Key Points
Cumulative recording of proliferation and differentiation in situ shows that primitive HSCs are not activated by inflammation or blood loss.
Abstract
Hematopoietic stem cells (HSCs) are the ultimate source of blood and immune cells, and transplantation reveals their unique potential to regenerate all blood lineages lifelong. HSCs are considered a quiescent reserve population under homeostatic conditions, which can be rapidly activated by perturbations to fuel blood regeneration. In accordance with this concept, inflammation and loss of blood cells were reported to stimulate the proliferation of HSCs, which is associated with a decline in their transplantation potential. To investigate the contribution of primitive HSCs to the hematopoietic stress response in the native environment, we use fate mapping and proliferation tracking mouse models. Although primitive HSCs were robustly activated by severe myeloablation, they did not contribute to the regeneration of mature blood cells in response to prototypic hematopoietic emergencies, such as acute inflammation or blood loss. Even chronic inflammatory stimulation, which triggered vigorous HSC proliferation, only resulted in a weak contribution of HSCs to mature blood cell production. Thus, our data demonstrate that primitive HSCs do not participate in the hematopoietic recovery from common perturbations and call for the reevaluation of the concept of HSC-driven stress responses.
Introduction
The hematopoietic system continuously generates enormous numbers of mature blood cells,1 and its output can be substantially accelerated by blood loss or infections. Hematopoietic stem cells (HSCs), which reside at the top of the hematopoietic hierarchy, give rise to mature blood cells via diverse progenitor intermediates. Upon transplantation, only this somatic stem cell population is able to reconstitute all blood cell lineages lifelong, which is the basis for therapeutic or experimental HSC transplantation.2,3 Although hematopoiesis after transplantation is driven by a few HSC clones,4-6 fate tracking of native hematopoiesis in adult mice revealed only rare but continuous and polyclonal HSC differentiation.6-9 Moreover, murine HSCs remain largely quiescent in adult steady-state hematopoiesis.10,11 A recent study revealed the enormous regenerative capacity of progenitor populations, which despite lack of long-term transplantation potential, contribute lifelong to native hematopoiesis.12 Models of HSC depletion further emphasize the robustness of blood formation in the absence of HSCs.13,14 Currently, HSCs are perceived as a quiescent reserve population, which is essential to boost blood cell output in situations of acute demand.15-17 In response to infection or blood loss, HSCs are thought to be activated via pattern recognition and cytokine receptors and participate in hematopoietic stress responses, ultimately resulting in a gradual loss of their transplantation potential (as reviewed previously18).
Here, we used mouse models of fate and proliferation tracking to study HSC activity in response to prototypic hematopoietic perturbations. We found that acute inflammatory signaling resulting in massively enhanced blood cell output was reflected in little, if any, increase in the contribution of primitive HSCs. Similarly, hematopoietic stress caused by the extensive depletion of mature blood cells did not trigger accelerated stem cell proliferation or differentiation. Only pharmacological or irradiation-induced severe depletion of hematopoietic progenitors triggered a robust plus in HSC activity. We thus argue against primitive HSCs constituting a reserve population that boosts hematopoiesis in response to acute inflammation or blood cell loss and propose that they may only be needed in situations of extreme distress.
Methods
Mice
All animal experiments were performed in accordance with institutional guidelines and the German Law for Protection of Animals, approved by the Landesdirektion Dresden (TVV 91/2017). Mice were housed in individually ventilated cages under a specific pathogen-free environment at the Experimental Center of the Medical Faculty, TU Dresden. R26rtTA/rtTA/Col1A1H2B-GFP/H2B-GFP19 mice were induced with doxycycline (DOX, 2 g/kg) via chow (Ssniff Spezialdiäten) for 1 to 2 weeks ad libitum. Fgd5ZsGreen:CreERT2/wt/R26LSL-tdRFP/LSL-tdRFP20,21 mice were induced by oral gavage of tamoxifen (TAM, 0.2 mg/g body weight [BW]) twice 3 to 4 days apart. Fate mapping and transplantation data from Fgd5ZsGreen:CreERT2/R26LSL-tdRFPmice shown in Figure 1C-D and supplemental Figure 2, which is available on the Blood website, and 5-fluorouracil (5-FU) perturbation data shown in Figure 1G-P and supplemental Figure 3C-G was previously published.22 5-FU (150 μg/g BW; Applichem) was administered via IV injection. Whole-body irradiation was performed using an Yxlon Maxi Shot X-ray tube at a dose of 2 Gy. Recombinant human granulocyte-colony stimulating factor (G-CSF; filgrastim) (0.3 μg/g BW; Neupogen, Amgen) was administered by subcutaneous (SC) injection on 5 consecutive days. Polyinosinic:polycytidylic acid (pI:C; 5 μg/g BW; Invivogen) was administered via intraperitoneal (IP) injection either 1 (“single” protocol) or 8 times (“repetitive” protocol, 2 injections per week). Lipopolysaccharide (LPS) (from Escherichia coli O111:B4, Invivogen; 35 μg per mouse in 2 out of 4 experiments and adjusted to 1.4 μg/g BW in the remaining experiments) was IP injected. Rabbit antimouse thrombocyte serum (50 to 70 μL per mouse in a total volume of 150 μL; effective dosage was pre-titrated for each batch of serum; WAK-Chemie Medical GmbH) or mock serum was administered IP either once (“acute” protocol) or 7 times (“chronic” protocol, injection every other day). Phenylhydrazine (PHZ, 40 μg/g BW; Sigma-Aldrich) was IP injected twice on 2 consecutive days.
Fate mapping and proliferation tracking of perturbed hematopoiesis. (A) How perturbations of hematopoiesis alter contribution of a primitive HSC subset will be studied by fate mapping (red dots and arrow) and proliferation tracking (green shades and arrow). (B) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP mouse model for fate mapping of HSCs. (C-D) RFP labeling of HSPCs isolated from TAM-induced Fgd5ZsGreen:CreERT2/R26LSL-tdRFP mice. Data from Morcos et al.22 (C) Initial labeling frequencies of BM HSPCs 10 days after induction. (D) Labeling of total HSCs (black line) relative to ES HSCs (dotted line, 8-20 mice per time point). (E) R26rtTA/Col1A1H2B-GFP mouse model for proliferation tracking of HSPCs. (F) Representative H2B-GFP histograms of ES HSCs isolated from 5-FU–perturbed and control mice (supplemental Figure 3C-E). (G-M) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP mice were TAM-induced and perturbed with either 2 Gy γ-radiation (n = 4), 5-FU (n = 5) or left untreated (n = 5). Experiment scheme (G), PB lymphocyte count 7 days after irradiation (H), PB reticulocyte numbers 7 days after 5-FU (I), and ratios of relative BM compartment sizes (% cells among total lin− BM cells) between perturbed and control (dotted line) animals (J-K) are shown. (L) Percentages of RFP+ ES HSCs. (M) Fraction of RFP-labeled cells relative to ES HSCs (significant differences between untreated and either 5-FU–perturbed or irradiated mice was calculated, all comparisons between unperturbed and irradiated mice are not significant). (N-O) R26rtTA/Col1A1H2B-GFP animals were doxycycline (DOX)–pulsed and exposed to either 2 Gy (n = 5), 5-FU (n = 5), or left untreated (n = 6). Experiment scheme (N) and numbers of additional divisions in response to perturbation (O) are shown (supplemental Figure 3C-E). (P) Transformation of data shown in panel M to visualize the net effects of myeloablation on label propagation. Mean (dark gray line) and variance (standard deviation [SD], shaded area) of control mice (n = 41 from 8 independent experiments) are shown (supplemental Figure 3F-H). CMP, common myeloid progenitor; Ery, erythrocyte; MkP, megakaryocyte progenitor; Neut, neutrophil; Plt, platelet; PMN, polymorphonuclear cell.
Fate mapping and proliferation tracking of perturbed hematopoiesis. (A) How perturbations of hematopoiesis alter contribution of a primitive HSC subset will be studied by fate mapping (red dots and arrow) and proliferation tracking (green shades and arrow). (B) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP mouse model for fate mapping of HSCs. (C-D) RFP labeling of HSPCs isolated from TAM-induced Fgd5ZsGreen:CreERT2/R26LSL-tdRFP mice. Data from Morcos et al.22 (C) Initial labeling frequencies of BM HSPCs 10 days after induction. (D) Labeling of total HSCs (black line) relative to ES HSCs (dotted line, 8-20 mice per time point). (E) R26rtTA/Col1A1H2B-GFP mouse model for proliferation tracking of HSPCs. (F) Representative H2B-GFP histograms of ES HSCs isolated from 5-FU–perturbed and control mice (supplemental Figure 3C-E). (G-M) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP mice were TAM-induced and perturbed with either 2 Gy γ-radiation (n = 4), 5-FU (n = 5) or left untreated (n = 5). Experiment scheme (G), PB lymphocyte count 7 days after irradiation (H), PB reticulocyte numbers 7 days after 5-FU (I), and ratios of relative BM compartment sizes (% cells among total lin− BM cells) between perturbed and control (dotted line) animals (J-K) are shown. (L) Percentages of RFP+ ES HSCs. (M) Fraction of RFP-labeled cells relative to ES HSCs (significant differences between untreated and either 5-FU–perturbed or irradiated mice was calculated, all comparisons between unperturbed and irradiated mice are not significant). (N-O) R26rtTA/Col1A1H2B-GFP animals were doxycycline (DOX)–pulsed and exposed to either 2 Gy (n = 5), 5-FU (n = 5), or left untreated (n = 6). Experiment scheme (N) and numbers of additional divisions in response to perturbation (O) are shown (supplemental Figure 3C-E). (P) Transformation of data shown in panel M to visualize the net effects of myeloablation on label propagation. Mean (dark gray line) and variance (standard deviation [SD], shaded area) of control mice (n = 41 from 8 independent experiments) are shown (supplemental Figure 3F-H). CMP, common myeloid progenitor; Ery, erythrocyte; MkP, megakaryocyte progenitor; Neut, neutrophil; Plt, platelet; PMN, polymorphonuclear cell.
Cell preparation
Whole bone marrow (BM) cells were extracted by crushing long bones with mortar and pestle using phosphate-buffered saline (PBS), 2% fetal calf serum (FCS), and 2 mM EDTA and filtering twice after erythrocyte lysis in hypotonic NH4Cl buffer. Hematopoietic lineage-positive (lin+) cells were depleted from samples with the lineage cell depletion kit (Miltenyi Biotec).
Peripheral blood (PB) was drawn into glass capillaries by retro-bulbar puncture directly into EDTA-coated tubes (Sarstedt). For the identification of red fluorescent protein–positive (RFP+) platelets and erythrocytes, 1 to 2 μL of whole blood was mixed with PBS, 2% FCS, and 2 mM EDTA and incubated with monoclonal antibodies. For PB leukocyte analysis, erythrocyte lysis in hypotonic NH4Cl buffer was performed twice for 5 minutes before cells were stained with monoclonal antibodies. For hemograms, blood was diluted 1:5 in an isotonic NaCl solution and analyzed on an XT-2000i Vet analyzer (Sysmex).
Spleen cell suspensions were prepared by mashing spleens using PBS, 2% FCS, and 2 mM EDTA through a 100 μm mesh and subsequent erythrocyte lysis in hypotonic NH4Cl buffer.
Peritoneal cells were obtained by flushing the peritoneal cavity with 4 mL PBS, 2% FCS, and 2 mM EDTA and filtering the aspirate through a 100 μm mesh.
Flow cytometry
Cell suspensions from BM, spleen, peritoneal lavage, or PB samples were stained with antibodies (supplemental Table 1) in PBS, 2% FCS, and 2 mM EDTA for 30 to 40 minutes, washed twice, and analyzed on either FACS Canto, ARIA II SORP, Aria II, ARIA III (all from BD Biosciences, Heidelberg, Germany) or MACSquant (Miltenyi Biotec) flow cytometers. FlowJo V9.9 and V10 software (Tree Star) was used for data analysis, and gates (supplemental Figure 1A-D) were set using Fluorescence-Minus-One controls. Gating of RFP+ cells was guided by negative (Cre− animal) and positive (germ line STOP-excised R26tdRFP mouse with ubiquitous RFP expression) controls. Absolute numbers of lin− BM cells were determined using a MACSquant flow cytometer.
Measurement of PB cytokine concentrations
PB samples were centrifuged (10 minutes, 1000g) within 30 minutes of blood collection. Plasma supernatants were frozen at −20 °C until analysis. Plasma cytokine concentration was measured using the LEGENDplex Mouse Inflammation Panel (13-plex, BioLegend), according to the manufacturer’s instructions. Flow cytometric analysis was performed on a MACSquant flow cytometer; data analysis was performed by the LEGENDplex Data Analysis Software Suite.
Data normalization and statistics
RFP label propagation data from BM and PB populations of induced Fgd5ZsGreen:CreERT2/R26LSL-tdRFP mice was normalized to the label in HSCs with a high surface expression of CD201 (EPCR) and Sca-1 (ES HSCs) of the same animal. The percentage of label increase caused by the stress response was calculated by subtracting the normalized arithmetic mean of label in control animals from the normalized label in a treated specimen (supplemental Figure 3F). The percentage of label increase was summarized in radar charts (Figures 1P, 2P, and 3M). Gray shaded areas provide an estimate of data variation (standard deviation calculated from unperturbed control animals, n = 41 from 8 experiments (supplemental Figure 3G-H).
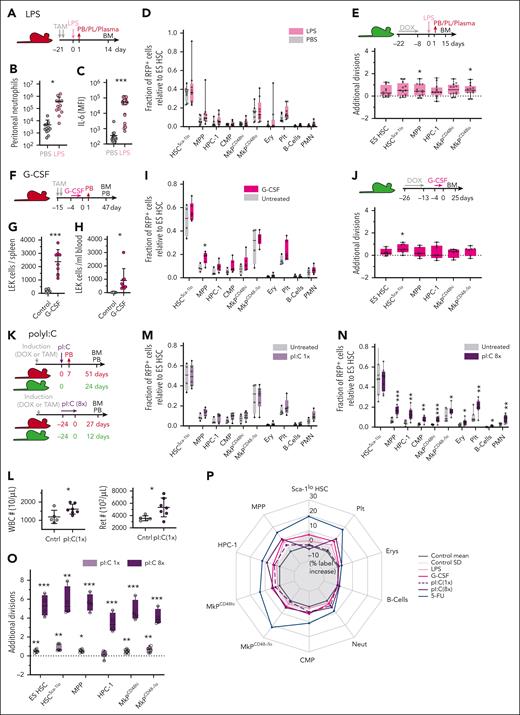
Limited contribution of HSCs to emergency myelopoiesis and type I IFN stimulation. (A-D) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP mice were TAM-induced and injected with LPS (n = 8) or PBS (n = 12). (B) Neutrophil count in the peritoneal cavity 24 hours after LPS/PBS injection. (C) Plasma levels of interleukin 6 (IL-6) 90 minutes after LPS/PBS injection. (D) Fraction of RFP-labeled cells (relative to ES HSCs) between LPS and saline-treated animals. Data from 2 independent experiments. (E) Labeled R26rtTA/Col1A1H2B-GFP animals were IP injected with LPS (n = 14) or PBS (n = 13) and the average number of additional divisions in response to LPS was determined. Data from 2 independent experiments. (F-J) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP (in panels F and I, n = 4-6 per condition) and R26rtTA/Col1A1H2B-GFP (in panels G, H, and J, n = 4-7 per condition) mice were subcutaneously injected with G-CSF or PBS for 5 consecutive days. Numbers of LEK HSPCs in spleen (G) or PB (H) of R26rtTA/Col1A1H2B-GFP animals on d6. (I) Fraction of RFP-labeled cells (relative to ES HSCs). (J) Average number of additional divisions in response to G-CSF. (K-O) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP (in panels K-N, n = 4-8 per condition) and R26rtTA/Col1A1H2B-GFP (in panel O, n = 4-7 per condition) mice were IP injected with pI:C or PBS following either a single (once) or repetitive (8 times) administration protocol. (L) PB leukocyte and reticulocyte numbers 7 days after 1 time pI:C. (M-N) Fraction of RFP-labeled cells (relative to ES HSCs). (O) Average number of additional divisions in response to pI:C. (P) Net effects of LPS, G-CSF, or pI:C perturbation on RFP label propagation (% label increase relative to ES HSCs; transformation and display of data as in Figure 1P). MFI, mean fluorescence intensity; PL, peritoneal lavage.
Limited contribution of HSCs to emergency myelopoiesis and type I IFN stimulation. (A-D) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP mice were TAM-induced and injected with LPS (n = 8) or PBS (n = 12). (B) Neutrophil count in the peritoneal cavity 24 hours after LPS/PBS injection. (C) Plasma levels of interleukin 6 (IL-6) 90 minutes after LPS/PBS injection. (D) Fraction of RFP-labeled cells (relative to ES HSCs) between LPS and saline-treated animals. Data from 2 independent experiments. (E) Labeled R26rtTA/Col1A1H2B-GFP animals were IP injected with LPS (n = 14) or PBS (n = 13) and the average number of additional divisions in response to LPS was determined. Data from 2 independent experiments. (F-J) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP (in panels F and I, n = 4-6 per condition) and R26rtTA/Col1A1H2B-GFP (in panels G, H, and J, n = 4-7 per condition) mice were subcutaneously injected with G-CSF or PBS for 5 consecutive days. Numbers of LEK HSPCs in spleen (G) or PB (H) of R26rtTA/Col1A1H2B-GFP animals on d6. (I) Fraction of RFP-labeled cells (relative to ES HSCs). (J) Average number of additional divisions in response to G-CSF. (K-O) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP (in panels K-N, n = 4-8 per condition) and R26rtTA/Col1A1H2B-GFP (in panel O, n = 4-7 per condition) mice were IP injected with pI:C or PBS following either a single (once) or repetitive (8 times) administration protocol. (L) PB leukocyte and reticulocyte numbers 7 days after 1 time pI:C. (M-N) Fraction of RFP-labeled cells (relative to ES HSCs). (O) Average number of additional divisions in response to pI:C. (P) Net effects of LPS, G-CSF, or pI:C perturbation on RFP label propagation (% label increase relative to ES HSCs; transformation and display of data as in Figure 1P). MFI, mean fluorescence intensity; PL, peritoneal lavage.
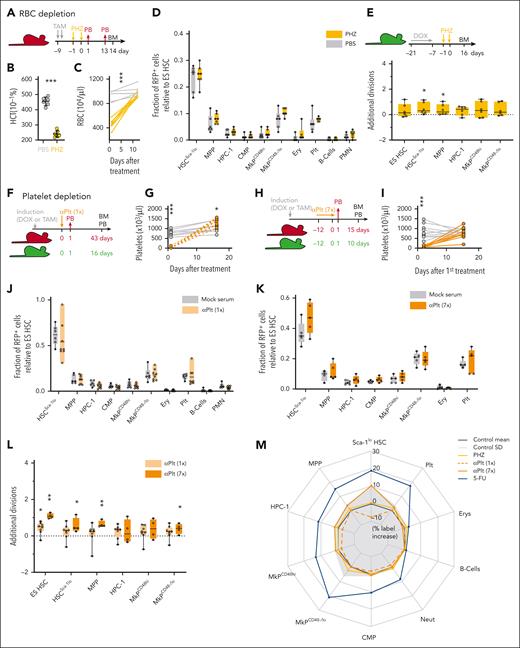
Limited contribution of HSCs to recovery from blood loss. (A-E) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP (in panels A-D, n = 6-7 per condition) and R26rtTA/Col1A1H2B-GFP (in panel E, n = 6-7 per condition) mice were IP injected with phenylhydrazine (PHZ) or PBS. Hematocrit (HCT) 24 hours after PHZ application (B) and PB RBC numbers (C) are shown. (D) Fraction of RFP-labeled cells (relative to ES HSCs). (E) Average number of additional divisions in response to PHZ. (F-L) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP (in panels F-K, n = 4-8 per condition) and R26rtTA/Col1A1H2B-GFP (in panel L, n = 4-8 per condition) mice were treated with antiplatelet (αPlt) serum or control serum following a single (once in panels F-G, J, and L) or repetitive (7 times in panels H-I and K-L) application protocol. (G-I) Platelet numbers in PB. (J-K) Fraction of RFP-labeled cells (relative to ES HSCs). (L) Average number of additional divisions in response to platelet depletion. (M) Net effects of RBC or platelet depletion on RFP label propagation (% label increase relative to ES HSCs; transformation and display of data as in Figure 1P).
Limited contribution of HSCs to recovery from blood loss. (A-E) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP (in panels A-D, n = 6-7 per condition) and R26rtTA/Col1A1H2B-GFP (in panel E, n = 6-7 per condition) mice were IP injected with phenylhydrazine (PHZ) or PBS. Hematocrit (HCT) 24 hours after PHZ application (B) and PB RBC numbers (C) are shown. (D) Fraction of RFP-labeled cells (relative to ES HSCs). (E) Average number of additional divisions in response to PHZ. (F-L) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP (in panels F-K, n = 4-8 per condition) and R26rtTA/Col1A1H2B-GFP (in panel L, n = 4-8 per condition) mice were treated with antiplatelet (αPlt) serum or control serum following a single (once in panels F-G, J, and L) or repetitive (7 times in panels H-I and K-L) application protocol. (G-I) Platelet numbers in PB. (J-K) Fraction of RFP-labeled cells (relative to ES HSCs). (L) Average number of additional divisions in response to platelet depletion. (M) Net effects of RBC or platelet depletion on RFP label propagation (% label increase relative to ES HSCs; transformation and display of data as in Figure 1P).
Histone 2b-green fluorescent protein (H2B-GFP) label retention data from R26rtTA/Col1A1H2B-GFP mice was normalized to express the number of additional divisions in perturbed vs control animals by subtracting the H2B-GFP mean fluorescence intensity of a specific animal and population from the corresponding mean fluorescence intensity thereof in control animals (supplemental Figure 3D-E). For more details on data normalization, refer to supplemental Methods.
All graphs shown in main or supplementary figures are designed with the following properties: individual mice are shown; box-plots show medians, lower, and upper quartiles; dotted lines represent means of untreated controls; bar graphs show means; significance between perturbed and control mice was calculated by an unpaired Student t test except for supplemental Figures 2D, 3B, and 4R, in which a 1-way analysis of variance with Tukey’s post-hoc test was applied (∗P ≥ .05; ∗∗P ≥ .01, ∗∗∗P ≥ .001, nonsignificant differences are not indicated).
Results
Fate mapping and proliferation tracking of perturbed hematopoiesis
To investigate the contribution of primitive HSCs to hematopoietic stress responses, we used the Fgd5ZsGreen:CreERT2/R26LSL-tdRFP fate mapping mouse model,20,21 in which TAM selectively induces inheritable RFP expression in adult immunophenotypic (lin− Sca-1+ Kit+ [LSK] CD48−/lo CD150+) (Figure 1A-C; see supplemental Figure 1A-D for gating) HSCs.22 As previously established,22 this model preferentially labels a small subpopulation of HSCs at the apex of the hematopoietic hierarchy identified by high surface expression of CD201 (EPCR) and Sca-1 (termed “ES HSCs” or “tip” HSCs). ES HSCs overlapped with other HSC subsets known for high transplantation potential, namely Sca-1hi HSCs23 and Fgd5+ HSCs21,24 (supplemental Figure 2A-C) and expressed the highest levels of Fgd5 reporter (supplemental Figure 2D). In addition, ES HSCs performed better upon transplantation than HSCs, which were solely selected based on Fgd5 reporter expression (supplemental Figure 2E-G). To monitor the propagation of label from ES HSCs to their progeny and to adjust for the interindividual variability of labeling efficiency, we calculated the fraction of ES HSC–derived label in each cell population under investigation. This approach revealed that ES HSCs constantly gave rise to other HSC subsets (Figure 1D; supplemental Figure 2G), as evidenced by the equilibration of their labeling within the first year of life and revealing that the system initially indeed labeled “tip” HSCs.22,25 Interestingly, long-term fate mapping also revealed that a considerable offset in labeling between all HSC subsets and multipotent progenitors (MPPs) persisted during 2 years of chase, implying a bottleneck of differentiation between these compartments (supplemental Figure 2G). Of note, several studies that reported the active contribution of HSC to steady-state hematopoiesis26,27 used the Fgd5ZsGreen:CreERT2 allele in combination with the R26LSL-tdTom Cre excision reporter (“Ai14”28) for labeling of hematopoietic stem and progenitor cells (HSPCs). However, this R26LSL-tdTom reporter has a lower activation threshold29 than the R26LSL-tdRFP reporter used in our study, which explains the qualitative and quantitative differences in labeling of both Fgd5-driven fate mapping models. In sum, induction of the Fgd5ZsGreen:CreERT2/R26LSL-tdRFP model results in HSC-specific labeling, which is initially enriched in ES HSCs, a population of primitive HSCs with high transplantation potential.
In addition to HSC label propagation, we determined the divisional history of HSPCs using R26rtTA/Col1A1H2B-GFP mice (Figure 1E).19,30 DOX administration to these mice induces ubiquitous H2B-GFP labeling. After withdrawal of the inducer, each cell division halves H2B-GFP intensity until background fluorescence levels are reached (Figure 1F).31
Acute inflammatory signaling under stress conditions perturbs HSC surface marker expression (supplemental Figure 3A-B) and results in contamination of the immunophenotypic HSC population with progenitor cells.24,32 However, both reporter mouse models record either cell division (H2B-GFP dilution) or contribution of primitive HSCs (RFP label propagation) in a cumulative fashion and can be read out after the acutely perturbed hematopoietic system has returned to steady state and faithful marker expression is restored.
Myeloablation stimulates ES HSC activity
The hematopoietic system is particularly susceptible to ionizing radiation or chemotherapy, procedures that rapidly deplete cycling HSPCs and are commonly referred to as myeloablation. To investigate the contribution and proliferation of primitive HSCs in response to this perturbation, we subjected previously induced Fgd5ZsGreen:CreERT2/R26LSL-tdRFP as well as R26rtTA/Col1A1H2B-GFP reporter mice to either 2 Gy γ-radiation or 5-FU (Figure 1G-P; supplemental Figure 3C-H). To control for successful myeloablation, PB analysis 7 days later revealed a profound reduction of lymphocytes or reticulocytes (Figure 1H and I, respectively). We analyzed the composition of the lin− BM compartment 5 weeks after perturbation and found a significant reduction of HSCs and MPPs after irradiation, whereas all compartments of 5-FU–exposed BM appeared normal (Figure 1J-K). ES HSCs represent the ultimate source of propagated label and therefore serve as the reference for normalization. Myeloablation did not alter the RFP labeling of ES HSCs (Figure 1L) but strongly accelerated label propagation from ES HSCs to Sca-1lo HSCs (Figure 1M; supplemental Figure 2G), which comprise more differentiated cells with low transplantation potential.22,23,33 This label also reached progenitors and, to a lesser extent, mature blood cells. To extract the net effect of both perturbations on proliferation, we calculated the additional divisions of HSPCs in myeloablated animals compared with controls. This revealed ∼3 additional divisions in HSCs, including ES HSCs in response to both perturbations (Figure 1N-O; supplemental Figure 3C-E). γ-radiation resulted in massive proliferation of MPPs and HPC-1s, thereby diluting the H2B-GFP label to the range of background controls and likely exceeding the maximum number of ∼4 to 6 traceable divisions. Likewise, we calculated the net effect caused by perturbation on label propagation by subtracting the labeling of controls from the labeling of perturbed animals and plotted this data as a radar chart (Figure 1P; supplemental Figure 3F-H). Taken together, our models faithfully reported the accelerated proliferation and differentiation of ES HSCs expected upon myeloablation.7,9,10
Rare contribution of ES HSCs to emergency myelopoiesis
Although myeloablation proved a potent but rather artificial stimulus for ES HSC activation, inflammatory signaling resulting from infection represents a more natural hematopoietic stress response. LPS is a major molecular pattern of gram-negative bacteria and triggers the expression of inflammatory cytokines via the activation of Toll-like receptor 4.34 HSCs were reported to directly sense LPS, thereby entering the cell cycle to initiate emergency myelopoiesis, and LPS exposure in vivo caused loss of HSC transplantation potential (as previously reviewed18). We IP injected TAM-induced Fgd5ZsGreen:CreERT2/R26LSL-tdRFP mice with a single dose of LPS and demonstrated the induction of emergency myelopoiesis by rapid recruitment of neutrophils to the injection site and increased interleukin 6 levels in PB plasma (Figure 2A-C). Unexpectedly, the massive acceleration of blood cell output upon LPS exposure was not reflected in faster label propagation from ES HSCs throughout the hematopoietic system (Figure 2D; supplemental Figure 4A-D). Although there was a slight label increase in Sca-1lo HSCs, MPPs, and common myeloid progenitors, labeling of mature erythrocytes and leukocytes did not increase, demonstrating that label from ES HSCs does not reach mature myeloid cell populations even within 2 weeks after LPS-induced emergency myelopoiesis. Consistent with HSC fate mapping, LPS injection did not induce robust proliferation of early HSPCs (Figure 2E; supplemental Figure 4E). The analysis of TAM-induced fate mapping mice 24 hours after LPS injection excluded the immediate propagation of HSC-derived label to progenitors, arguing against a rapid and transient differentiation wave of primitive HSCs (supplemental Figure 4F-J).
G-CSF induces myeloid differentiation, as well as HSPC mobilization35, and G-CSF secretion by endothelial cells in response to systemic LPS administration was identified as a key event of emergency myelopoiesis.36 We injected previously labeled Fgd5ZsGreen:CreERT2/R26LSL-tdRFP and R26rtTA/Col1A1H2B-GFP mice for 5 consecutive days with G-CSF (Figure 2F-J; supplemental Figure 4K-N) and demonstrated mobilization of BM HSPCs to the spleen and PB (Figure 2G-H) on day 6. BM analysis conducted 47 and 25 days after G-CSF administration, respectively, uncovered a pattern of RFP label propagation (Figure 2I; supplemental Figure 4K-M) and HSPC proliferation (Figure 2J; supplemental Figure 4N), which resembled the condition in our LPS-treated animals. Specifically, we found moderately increased labeling of MPPs, but the ES HSC–derived label did not reach mature blood lineages. Again, G-CSF did not robustly stimulate additional divisions in HSCs and early progenitor cells. Because HSPC egress from the BM to the periphery is a major effect of G-CSF35 and has previously been linked to cell proliferation,37,38 we investigated the divisional activity of HSPCs directly after mobilization (supplemental Figure 4O-R) and detected a similar divisional history (≤1 additional division) of both mobilized and control HSCs analyzed in the BM and spleen. In contrast, the comparison of PB and BM in G-CSF–mobilized animals revealed extensive proliferation of PB HSCs (supplemental Figure 4O-P) which expressed surface markers linked to proliferation and differentiation (supplemental Figure 4Q-R), suggesting selective mobilization of less primitive HSCs into the bloodstream.
Type I IFN stimulates ES HSC divisions but little differentiation
Acute type I interferon (IFN) exposure stimulates the cell cycle entry of quiescent HSCs.39 The synthetic double-stranded RNA analogue pI:C induces type I IFN and inflammatory cytokines via Toll-like receptor 3 activation and recapitulates key features of a viral infection. Repetitive pI:C application was shown to attrite HSC transplantation potential.40 To induce acute or prolonged type I IFN signaling, we injected labeled fate mapping and proliferation tracking mice once or repetitively (8 times) with pI:C (Figure 2K-O; supplemental Figure 4S-Z), which resulted in an increase of PB leukocytes and reticulocytes that was persistent until 7 days after injection (Figure 2L). ES HSC label propagation was not accelerated upon a single administration of pI:C (Figure 2M; supplemental Figure 4U). Repetitive pI:C injections resulted in moderate, albeit significant, increases of labeled cells in all analyzed progenitor and mature blood cell populations, with the exception of more committed Sca-1lo HSCs (Figure 2N; supplemental Figure 4Y). Importantly, pI:C robustly stimulated the mitotic activity of HSPCs in a dose-dependent manner, with at least 5 additional divisions on average in HSCs and MPPs upon repetitive pI:C application (Figure 2O; supplemental Figure 4V,Z). Taken together, acute inflammatory cytokine signaling mimicking bacterial or viral infections did not induce a robust contribution of primitive HSCs to mature blood cells (Figure 2P). Only in response to prolonged type I IFN signaling did HSPCs vigorously proliferate, but compared with myeloablation, which robustly accelerated label acquisition throughout the hierarchy, this resulted in weaker propagation of the ES HSC–derived label.
Massive loss of erythrocytes and platelets does not stimulate ES HSC output
Red blood cells (RBCs) constitute 90% of all blood cells and account for 80% to 90% of all daily renewed cells in mice and men.41 To model acute blood loss, we injected our labeled reporter mouse models with 2 doses of PHZ (Figure 3A-E; supplemental Figure 5A-D), which destroys RBCs and potentially elicits inflammatory side effects.42 One day after PHZ application, we observed halved hematocrit levels and RBC numbers, which equals a loss of ∼7.5 × 109 cells per mouse, but complete regeneration was achieved by day 14 (Figure 3B-C). However, in PHZ-perturbed mice, we did not observe increased label propagation from ES HSC (Figure 2D; supplemental Figure 5A-C), and HSPC proliferation was not accelerated (Figure 3E; supplemental Figure 5D), which revealed that billions of additional RBCs were regenerated without significant input from HSCs.
Similar to RBCs, low platelet counts have potentially lethal consequences. To model acute as well as chronic thrombocytopenia, we depleted platelets by either a single or repeated injection of antiplatelet serum (Figure 3F-L; supplemental Figure 5E-L). Platelet numbers were completely recovered within 14 days after treatment (Figure 3G,I), but ES HSC–derived label propagation was neither accelerated by acute nor chronic platelet depletion (Figure 3J-K; supplemental Figure 5G,K). Antiplatelet serum dose-dependently induced less than or equal to a single additional division on average in early HSPCs (Figure 3L; supplemental Figure 5H,L), as previously reported.43 Taken together, depletion of mature platelets and RBCs did not elicit a substantial contribution of primitive HSCs to mature blood cells (Figure 3M).
Discussion
Primitive HSCs with high transplantation potential are regarded as a reserve population that directly fuels emergency hematopoiesis.15-17 We investigated the contribution of these cells to hematopoietic stress responses under native conditions and found that the flux from ES HSCs to their immediate progeny did only marginally increase in response to perturbations, and did not reach more distant populations, thereby raising the question of how hematopoietic stress responses are hierarchically organized.
Transiently boosted output rates from ES HSCs during emergency can be captured by fate mapping, as they accelerate label equilibration between ES HSCs and progenitors and result in permanently altered labeling of ES HSC progeny. Our Fgd5ZsGreen:CreERT2/R26LSL-tdRFP fate mapping model labels ∼10% of ES HSCs, which then serve as a proxy for the entire population. This assumption is substantiated by the following observations: (1) the labeling of ES HSCs and total immunophenotypic HSC completely equilibrated starting from 1 year after induction (Figure 1D), which must result from similar contribution of labeled and unlabeled ES HSCs; (2) this label steadily propagated in a linear fashion to all mature blood cell lineages; and (3) most of the long-term transplantation potential is confined to ES HSCs and similar in labeled and unlabeled ES HSCs (supplemental Figure 2E-F). How blood regeneration can evoke increased input from HSCs is exemplified by myeloablation, which represents a severe, potentially life-threatening perturbation and robustly accelerated HSPC proliferation, as well as the propagation of the ES HSC–derived label. In addition, this demonstrated the feasibility of both H2B-GFP– and Cre/loxP-based tracking strategies and argued against previously reported adverse effects of label induction.44-46 Of note, even in response to myeloablative irradiation or chemotherapy, only a minority of ES HSCs differentiated into their immediate progeny (Figure 1M). Likewise, upon transplantation, HSCs on their own are insufficient to ensure the short-term survival of the myeloablated host, and erythromyeloid progenitors were shown to be crucial for initial radioprotection until HSCs provide long-term repopulation.47 Hence, HSCs are not able to rapidly generate sufficient numbers of mature blood cells, despite their superior self-renewal potential.
In contrast to myeloablation, primitive HSCs only marginally contributed to mature blood cell production under conditions mimicking an acute bacterial or viral infection. Importantly, ES HSC–derived label did not reach mature myeloid cells faster upon LPS- or G-CSF–induced perturbation, demonstrating that this stress response was achieved without direct input from primitive HSCs. It is conceivable that less primitive HSC subsets (eg, Sca-1lo HSCs) respond to emergency myelopoiesis independently from ES HSCs. Although our fate mapping mouse model preferentially labels ES HSCs, we still observe considerable labeling of Sca-1lo HSCs (three- to fivefold higher than in MPPs). Therefore, a significant contribution of non–ES HSC would be traceable, but we found that the labeling offset between HSC subsets and MPPs resisted all perturbations, implying that all HSCs did not considerably increase their output and regeneration was initiated by MPPs, as recently demonstrated.48
Hematopoietic perturbations could result in a rapid and direct differentiation of HSCs into short-lived progeny without considerable self-renewal. Our fate mapping model may not capture transient HSC progeny or input emanating from a few HSCs. Such behavior would assume that stress-induced HSC progeny has fundamentally altered differentiation and self-renewal properties at all hierarchical levels. However, our fate mapping experiments revealed a stable and increased labeling of short-lived granulocytes and platelets 5 weeks after myeloablation (Figure 1M), arguing for similar properties of HSC progeny under stress and homeostatic conditions. Moreover, BM analysis 24 hours after LPS perturbation did not reveal a label increase in myeloid progenitors, providing evidence against a transient wave of mature myeloid progeny originating directly from HSCs (supplemental Figure 4F-J).
Although acute LPS or G-CSF stimulation provoked only minimal cell division of HSPCs, type I IFN exposure robustly induced proliferation, as previously reported.39,40 Given the massive proliferation of HSPCs in response to escalating doses of pI:C, this protocol induced significant but surprisingly little differentiation of labeled ES HSCs. We speculate that a massive type I IFN response provoked cell death in HSPCs and that proliferation of residual cells compensated for this loss without simultaneously increasing the differentiation flux from ES HSCs. Indeed, exaggerated type I IFN was shown to induce cell death.49 However, HSCs were reported to be refractory to its destructive effects.50,51 This may explain why chronic pI:C perturbation resulted in only marginally higher RFP label propagation than the acute pI:C protocol.
Our finding that primitive HSCs participate rarely, if at all, in hematopoietic stress responses contrasts with the frequent observation that acute infection and inflammation stimulate cell cycle entry and profoundly reduce HSC transplantation potential (as previously reviewed18). This phenomenon has usually been linked to attrition of HSC during stress responses52 and can be exacerbated further by applying chronic or severe perturbation protocols. Our results now show that primitive HSCs with high transplantation potential do not robustly proliferate or differentiate in response to acute inflammation. This discrepancy might be explained by acute inflammatory signaling confounding surface marker expression and resulting in contamination of the HSC population with cycling progenitors. Accordingly, cell cycle entry as well as attrition of HSC transplantation potential in response to inflammation were not observed when HSCs were defined by Fgd5,24,53 CD86,32 or EPCR expression.53
The main regenerative burden of the hematopoietic system under steady-state conditions is the production of RBCs and platelets.1 Interestingly, the rapid recovery after a severe and potentially life-threatening reduction of either cell type occurred without significant ES HSC input. RBCs can solely be replaced by a massive proliferation of progenitor cells, whereas thousands of platelets are generated by a single megakaryocyte. Accordingly, the direct differentiation of HSCs into megakaryocytes is well documented.22,54-56 Both amplification strategies, however, might be too slow for emergency responses emanating from the relatively small HSC population. To rapidly put out 109 RBCs, 104 HSCs would have to simultaneously undergo massive expansion and maturation within several days. Similarly, the process of endoreplication in nascent megakaryocytes might take too long to boost emergency thrombopoiesis. Reports of an inflammation-induced HSC-like megakaryocyte progenitor population could result from omitting Sca-1 from the HSPC gating strategy.57 Thus, these cells could actually correspond to CD48-/lo megakaryocyte progenitors,22 which immunophenotypically resemble HSCs except for lacking Sca-1 and EPCR expression.
Our results argue that primitive HSCs do not represent a reserve population for commonly encountered hematopoietic challenges, such as blood loss and inflammation, and only chronic inflammatory or myeloablative hematopoietic stress warrants increased HSC input. Instead, we speculate that primitive HSCs slowly differentiate or “trickle-down” to constantly replenish and rejuvenate progenitor pools at a very low rate, whereas acute stress conditions only mildly amplify this process. Consequently, many hematopoietic perturbations must be primarily compensated by increased self-renewal and contribution of progenitors. Given the underappreciated self-renewal capacity and longevity of MPPs under native conditions,12 these cells are in principle well equipped to compensate hematopoietic perturbations independent of HSCs. The fact that only a minor fraction of humans ever suffer from BM failure in spite of the lifelong recurrence of infections also argues for a reevaluation of the concept of stress-induced HSC attrition.
Acknowledgments
The authors thank Christa Haase, Livia Schulze, and Luisa Röbisch for expert technical assistance, and Hans-Reimer Rodewald and Thomas Höfer for sharing of unpublished data.
This work was supported by funding from the German Research Council (DFG, GE3038/1-1) (A.G.) and (TR237 [B17]) (A.R.).
Authorship
Contribution: C.M.M. performed experiments with help from N.D. and M.C.; T.G. provided methodology; A.G. conceived and supervised the study; A.G. and C.M.M. wrote the paper; and A.R. revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Gerbaulet, Institute for Immunology, Medical Faculty, TU Dresden, Fetscherstraße 74, 01307 Dresden, Germany; e-mail: alexander.gerbaulet@tu-dresden.de.
References
Author notes
Data are available on request from the corresponding author, Alexander Gerbaulet (alexander.gerbaulet@tu-dresden.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Fate mapping and proliferation tracking of perturbed hematopoiesis. (A) How perturbations of hematopoiesis alter contribution of a primitive HSC subset will be studied by fate mapping (red dots and arrow) and proliferation tracking (green shades and arrow). (B) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP mouse model for fate mapping of HSCs. (C-D) RFP labeling of HSPCs isolated from TAM-induced Fgd5ZsGreen:CreERT2/R26LSL-tdRFP mice. Data from Morcos et al.22 (C) Initial labeling frequencies of BM HSPCs 10 days after induction. (D) Labeling of total HSCs (black line) relative to ES HSCs (dotted line, 8-20 mice per time point). (E) R26rtTA/Col1A1H2B-GFP mouse model for proliferation tracking of HSPCs. (F) Representative H2B-GFP histograms of ES HSCs isolated from 5-FU–perturbed and control mice (supplemental Figure 3C-E). (G-M) Fgd5ZsGreen:CreERT2/R26LSL-tdRFP mice were TAM-induced and perturbed with either 2 Gy γ-radiation (n = 4), 5-FU (n = 5) or left untreated (n = 5). Experiment scheme (G), PB lymphocyte count 7 days after irradiation (H), PB reticulocyte numbers 7 days after 5-FU (I), and ratios of relative BM compartment sizes (% cells among total lin− BM cells) between perturbed and control (dotted line) animals (J-K) are shown. (L) Percentages of RFP+ ES HSCs. (M) Fraction of RFP-labeled cells relative to ES HSCs (significant differences between untreated and either 5-FU–perturbed or irradiated mice was calculated, all comparisons between unperturbed and irradiated mice are not significant). (N-O) R26rtTA/Col1A1H2B-GFP animals were doxycycline (DOX)–pulsed and exposed to either 2 Gy (n = 5), 5-FU (n = 5), or left untreated (n = 6). Experiment scheme (N) and numbers of additional divisions in response to perturbation (O) are shown (supplemental Figure 3C-E). (P) Transformation of data shown in panel M to visualize the net effects of myeloablation on label propagation. Mean (dark gray line) and variance (standard deviation [SD], shaded area) of control mice (n = 41 from 8 independent experiments) are shown (supplemental Figure 3F-H). CMP, common myeloid progenitor; Ery, erythrocyte; MkP, megakaryocyte progenitor; Neut, neutrophil; Plt, platelet; PMN, polymorphonuclear cell.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/20/10.1182_blood.2022018996/2/m_blood_bld-2022-018996-gr1.jpeg?Expires=1769106129&Signature=jb1e6DbkfhJD572ds~vxchBh8JVd5fYPSkdVgKv3fqo7bTJSpGN2sFlo4mNdHewO7Pl7Uu01GC7yJn0~BRCxKp1-fREU22bHN50mwqS2E-gU-HF-V7SpCBbOi-z1We1h70SUQev957sGjf6Pc5Y-EYrVdFdu6puUOimSZo-WZ1zo~0ybu3ThvaB~-CS2bL0Li3uAIFqMI74b0T~-9QLriVFJbyxFJw~2uDdc1e2ugNk3RoVPywAi1GlNq266zU3BrEda-OWcoemyIkAdjY0EMaAxYNSNyYafjNkQy3XuMplVRF~wG2JqKNoTYQJz1wVAmEcJCkG7S5QCPy-mKES4zw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal