacer (S), and 2 CUB (CUB1,2) domains and VWF subunit with stretched A2 domain. ADAMTS13 inhibiting antispacer domain antibodies (1) bind to the spacer domain epitope (exosite 3), not merely inhibit exosite 3 interaction with VWF A2 domain (2), but exert an allosteric effect on the metalloprotease domain (3), impairing its access to the Y1605-M1606 cleavage site in the VWF A2 domain (4). Professional illustration by Patrick Lane, ScEYEnce Studios.
Proposed mechanism of ADAMTS13 inhibition by antispacer domain autoantibodies. ADAMTS13 with metalloprotease (M), disintegrin (D), 8 thrombospondin-type 1 (T1-8), cysteine-rich (C), spacer (S), and 2 CUB (CUB1,2) domains and VWF subunit with stretched A2 domain. ADAMTS13 inhibiting antispacer domain antibodies (1) bind to the spacer domain epitope (exosite 3), not merely inhibit exosite 3 interaction with VWF A2 domain (2), but exert an allosteric effect on the metalloprotease domain (3), impairing its access to the Y1605-M1606 cleavage site in the VWF A2 domain (4). Professional illustration by Patrick Lane, ScEYEnce Studios.
Halkidis et al reexamined the mechanism of ADAMTS13 inhibition by the same 3 scFvs, scFv 4-20, 4-16, and 3-1, by formal enzyme kinetic assays. Recombinant ADAMTS13, truncated variants, and ADAMTS13 in plasma were functionally tested with the fluorescence resonance energy transfer system von Willebrand factor 73 (FRETS-VWF73) peptide substrate in the presence and absence of the inhibiting scFvs. Using varying FRETS-VWF73 substrate concentrations, they studied the influence of the scFvs on the kinetic parameters maximum enzyme velocity (Vmax, reflecting the catalytic activity) and substrate concentration giving half-maximal Vmax (K 0.5, reflecting the Michaelis constant). Unexpectedly, the 3 scFvs predominantly reduced the Vmax and only minimally increased K 0.5. This, however, was not the predicted outcome from the original publication.7 These findings suggest that the scFvs induced an allosteric inhibition of the metalloprotease domain and had no or less influence on the substrate (VWF and specifically FRETS-VWF73) binding to ADAMTS13. Therefore, the earlier hydrogen-deuterium exchange data from 2015 were reanalyzed. These had initially been interpreted as showing deuterium uptake alterations upon adding scFvs to ADAMTS13 exclusively in the spacer domain epitopic loops7 but now revealed clear deuterium uptake changes in several oligopeptide regions of the metalloprotease domain as well. This supports the model of an allosteric effect of the scFvs binding to the ADAMTS13 spacer domain across the consecutive cysteine-rich, first thrombospondin-1, and disintegrin domains (see figure).
There are 2 important messages from this article. For the TTP expert, it is noteworthy that anti-ADAMTS13 spacer domain antibodies binding to peptide sequences in the ADAMTS13 exosite 3, which directly binds to the distal A2 domain of the VWF substrate,8,9 are not merely hindering substrate binding but seem to allosterically change the far-away metalloprotease domain, inhibiting its access to the proteolytic bond Tyr1605–Met1606 (see figure). For any clinician-scientist, this study demonstrates that established and published “facts” are better regarded as a “model” meriting reinvestigation with confirmatory experiments and reanalysis of existing data. The X. L. Zheng group is to be congratulated for having done so.
Conflict-of-interest disclosure: B.L. is chairman of data monitoring committees of studies investigating rADAMTS13 for the treatment of congenital and acquired TTP (Takeda), chairman of a steering committee investigating global impact of congenital TTP (Takeda), and chairman of the data monitoring committee of a study investigating caplacizumab for the treatment of iTTP without plasma exchange (Sanofi).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal