Key Points
OS for relapsed/refractory Hodgkin lymphoma after autologous HCT has improved after the approval of BV and the PD-1 inhibitors.
PD-1 inhibitor-based salvage regimens before autologous HCT improved PFS in multivariable analysis.
Abstract
The treatment landscape of relapsed/refractory (R/R) classic Hodgkin lymphoma (cHL) has evolved significantly over the past decade after the approval of brentuximab vedotin (BV) and the programmed death-1 (PD-1) inhibitors. We evaluated how outcomes and practice patterns have changed for patients with R/R cHL who underwent autologous hematopoietic cell transplantation (AHCT) at our institution from 2011 to 2020 (N = 183) compared with those from 2001 to 2010 (N = 159) and evaluated prognostic factors for progression-free survival (PFS) and overall survival (OS) in both eras. OS was superior in the modern era with a trend toward lower nonrelapse mortality beyond 2 years after transplant. Among patients who progressed after AHCT, 4-year postprogression survival increased from 43.3% to 71.4% in the modern era, reflecting increasing use of BV and the PD-1 inhibitors. In multivariable analysis for patients that underwent transplant in the modern era, age ≥45 years, primary refractory disease, and lack of complete remission pre-AHCT were associated with inferior PFS, whereas receipt of a PD-1 inhibitor-based regimen pre-AHCT was associated with superior PFS. Extranodal disease at relapse was associated with inferior OS. Our study demonstrates improved survival for R/R cHL after AHCT in the modern era attributed to more effective salvage regimens allowing for better disease control pre-AHCT and improved outcomes for patients who progressed after AHCT. Excellent outcomes were observed with PD-1 inhibitor-based salvage regimens pre-AHCT and support a randomized trial evaluating immunotherapy in the second line setting.
Introduction
Most patients with classic Hodgkin lymphoma (cHL) are cured with frontline chemotherapy, but ∼10% to 20% relapse whereas another 5% to 10% have primary refractory disease.1-3 For transplant-eligible patients with relapsed or refractory (R/R) cHL, the standard of care is salvage therapy followed by autologous hematopoietic cell transplantation (AHCT), which cures over half of patients with superior progression-free survival (PFS) compared with chemotherapy alone.4,5 Achieving a metabolic complete remission (CR) by positron emission tomography (PET) before AHCT is a key prognostic factor associated with superior 5-year PFS of 75% vs 31% for patients with residual PET-positive disease.6
Over the past decade, the following 3 novel agents have been approved by the US Food and Drug Administration for R/R cHL: the anti-CD30 antibody-drug conjugate brentuximab vedotin (BV), approved in 2011, and 2 programmed death-1 (PD-1) inhibitors, nivolumab and pembrolizumab, approved in 2016 and 2017, respectively. These agents were initially evaluated in patients with multiply R/R cHL with progressive disease (PD) after AHCT and demonstrated high response rates ranging from 69% to 75% with CR rates of 16% to 34%.7-10 As single agents after failure of AHCT, the median PFS with BV and the PD-1 inhibitors is ∼9 months and 14 months, respectively.11-13
More recently, trials have integrated BV and the PD-1 inhibitors into earlier lines of therapy including as post-AHCT maintenance, first salvage before AHCT, and in the frontline setting. In the phase 3 AETHERA trial, BV maintenance after AHCT led to superior 5-year PFS of 59% vs 41% with placebo in patients at high risk for relapse or PD.14,15 Pembrolizumab maintenance and combined BV/nivolumab maintenance have also been evaluated in phase 2 trials with excellent 18-month PFS of 82% to 95%.16,17 In the second line setting, BV has been combined with platinum-based chemotherapy,18-20 bendamustine,21,22 or nivolumab,23,24 with high CR rates of 67% to 83% and 3-year PFS of 67% to 91% in patients proceeding to consolidative AHCT. Pembrolizumab or nivolumab has also been combined with platinum or gemcitabine-based chemotherapy with high CR rates of 87% to 95% and 2-year PFS of 88% to 100% in patients proceeding to AHCT.25-27 In the frontline setting, BV in combination with doxorubicin, vinblastine, and dacarbazine (BV-AVD) was US Food and Drug Administration approved in 2018 for stage 3-4 cHL based on the ECHELON-1 trial, demonstrating a PFS benefit over doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD).28 An overall survival (OS) benefit of BV-AVD over ABVD was also recently reported with mature 6-year follow-up.29 Recent trials from the United States and Germany have demonstrated excellent outcomes with pembrolizumab or nivolumab in combination with AVD in the frontline setting for early stage unfavorable and advanced cHL with 2-year PFS ranging from 83% to 100%.30-33
Given these significant developments over the past decade, we evaluated how outcomes and practice patterns have changed for R/R cHL patients who underwent AHCT at our institution from 2011 to 2020 compared with 2001 to 2010, reflecting increasing use of novel agents in the modern era. We also evaluated prognostic factors for PFS and OS in both eras and describe a new prognostic model for PFS after AHCT in the modern era.
Methods
Patients
We included all consecutive patients with R/R cHL who underwent AHCT at Stanford Hospital between September 2001 and December 2020, using a uniform conditioning regimen of gemcitabine, vinorelbine, carmustine (BCNU), etoposide, and cyclophosphamide (GN-BVC). We divided the cohort into the following 2 treatment eras: patients undergoing AHCT between 2001 to 2010 and between 2011 to 2020, reflecting increasing use of novel agents in the latter era (hereafter referred to as the modern era). GN-BVC conditioning was administered as previously described,34 with further reduction in the dose of BCNU from 350 mg/m2 to 300 mg/m2 implemented on 10 August 2017. All patients were enrolled on transplant protocols approved by the Stanford University Institutional Review Board or on treatment plans, and provided informed consent in accordance with the Declaration of Helsinki. The censoring date was 31 December 2021, allowing a minimum follow-up of 1 year for all patients. Patients with nodular lymphocyte-predominant Hodgkin lymphoma were excluded.
Study end points
The study end points included OS, PFS, and the cumulative incidence of relapse and nonrelapse mortality (NRM). These end points were evaluated for the entire cohort and compared between eras. OS was defined as the time from AHCT to death from any cause. PFS was defined as the time from AHCT to the first observation of relapse/PD or death. PFS was evaluated for clinically relevant subgroups based on the initial disease-free interval, remission status before AHCT, number of adverse risk factors as defined in the AETHERA trial, and the type of salvage therapy administered immediately before AHCT. Pretransplant remission status was assessed by PET imaging for all patients using the International Working Group criteria for patients who received transplantation before 2014 and using the Lugano criteria for patients transplanted thereafter.35-37 For patients who received transplantation in the modern era, a Deauville score of 1 to 3 was considered a metabolic CR.38 NRM was defined as death from any cause other than relapse/PD. For patients experiencing PD after AHCT, we evaluated postprogression survival (PPS), defined as the time from relapse/PD to death from any cause. Causes of death and notable treatment-related toxicities, including pneumonitis, hepatic veno-occlusive disease (VOD), therapy-related myelodysplastic syndrome (MDS) or acute leukemia, other second cancers, and clinically significant immune-related adverse events, were recorded.
Statistical analysis
Standardized mean differences were used to compare patient characteristics and practice patterns between eras. For time-to-event analyses, the Kaplan-Meier method was used to estimate the probabilities of OS, PFS, and PPS. The cumulative incidences of relapse and NRM were estimated using the competing risk method. Relapse was treated as a competing risk for NRM, and death was treated as a competing risk for relapse. Log-rank tests were used to detect differences between groups. Cox regression analysis was used to identify variables associated with PFS and OS in both eras. Twelve variables were included in the Cox models including age at AHCT, race/ethnicity, initial disease stage, bulky disease at diagnosis (mediastinal mass ratio ≥0.33 or any nodal conglomerate ≥10 cm), B symptoms at diagnosis, extranodal disease at diagnosis, initial disease-free interval, B symptoms at relapse, extranodal disease at relapse, number of salvage regimens before AHCT, last salvage regimen before AHCT, and remission status by PET before AHCT. Variable selection was performed via the lasso method. All P values are 2 tailed with values <.05 considered significant. Statistical analyses were performed using R Statistical Software (Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
The baseline patient characteristics are shown in Table 1. A total of 342 patients underwent AHCT, including 159 patients who received transplantation between 2001 and 2010 and 183 who received transplantation between 2011 and 2020. The median age at AHCT was 33 years (range, 18-69 years). Fifty-five percent were male and 62% were White, non-Hispanic. Race and ethnicity differed by era with a decrease in the proportion of White, non-Hispanic patients undergoing AHCT and increase in Asian/Pacific Islander and mixed-race patients undergoing AHCT in the modern era (P < .001). At initial diagnosis, most patients in both eras had advanced stage disease and B symptoms. Bulky disease and extranodal involvement were present in 44% and 29%, respectively. Frontline therapy differed by era with increasing use of ABVD and decreasing use of Stanford V in the modern era (P < .001).
Patient characteristics
| . | All patients (N = 342) . | AHCT in 2001-2010 (N = 159) . | AHCT in 2011-2020 (N = 183) . | P value∗ . | Standardized mean difference∗ . |
|---|---|---|---|---|---|
| Demographics | |||||
| Age at AHCT, median (range) | 33 (18-69) | 33 (18-68) | 33 (19-69) | .924 | 0.5% |
| Male sex, N (%) | 187 (55%) | 91 (57%) | 96 (52%) | .438 | 9.6% |
| White, non-Hispanic | 211 (62%) | 116 (73%) | 95 (52%) | <.001 | 53.8% |
| Hispanic | 60 (18%) | 25 (16%) | 35 (19%) | ||
| Asian/Pacific Islander | 35 (10%) | 12 (8%) | 23 (13%) | ||
| Black or African American | 11 (3%) | 3 (2%) | 8 (4%) | ||
| Mixed race | 25 (7%) | 3 (2%) | 22 (12%) | ||
| Histology | |||||
| Nodular sclerosis | 296 (87%) | 143 (90%) | 153 (84%) | .127 | 29.6% |
| Mixed cellularity | 22 (6%) | 7 (4%) | 15 (8%) | ||
| Lymphocyte rich | 8 (2%) | 1 (1%) | 7 (4%) | ||
| Lymphocyte depleted | 1 (<1%) | 1 (1%) | 0 (0%) | ||
| cHL NOS | 15 (4%) | 7 (4%) | 8 (4%) | ||
| Baseline disease characteristics | |||||
| Ann Arbor stage 3-4 | 184 (54%) | 82 (52%) | 102 (56%) | .508 | 8.4% |
| B symptoms | 194 (57%) | 96 (60%) | 98 (54%) | .246 | 13.8% |
| Bulky disease | 151 (44%) | 75 (47%) | 76 (42%) | .348 | 11.4% |
| Extranodal disease | 98 (29%) | 42 (26%) | 56 (31%) | .463 | 9.3% |
| Frontline therapy | |||||
| ABVD | 260 (76%) | 100 (63%) | 160 (87%) | <.001 | 62.2% |
| Stanford V | 64 (19%) | 49 (31%) | 15 (8%) | ||
| Escalated BEACOPP | 8 (2%) | 5 (3%) | 3 (2%) | ||
| Other regimen | 10 (3%) | 5 (3%) | 5 (3%) | ||
| Response to frontline therapy | |||||
| Primary refractory disease | 94 (27%) | 44 (28%) | 50 (27%) | .934 | 4.0% |
| Relapse <1 y after treatment | 122 (36%) | 58 (36%) | 64 (35%) | ||
| Relapse ≥1 y after treatment | 126 (37%) | 57 (36%) | 69 (38%) | ||
| Number of AETHERA risk factors | |||||
| 0 risk factors | 89 (26%) | 40 (25%) | 49 (27%) | .054 | 30.4% |
| 1 risk factor | 123 (36%) | 54 (34%) | 69 (38%) | ||
| 2 risk factors | 102 (30%) | 57 (36%) | 45 (25%) | ||
| 3 risk factors | 28 (8%) | 8 (5%) | 20 (11%) | ||
| Number of salvage regimens before AHCT | |||||
| 1 salvage regimen | 257 (75%) | 128 (80%) | 129 (70%) | .022 | 30.7% |
| 2 salvage regimens | 68 (20%) | 28 (18%) | 40 (22%) | ||
| 3 or more salvage regimens | 17 (5%) | 3 (2%) | 14 (8%) | ||
| Last salvage regimen before AHCT | |||||
| Platinum-based regimen | 232 (68%) | 143 (90%) | 89 (49%) | <.001 | 119.5% |
| Gemcitabine-based regimen | 35 (10%) | 8 (5%) | 27 (15%) | ||
| BV-based regimen† | 36 (11%) | 0 (0%) | 36 (20%) | ||
| PD-1 inhibitor-based regimen‡ | 27 (8%) | 0 (0%) | 27 (15%) | ||
| Other regimen | 12 (3%) | 8 (5%) | 4 (2%) | ||
| Remission status before AHCT | |||||
| CR | 178 (52%) | 67 (42%) | 111 (61%) | <.001 | 45.2% |
| PR | 144 (42%) | 76 (48%) | 68 (37%) | ||
| SD/PD | 20 (6%) | 16 (10%) | 4 (2%) |
| . | All patients (N = 342) . | AHCT in 2001-2010 (N = 159) . | AHCT in 2011-2020 (N = 183) . | P value∗ . | Standardized mean difference∗ . |
|---|---|---|---|---|---|
| Demographics | |||||
| Age at AHCT, median (range) | 33 (18-69) | 33 (18-68) | 33 (19-69) | .924 | 0.5% |
| Male sex, N (%) | 187 (55%) | 91 (57%) | 96 (52%) | .438 | 9.6% |
| White, non-Hispanic | 211 (62%) | 116 (73%) | 95 (52%) | <.001 | 53.8% |
| Hispanic | 60 (18%) | 25 (16%) | 35 (19%) | ||
| Asian/Pacific Islander | 35 (10%) | 12 (8%) | 23 (13%) | ||
| Black or African American | 11 (3%) | 3 (2%) | 8 (4%) | ||
| Mixed race | 25 (7%) | 3 (2%) | 22 (12%) | ||
| Histology | |||||
| Nodular sclerosis | 296 (87%) | 143 (90%) | 153 (84%) | .127 | 29.6% |
| Mixed cellularity | 22 (6%) | 7 (4%) | 15 (8%) | ||
| Lymphocyte rich | 8 (2%) | 1 (1%) | 7 (4%) | ||
| Lymphocyte depleted | 1 (<1%) | 1 (1%) | 0 (0%) | ||
| cHL NOS | 15 (4%) | 7 (4%) | 8 (4%) | ||
| Baseline disease characteristics | |||||
| Ann Arbor stage 3-4 | 184 (54%) | 82 (52%) | 102 (56%) | .508 | 8.4% |
| B symptoms | 194 (57%) | 96 (60%) | 98 (54%) | .246 | 13.8% |
| Bulky disease | 151 (44%) | 75 (47%) | 76 (42%) | .348 | 11.4% |
| Extranodal disease | 98 (29%) | 42 (26%) | 56 (31%) | .463 | 9.3% |
| Frontline therapy | |||||
| ABVD | 260 (76%) | 100 (63%) | 160 (87%) | <.001 | 62.2% |
| Stanford V | 64 (19%) | 49 (31%) | 15 (8%) | ||
| Escalated BEACOPP | 8 (2%) | 5 (3%) | 3 (2%) | ||
| Other regimen | 10 (3%) | 5 (3%) | 5 (3%) | ||
| Response to frontline therapy | |||||
| Primary refractory disease | 94 (27%) | 44 (28%) | 50 (27%) | .934 | 4.0% |
| Relapse <1 y after treatment | 122 (36%) | 58 (36%) | 64 (35%) | ||
| Relapse ≥1 y after treatment | 126 (37%) | 57 (36%) | 69 (38%) | ||
| Number of AETHERA risk factors | |||||
| 0 risk factors | 89 (26%) | 40 (25%) | 49 (27%) | .054 | 30.4% |
| 1 risk factor | 123 (36%) | 54 (34%) | 69 (38%) | ||
| 2 risk factors | 102 (30%) | 57 (36%) | 45 (25%) | ||
| 3 risk factors | 28 (8%) | 8 (5%) | 20 (11%) | ||
| Number of salvage regimens before AHCT | |||||
| 1 salvage regimen | 257 (75%) | 128 (80%) | 129 (70%) | .022 | 30.7% |
| 2 salvage regimens | 68 (20%) | 28 (18%) | 40 (22%) | ||
| 3 or more salvage regimens | 17 (5%) | 3 (2%) | 14 (8%) | ||
| Last salvage regimen before AHCT | |||||
| Platinum-based regimen | 232 (68%) | 143 (90%) | 89 (49%) | <.001 | 119.5% |
| Gemcitabine-based regimen | 35 (10%) | 8 (5%) | 27 (15%) | ||
| BV-based regimen† | 36 (11%) | 0 (0%) | 36 (20%) | ||
| PD-1 inhibitor-based regimen‡ | 27 (8%) | 0 (0%) | 27 (15%) | ||
| Other regimen | 12 (3%) | 8 (5%) | 4 (2%) | ||
| Remission status before AHCT | |||||
| CR | 178 (52%) | 67 (42%) | 111 (61%) | <.001 | 45.2% |
| PR | 144 (42%) | 76 (48%) | 68 (37%) | ||
| SD/PD | 20 (6%) | 16 (10%) | 4 (2%) |
Bold values denote statistical significance at the P < .05 level.
BEACOPP, bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisone; N, number of patients; NOS, not otherwise specified.
P values and standardized mean difference values indicate a comparison between patients undergoing AHCT in 2001 to 2010 vs 2011 to 2020.
Includes BV alone or in combination with chemotherapy (eg, BV/bendamustine, BV-ICE).
Includes PD-1 inhibitor alone or in combination with other agents (eg, pembrolizumab, nivolumab/BV).
Seventy-three percent of patients relapsed and 27% had primary refractory disease. Seventy-four percent had ≥1 adverse risk factors as defined in the AETHERA trial (primary refractory disease, first CR [CR1] duration <1 year, or extranodal disease at relapse).14 Patients received a median of 2 systemic therapies before AHCT (range, 1-6 therapies), and 25% received multiple salvage regimens before AHCT. More patients who received transplantation in the modern era received ≥2 salvage regimens before AHCT (P = .022). The choice of salvage therapy varied by era with significant decrease in the use of platinum-based chemotherapy and increase in the use of regimens incorporating BV and/or PD-1 inhibitors (P < .001) (supplemental Table 1, available on the Blood website). Fifty-two percent of patients were in metabolic CR at the time of AHCT, with a higher percentage of patients who received transplantation in CR in the modern era (P < .001).
Use of novel agents, radiotherapy, and allogeneic HCT by era
The use of novel agents and radiotherapy differed significantly by era (Table 2). For patients who received transplantation in the modern era, 57% received BV at any time point compared with 9% of those who received transplantation between 2001 and 2010 (P < .001). In the modern era, BV was more commonly administered before AHCT, whereas 14% received BV maintenance per the AETHERA trial. Thirty-one percent of patients who underwent transplantation in the modern era received a PD-1 inhibitor compared with only 1% of patients who received transplantation from 2001 to 2010 (P < .001). PD-1 inhibitors were more commonly administered pre-AHCT as a component of salvage therapy. The use of radiotherapy at any time point decreased between eras from 73% to 32% (P < .001), including a decline in both pretransplant radiotherapy and posttransplant consolidation. Radiotherapy field size decreased after the transition from involved field radiotherapy (IFRT) to involved site radiotherapy (ISRT), adopted in 2014. Radiotherapy doses also decreased between eras from a median of 36 Gy to 30 Gy. Thirty-eight patients (11%) underwent nonmyeloablative allogeneic HCT for PD after AHCT, and their outcomes have been previously published.39,40 There was no difference in the use of allogeneic HCT between eras (P = .53).
Use of novel agents, radiotherapy, and allogeneic HCT by era
| . | All patients (N = 342) . | AHCT in 2001-2010 (N = 159) . | AHCT in 2011-2020 (N = 183) . | P value∗ . | Standardized mean difference∗ . |
|---|---|---|---|---|---|
| BV | |||||
| Received at any time point | 120 (35%) | 15 (9%) | 105 (57%) | <.001 | 118.0% |
| Before transplant | 78 (23%) | 0 (0%) | 78 (43%) | <.001 | 121.9% |
| After transplant | 59 (17%) | 15 (9%) | 44 (24%) | .001 | 39.9% |
| Maintenance therapy | 25 (7%) | 0 (0%) | 25 (14%) | <.001 | 56.3% |
| For PD | 34 (10%) | 15 (9%) | 19 (10%) | .911 | 3.2% |
| PD-1 inhibitor | |||||
| Received at any time point | 58 (17%) | 1 (1%) | 57 (31%) | <.001 | 91.9% |
| Before transplant | 38 (11%) | 0 (0%) | 38 (21%) | <.001 | 72.4% |
| After transplant | 20 (6%) | 1 (1%) | 19 (10%) | <.001 | 43.8% |
| Radiotherapy | |||||
| Received at any time point | 175 (51%) | 116 (73%) | 59 (32%) | <.001 | 89.3% |
| Before transplant | 127 (37%) | 85 (53%) | 42 (23%) | <.001 | 66.1% |
| After transplant | 60 (18%) | 40 (25%) | 20 (11%) | .001 | 37.7% |
| Consolidation | 33 (10%) | 24 (15%) | 9 (5%) | .003 | 34.4% |
| For PD | 27 (8%) | 16 (10%) | 11 (6%) | .236 | 14.9% |
| Allogeneic HCT | |||||
| For progression after AHCT | 38 (11%) | 20 (13%) | 18 (10%) | .527 | 8.7% |
| . | All patients (N = 342) . | AHCT in 2001-2010 (N = 159) . | AHCT in 2011-2020 (N = 183) . | P value∗ . | Standardized mean difference∗ . |
|---|---|---|---|---|---|
| BV | |||||
| Received at any time point | 120 (35%) | 15 (9%) | 105 (57%) | <.001 | 118.0% |
| Before transplant | 78 (23%) | 0 (0%) | 78 (43%) | <.001 | 121.9% |
| After transplant | 59 (17%) | 15 (9%) | 44 (24%) | .001 | 39.9% |
| Maintenance therapy | 25 (7%) | 0 (0%) | 25 (14%) | <.001 | 56.3% |
| For PD | 34 (10%) | 15 (9%) | 19 (10%) | .911 | 3.2% |
| PD-1 inhibitor | |||||
| Received at any time point | 58 (17%) | 1 (1%) | 57 (31%) | <.001 | 91.9% |
| Before transplant | 38 (11%) | 0 (0%) | 38 (21%) | <.001 | 72.4% |
| After transplant | 20 (6%) | 1 (1%) | 19 (10%) | <.001 | 43.8% |
| Radiotherapy | |||||
| Received at any time point | 175 (51%) | 116 (73%) | 59 (32%) | <.001 | 89.3% |
| Before transplant | 127 (37%) | 85 (53%) | 42 (23%) | <.001 | 66.1% |
| After transplant | 60 (18%) | 40 (25%) | 20 (11%) | .001 | 37.7% |
| Consolidation | 33 (10%) | 24 (15%) | 9 (5%) | .003 | 34.4% |
| For PD | 27 (8%) | 16 (10%) | 11 (6%) | .236 | 14.9% |
| Allogeneic HCT | |||||
| For progression after AHCT | 38 (11%) | 20 (13%) | 18 (10%) | .527 | 8.7% |
Bold values denote statistical significance at the P < .05 level.
P values and standardized mean difference values indicate a comparison between patients undergoing AHCT in 2001 to 2010 vs 2011 to 2020.
Survival, relapse, and NRM by era
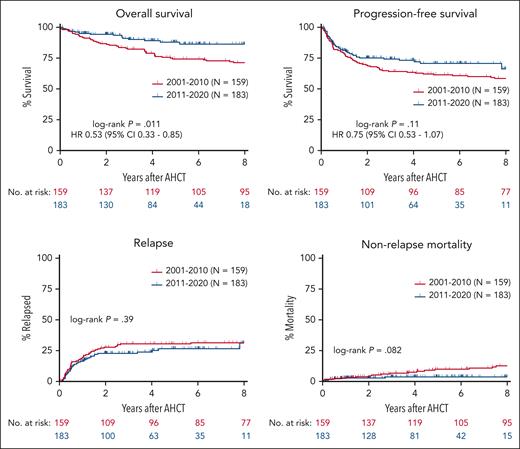
Patient outcomes stratified by treatment era are shown in Figure 1. Median follow-up for the entire cohort was 5.9 years (11.6 years for patients who received transplantation in 2001-2010 and 4.0 years for those in 2011-2020). OS was superior for patients who received transplantation in the modern era (4-year OS 89.1% vs 79.0%, hazard ratio [HR] 0.53; 95% confidence interval [CI], 0.33-0.85; P = .011) without a statistically significant difference in PFS (4-year PFS 73.1% vs 63.3%, HR 0.75; 95% CI, 0.53-1.07; P = .11). Given the higher percentage of patients who received transplantation in CR in the modern era, outcomes were evaluated separately for patients in CR and in partial response (PR) before AHCT (supplemental Figure 1). OS and PFS did not differ by era for CR in patients who received transplantation. However, among patients who received transplantation in PR, OS was superior in the modern era (4-year OS 88.2% vs 74.6%, HR 0.47; 95% CI, 0.24-0.92; P = .042). In both eras, most patients who received transplantation in PR converted to CR after AHCT (60.3% and 52.6% in 2011-2020 and 2001-2010, respectively). The cumulative incidence of relapse did not differ by era (4-year estimates 23.9% vs 30.5%, P = .39). Early NRM was similar between eras (1-year estimates 2.5% vs 2.8%), but there was a trend toward lower NRM beyond 2 years after transplant in the modern era (4-year estimates 3.6% vs 7.0%, P = .082).
Survival, relapse, and nonrelapse mortality by era. Kaplan-Meier estimates of OS, PFS, and the cumulative incidence of relapse and nonrelapse mortality are shown for patients who underwent autologous transplantation in 2001 to 2010 (red) and 2011 to 2020 (blue).
Survival, relapse, and nonrelapse mortality by era. Kaplan-Meier estimates of OS, PFS, and the cumulative incidence of relapse and nonrelapse mortality are shown for patients who underwent autologous transplantation in 2001 to 2010 (red) and 2011 to 2020 (blue).
Postprogression survival by era
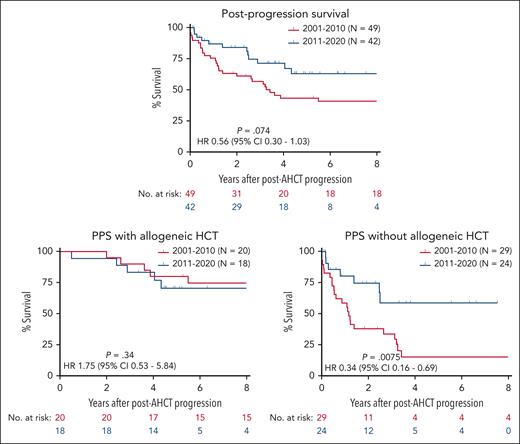
Forty-nine patients (31%) progressed after AHCT in the 2001 to 2010 cohort and 42 (23%) progressed in the 2011 to 2020 cohort. More patients who received transplantation in the modern era received BV (45% vs 31%) and PD-1 inhibitors (45% vs 4%) for relapse after AHCT, whereas the proportion undergoing subsequent allogeneic HCT was similar between eras (43% vs 41%). Among the patients who progressed after AHCT, there was a trend toward longer PPS in the modern era (4-year PPS 71.4% vs 43.3%, HR 0.56; 95% CI, 0.30-1.03; P = .074) (Figure 2). PPS was similar between eras for patients who underwent subsequent allogeneic HCT (4-year PPS of 83.3% vs 80.0%, P = .20). However, among patients who did not undergo allogeneic HCT, PPS was superior in the modern era (4-year PPS 58.8% vs 15.2%, HR 0.34; 95% CI, 0.16-0.69; P = .0075).
Postprogression survival by era. Kaplan-Meier estimates of postprogression survival are shown for patients who relapsed or progressed after autologous transplantation done in 2001 to 2010 (red) and 2011 to 2020 (blue). The bottom panels show postprogression survival for patients who underwent subsequent allogeneic transplantation (left) or no allogeneic transplantation (right).
Postprogression survival by era. Kaplan-Meier estimates of postprogression survival are shown for patients who relapsed or progressed after autologous transplantation done in 2001 to 2010 (red) and 2011 to 2020 (blue). The bottom panels show postprogression survival for patients who underwent subsequent allogeneic transplantation (left) or no allogeneic transplantation (right).
Causes of death and treatment-related toxicities
Causes of death and notable treatment-related toxicities are summarized in Table 3. Most patients died from PD in both eras. Leading causes of NRM included infections/sepsis, cardiovascular disease, and second cancers. Nineteen patients (5.6%) developed second cancers, which are summarized in supplemental Table 2. The most common second cancers included therapy-related MDS or acute leukemia and bone or soft tissue sarcomas, occurring in 1.8% and 0.9% of patients, respectively. Pneumonitis requiring corticosteroids within 100 days of AHCT occurred in 14% of patients and was attributed to BCNU in most cases. Among the 27 patients receiving PD-1 inhibitor-based salvage regimens before AHCT, posttransplant pneumonitis occurred in 5 patients (19%). Other clinically significant immune-related adverse events included thyroiditis requiring thyroid hormone replacement (N = 4), inflammatory arthritis (N = 3), new onset type 1 diabetes mellitus (N = 2), and inflammatory colitis (N = 2). Hepatic VOD was rare occurring in <1% of patients.
Causes of death and posttransplant toxicities by era
| . | All patients (N = 342) . | AHCT in 2001-2010 (N = 159) . | AHCT in 2011-2020 (N = 183) . |
|---|---|---|---|
| All-cause mortality | |||
| Total number of deaths | 74 | 55 | 19 |
| Median follow-up after AHCT | 5.9 y | 11.6 y | 4.0 y |
| 4-y OS estimate | 83.8% | 79.0% | 89.1% |
| Causes of death | |||
| PD, N (%)∗ | 33 (45%) | 23 (42%) | 10 (53%) |
| Infection/sepsis | 10 (14%) | 8 (15%) | 2 (11%) |
| Cardiovascular disease | 7 (9%) | 5 (9%) | 2 (11%) |
| Respiratory failure | 5 (7%) | 3 (5%) | 2 (11%) |
| Therapy-related MDS/leukemia | 3 (4%) | 2 (4%) | 1 (5%) |
| Other second cancer | 6 (8%) | 5 (9%) | 1 (5%) |
| Hepatic VOD | 2 (3%) | 1 (2%) | 1 (5%) |
| GVHD (post–allogeneic HCT) | 2 (3%) | 2 (4%) | 0 (0%) |
| Unknown/other | 6 (8%) | 6 (11%) | 0 (0%) |
| Post transplant toxicities | |||
| Pneumonitis, N (%)† | 49 (14%) | 23 (14%) | 26 (14%) |
| Therapy-related MDS/leukemia | 6 (2%) | 4 (3%) | 2 (1%) |
| Hepatic VOD | 3 (<1%) | 2 (1%) | 1 (<1%) |
| . | All patients (N = 342) . | AHCT in 2001-2010 (N = 159) . | AHCT in 2011-2020 (N = 183) . |
|---|---|---|---|
| All-cause mortality | |||
| Total number of deaths | 74 | 55 | 19 |
| Median follow-up after AHCT | 5.9 y | 11.6 y | 4.0 y |
| 4-y OS estimate | 83.8% | 79.0% | 89.1% |
| Causes of death | |||
| PD, N (%)∗ | 33 (45%) | 23 (42%) | 10 (53%) |
| Infection/sepsis | 10 (14%) | 8 (15%) | 2 (11%) |
| Cardiovascular disease | 7 (9%) | 5 (9%) | 2 (11%) |
| Respiratory failure | 5 (7%) | 3 (5%) | 2 (11%) |
| Therapy-related MDS/leukemia | 3 (4%) | 2 (4%) | 1 (5%) |
| Other second cancer | 6 (8%) | 5 (9%) | 1 (5%) |
| Hepatic VOD | 2 (3%) | 1 (2%) | 1 (5%) |
| GVHD (post–allogeneic HCT) | 2 (3%) | 2 (4%) | 0 (0%) |
| Unknown/other | 6 (8%) | 6 (11%) | 0 (0%) |
| Post transplant toxicities | |||
| Pneumonitis, N (%)† | 49 (14%) | 23 (14%) | 26 (14%) |
| Therapy-related MDS/leukemia | 6 (2%) | 4 (3%) | 2 (1%) |
| Hepatic VOD | 3 (<1%) | 2 (1%) | 1 (<1%) |
NA, not applicable.
Percentages are based on the total number of deaths.
Percentages are based on the total number of patients.
Prognostic factors for PFS and OS
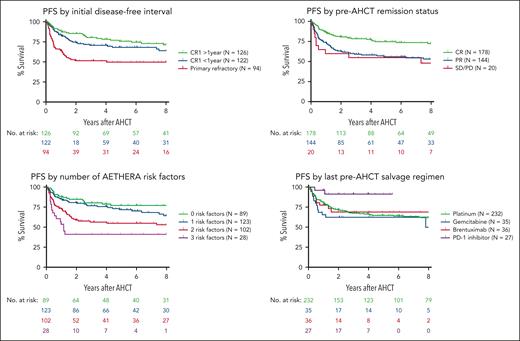
In univariable analyses, the initial disease-free interval, remission status before AHCT, number of AETHERA risk factors, and type of salvage therapy before AHCT were prognostic for PFS (Figure 3). For patients with CR1 >1 year, CR1 <1 year, and primary refractory disease, the 4-year PFS estimates were 78.0%, 70.5%, and 51.3%, respectively (P = .0002). For patients in CR, PR, or with stable disease (SD)/PD before AHCT, the 4-year PFS estimates were 77.7%, 58.3%, and 55.0%, respectively (P = .0008). The number of AETHERA risk factors (0, 1, 2, or 3) was also strongly prognostic with 4-year PFS estimates of 78.9%, 75.8%, 55.0%, and 41.2%, respectively (P < .0001). Outcomes were also compared based on the type of salvage regimen administered immediately before AHCT including platinum, gemcitabine, BV, and PD-1 inhibitor-based regimens. PFS was significantly higher among patients receiving PD-1 inhibitor-based regimens compared with those receiving platinum-based chemotherapy (91.3% vs 66.4% at 4 years, P = .026). Patients receiving gemcitabine or BV-based regimens had similar PFS to those receiving platinum-based chemotherapy.
Clinical factors impacting PFS. Kaplan-Meier estimates of PFS are shown for the entire cohort stratified by the initial disease-free interval after frontline therapy (top left panel), remission status before autologous transplantation (top right panel), number of adverse risk factors as defined in the AETHERA trial (bottom left panel), or the type of salvage therapy administered immediately before autologous transplantation (bottom right panel).
Clinical factors impacting PFS. Kaplan-Meier estimates of PFS are shown for the entire cohort stratified by the initial disease-free interval after frontline therapy (top left panel), remission status before autologous transplantation (top right panel), number of adverse risk factors as defined in the AETHERA trial (bottom left panel), or the type of salvage therapy administered immediately before autologous transplantation (bottom right panel).
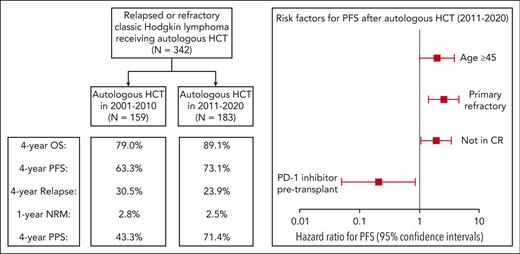
Cox proportional hazards models for PFS and OS were developed separately for each era. In multivariable analysis for patients who underwent transplantation from 2001 to 2010, the variables with a significant impact on PFS included primary refractory disease (HR 2.97; 95% CI, 1.35-6.54; P = .0068) and receipt of ≥2 salvage regimens (HR 4.55, 95%; CI, 2.10-9.89; P = .00013) (supplemental Table 3). Age ≥45 years was associated with inferior OS (HR 2.06; 95% CI, 1.16-3.65; P = .013). In multivariable analysis for patients who received transplantation in the modern era, the following 4 variables had a significant impact on PFS: age ≥45 years (HR 1.96; 95% CI, 1.01-3.84; P = .049), primary refractory disease (HR 2.58; 95% CI, 1.44-4.63; P = .0014), lack of CR before AHCT (HR 1.93; 95% CI, 1.06-3.50; P = .031), and receipt of a PD-1 inhibitor-based regimen before AHCT (HR 0.21; 95% CI, 0.05-0.80; P = .030) (Table 4). In the modern era, extranodal disease at relapse was associated with inferior OS (HR 3.12; 95% CI, 1.25-7.77; P = .014).
Multivariable analysis for PFS in the modern era
| Variable . | N (%) . | Hazard ratio (95% CI) . | P value . |
|---|---|---|---|
| Age <45 y | 146 (80%) | Reference | — |
| Age ≥45 y | 37 (20%) | 1.96 (1.01-3.84) | .049 |
| Relapsed | 133 (73%) | Reference | — |
| Refractory | 50 (27%) | 2.58 (1.44-4.63) | .0014 |
| Metabolic CR | 111 (61%) | Reference | — |
| Not in CR | 72 (39%) | 1.93 (1.06-3.50) | .031 |
| Chemotherapy pre-AHCT∗ | 156 (85%) | Reference | — |
| PD-1 inhibitor pre-AHCT† | 27 (15%) | 0.21 (0.05-0.86) | .030 |
| Variable . | N (%) . | Hazard ratio (95% CI) . | P value . |
|---|---|---|---|
| Age <45 y | 146 (80%) | Reference | — |
| Age ≥45 y | 37 (20%) | 1.96 (1.01-3.84) | .049 |
| Relapsed | 133 (73%) | Reference | — |
| Refractory | 50 (27%) | 2.58 (1.44-4.63) | .0014 |
| Metabolic CR | 111 (61%) | Reference | — |
| Not in CR | 72 (39%) | 1.93 (1.06-3.50) | .031 |
| Chemotherapy pre-AHCT∗ | 156 (85%) | Reference | — |
| PD-1 inhibitor pre-AHCT† | 27 (15%) | 0.21 (0.05-0.86) | .030 |
Bold values denote statistical significance at the P < .05 level.
N, number of patients.
Chemotherapy includes BV-based regimens but excludes regimens incorporating a PD-1 inhibitor.
Includes patients receiving a PD-1 inhibitor-based regimen as the last salvage therapy before AHCT.
Discussion
The treatment landscape of R/R cHL has evolved significantly over the past decade, now with numerous options for salvage therapy before AHCT along with new effective options for posttransplant consolidation and relapse. Our study provides a new benchmark for outcomes for patients with R/R cHL who received transplantation in the modern era with encouraging 4-year PFS and OS estimates of 73% and 89%, respectively. We demonstrate significant improvement in OS after AHCT using the same conditioning over the past decade, reflecting several factors including (1) more effective salvage regimens, particularly those incorporating immunotherapy, allowing for better disease control before AHCT, (2) more effective therapies for posttransplant relapse, and (3) a trend toward lower NRM beyond 2 years posttransplant.
Our study adds to the growing literature demonstrating particularly excellent outcomes with immunotherapy-based salvage regimens before AHCT.24-27 Recent studies suggest that PD-1 inhibitors can sensitize cHL to subsequent cytotoxic chemotherapy, supporting the use of immunotherapy in the pre-AHCT setting.41,42 In a multicenter retrospective study of patients with multiply R/R cHL who underwent AHCT after a PD-1 inhibitor-based regimen, 18-month PFS was 81% with response to PD-1 blockade having a greater prognostic impact on PFS than prior chemosensitivity.43 Another recent multicenter retrospective study demonstrated superior PFS for patients receiving BV/nivolumab or a PD-1 inhibitor alone before AHCT compared with those receiving platinum-based chemotherapy in univariable analysis.44 Our data support this observation with superior 4-year PFS of 91% vs 66% with pre-AHCT immunotherapy vs platinum-based chemotherapy, respectively. Receipt of pre-AHCT immunotherapy vs chemotherapy was also associated with superior PFS in multivariable analysis with a hazard ratio of 0.21 (95% CI, 0.05-0.80). These data are hypothesis generating and support consideration of PD-1 inhibitor-based regimens in the second line setting, particularly for patients with primary refractory disease. A phase 3 trial (ECOG-ACRIN 4211) is currently planned to compare a chemotherapy arm (investigator’s choice of ICE, GVD, or BV/bendamustine) with pembrolizumab combined with chemotherapy to definitively assess the role of second line immunotherapy in a prospective randomized trial.
Historically, patients who relapse after AHCT have poor outcomes with median OS 25 months and particularly poor outcomes for those with early relapse within 1 year of AHCT.45-48 We demonstrate improving outcomes for this high-risk population with 4-year PPS increasing from 43% to 71% in the modern era, and many patients achieving a durable CR with BV, PD-1 inhibitors, and/or allogeneic HCT. Recent studies including our own single center experience have demonstrated the utility of reduced intensity or nonmyeloablative allogeneic HCT to produce durable remissions for patients progressing after AHCT.39,40,49,50 Although the use of PD-1 inhibitors before allogeneic HCT can precipitate acute graft-versus-host disease (GVHD), recent studies indicate that posttransplant cyclophosphamide can mitigate this risk.49,50 A 6-week washout period between PD-1 blockade and allogeneic HCT is also recommended to mitigate the risk of GVHD.51
Regarding causes of death and posttransplant toxicities in the modern era, most patients died from PD with low NRM (2.5% at 1 year) and low rates of therapy-related MDS/leukemia (1%) and hepatic VOD (<1%). Pneumonitis requiring corticosteroids occurred in 14% of patients in both eras and in 19% of patients receiving PD-1 inhibitor-based regimens pre-AHCT. Recent studies suggest a higher incidence of engraftment syndrome in patients receiving PD-1 inhibitors before AHCT.25,52 We did not observe clinically significant engraftment syndrome among patients receiving pretransplant PD-1 inhibitors in this study, but clinicians should remain vigilant for this potential toxicity and consider early intervention with corticosteroids for unexplained fevers, rash, diarrhea, and/or elevated transaminases in the early posttransplant period. Although early NRM did not differ by era, we observed a trend toward lower NRM beyond 2 years posttransplant in the modern era. This observation may relate to various factors including changes in chemotherapy and/or radiotherapy practice patterns along with better supportive care. However, longer follow-up is needed to accurately compare late NRM and late effects of therapy including cardiovascular disease and second cancers.
Regarding prognostic factors in the modern era, our study demonstrated inferior PFS after AHCT for patients aged ≥45 years, with primary refractory disease, and not in metabolic CR at the time of AHCT. Although patients who received transplantation in metabolic CR had superior 4-year PFS of 78%, patients who received transplantation in PR had acceptable outcomes in this study with the majority converting to CR posttransplant and 4-year PFS is 58% in this cohort. These outcomes compare favorably to prior studies and support consideration of AHCT for these patients, particularly for those with a good PR after multiple salvage regimens.6 Although the number of salvage regimens before AHCT was strongly prognostic for PFS for patients who received transplantation between 2001 and 2010, the same adverse risk was not observed in the modern era, likely related to newer salvage regimens with alternative mechanisms of action, particularly immunotherapy. Consistent with a recent report, patients in our study with multiply R/R cHL after doxorubicin and platinum-based regimens were effectively salvaged with immunotherapy and AHCT with excellent PFS despite lack of chemosensitivity.43 Our study also validated the prognostic impact of the AETHERA risk factors on PFS, with extranodal disease at relapse also associated with inferior OS in multivariable analysis.
In conclusion, we demonstrate improved outcomes for R/R cHL after AHCT in the modern era after the approval of BV and the PD-1 inhibitors. We demonstrate particularly excellent outcomes with PD-1 inhibitor-based regimens pre-AHCT, supporting a randomized phase 3 trial comparing chemotherapy vs immunotherapy-based salvage regimens in the second line setting. Important limitations include the single center nature of the study, evolving response criteria over the past 2 decades, and potential introduction of selection bias as some patients were treated on clinical trials with BV/bendamustine or BV/nivolumab with or without ipilimumab. Expansion of this study through the Center for International Blood and Marrow Transplant Research is planned to further define changes in outcomes by treatment era and to validate prognostic factors for PFS and OS in the era of novel agents.
Acknowledgments
The authors thank Jonathon Cohen, Babis Andreadis, Wei Ai, Madhav Seshadri, and Bita Fakhri for their helpful comments and review of the manuscript.
Authorship
Contribution: M.A.S. wrote the manuscript; J.S.T. conducted statistical analyses; M.A.S., R.A.S., J.S.T., Y.L., R.L., R.H.A., and S.A. conceived the study and were in charge of overall direction and planning; and all authors provided important feedback and reviewed the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sally Arai, Division of Blood and Marrow Transplantation, Department of Medicine, Stanford University, 300 Pasteur Dr, Stanford, CA 94305; e-mail: sarai1@stanford.edu.
References
Author notes
Data are available on request from the corresponding author, Sally Arai (sarai1@stanford.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

Comments
Improving the Outcome of Relapsed/Refractory Classical Hodgkin's Lymphoma with the Advent of New Drugs
We read with great interest the manuscript by Spinner et al. (1) reporting improved outcomes for relapsed/refractory (R/R) classical Hodgkin lymphoma (HL) after autologous stem cell transplantation (ASCT) in the era of novel agents. We would like to congratulate the authors for this report confirming that brentuximab vedotin (BV) and checkpoint inhibitors (CPI) have changed the landscape of R/R HL (2). In this single institution study (1), the authors have clearly demonstrated that overall survival (OS) was superior in 183 patients with R/R HL who underwent ASCT between 2011-2020 as compared to that of 159 patients transplanted between 2001 and 2010 (4-year estimates: 89.1% vs 79.0%, p=0.011). Conversely, for 91 patients who progressed after ASCT, improvement in post progression survival in recent years did not reach statistical significance, likely reflecting the small sample size.
Salvage chemotherapy and ASCT results in the cure of around 50% of patients with HL failing first-line therapy (3). Historically, patients who progressed after ASCT had a poor outcome, with a median OS of around 1-2 years (4). To assess the most recent trends in survival after post-ASCT relapse, we conducted within the Lymphoma working party of the European Society of Blood and Marrow Transplantation (EBMT), a retrospective, registry-based, multicenter study that included 1781 adult patients with relapsed HL after ASCT, over a period of 12 years (from 2006 to 2017) (5). We found a significant improvement in the 4-year OS after relapse, which increased from 32% in patients who relapsed between 2006 and 2008 to 63% in those who relapsed between 2015 and 2017 (p=0.001) (5). Notably, the survival improvement over time was predominantly observed in patients who had an early relapse (within 12 months) after ASCT. This makes our findings even more impressive because, typically, shorter survival is associated with early relapse. This effect is likely due to the greater efficacy and novel mechanism of action of the new monoclonal antibodies (2). Our study represents the largest analysis to date assessing the outcomes and characteristics of patients with relapsed HL after ASCT, and our findings show that the survival of these patients has significantly improved over time. These large-scale real-world data can serve as a benchmark for future studies in this setting.
References
1- Spinner MA, Sica RA, Tamaresis JS, Lu Y, Chang C, Lowsky R, Frank MJ, Johnston LJ, Miklos DB, Muffly L, Negrin RS, Rezvani AR, Shiraz P, Shizuru JA, Weng WK, Binkley MS, Hoppe RT, Advani RH, Arai S. Improved outcomes for relapsed/refractory Hodgkin lymphoma after autologous transplantation in the era of novel agents. Blood. 2023 Mar 1:blood.2022018827.
2- Mohty R, Dulery R, Bazarbachi AH, Savani M, Hamed RA, Bazarbachi A, Mohty M. Latest advances in the management of classical Hodgkin lymphoma: the era of novel therapies. Blood Cancer J. 2021 Jul 9;11(7):126.
3- Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359:2065-71.
4- Moskowitz AJ, Perales MA, Kewalramani T, Yahalom J, Castro-Malaspina H, Zhang Z, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009;146:158-63.
5- Bazarbachi A, Boumendil A, Finel H, Khvedelidze I, Romejko-Jarosinska J, Tanase A, Akhtar S, Ben Othman T, Ma'koseh M, Afanasyev B, Cheikh J, Briones J, Gülbas Z, Hamladji RM, Elverdi T, Blaise D, Martínez C, Alma E, Halaburda K, Sousa AB, Glass B, Robinson S, Montoto S, Sureda A. The outcome of patients with Hodgkin lymphoma and early relapse after autologous stem cell transplant has improved in recent years. Leukemia. 2022 Jun;36(6):1646-1653.