In this issue of Blood, Davis et al1 report their experience with tixagevimab–cilgavimab preexposure prophylaxis in a cohort of 251 patients with chronic lymphocytic leukemia (CLL), B-cell lymphomas, multiple myeloma, or B-cell acute lymphoblastic leukemia.
With a case-fatality rate up to 34% in hospitalized patients in the prevaccine era2 and a significantly reduced response to vaccination, COVID-19 represents a major issue for patients with tumors of the hematopoietic and lymphoid tissues. Searching for additional strategies to better protect patients with hematological malignancies, the use of preexposure prophylaxis seemed a smart and feasible approach. In patients whose immune system function is compromised, passive immunization relying on effective neutralizing antibodies administered once to achieve protective antibody levels regardless of the B-cell function should mean long-term protection and reduced risk. Indeed, the PROVENT study showed a symptomatic infection rate of only 0.2% in a study population chosen because of a low probability of responding to vaccination or higher risk of exposure in those treated with tixagevimab–cilgavimab. There was a 77% reduction in the risk of symptomatic COVID-19 compared with placebo.3 That said, only 383 of 5197 (7.4%) subjects in the study were considered at high risk of infection because of a cancer diagnosis and only 24 (0.5%) because of an immunosuppressive disease.
The report of Davis et al provides relevant information on the complex area of SARS-CoV-2 infection prevention and management in the most difficult to protect category of patients with hematological malignancies, that is, those with B-cell lymphoproliferative disorders. Even though recent reports suggest a reduced incidence of severe SARS-CoV-2 infections with fewer patients in need of hospitalization and a lower case fatality rate, this group of patients, in particular those with active disease or those requiring treatment, remains more vulnerable to adverse clinical outcomes including severe infections and deaths.4 This vulnerability is potentially driven, at least in part, by the impaired response to SARS-CoV-2 vaccines with a seroconversion rate and T-cell response definitely lower than healthy subjects or people with solid tumors. The lowest rates were detected in patients with CLL, with seroconversion ranging from 23% to 66%5 and T-cell responses being present in 30% to 40%.6
At a median follow-up of 3 months from preexposure prophylaxis, Davis et al identified 27 (11%) subjects with confirmed COVID-19 breakthrough infection, with 22 (9%) at least 30 days after tixagevimab–cilgavimab administration. Interestingly, 63% of infected patients had received at least 3 doses of SARS-CoV-2 vaccine, the vast majority of the breakthrough infections occurred in patients who had recently received B-cell-depleting agents, were on active treatment, or were within 6 months of hematopoietic stem cell transplantation. Only 4 (15%) patients required hospitalization, and no COVID-19-related death was reported. It is important to note that 63% of the patients were treated with antiviral drugs, that is, nirmatrelvir–ritonavir, molnupiravir, or remdesivir, in a timely manner. Though no specific testing for the variants was done in this retrospective study, most infections occurred between June and August 2022 when Omicron variant BA.5 was dominant. These findings support the preclinical data showing reduced efficacy of tixagevimab–cilgavimab against Omicron BA.5 compared with BA.27 and support the administration of a 300-mg + 300-mg dosage of tixagevimab–cilgavimab (as per Food and Drug Administration label) to try to offset the potentially reduced neutralization activity against Omicron BA.4/5 also in patients.
In patients with hematological malignancies, at least 2 studies on tixagevimab–cilgavimab prophylaxis with less than 3 months of follow-up showed the occurrence of symptomatic COVID-19 in about 4% of patients.8,9 Reassuringly, 0 of 52 patients in one series8 and 2 of 1112 (0.2%) in the other9 died because of COVID-19, well below the 34% mortality rate in hospitalized patients that was observed in the initial wave of COVID-19.2 Furthermore, a very low hospitalization rate (5.9%) with no COVID-related death was recently described in a preprint publication that included a large series of immunocompromised patients, 45% of whom had a hematologic disease, after preexposure prophylaxis and administration of antiviral agents.10
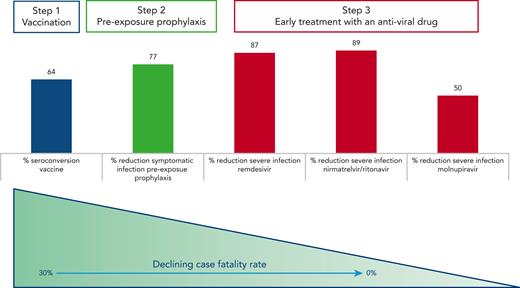
In conclusion, though the effectiveness of tixagevimab and cilgavimab in the Omicron era remains difficult to assess, the data by Davis and coworkers on patients with B-cell malignancies add to a growing body of evidence that clearly point to a dramatic improvement of the outcome of COVID-19 infection in fragile patients thanks to vaccination, preexposure prophylaxis, and early treatment with antiviral agents (see figure). We have won some battles against COVID-19 but not (yet) the war, as SARS-CoV-2 variants are rapidly changing over time. Thus, it is crucial to remain on the alert and continue to (1) inform and educate our patients on the infection risks and the importance of a timely diagnosis for administering the appropriate treatment and (2) investigate and test prophylactic and therapeutic strategies tailored in particular to protect the most vulnerable subgroups of patients, including those with impaired immune function and at higher risk of dismal outcome upon infection.
Sequence of interventions to prevent and successfully treat COVID-19 infection in patients with B-cell malignancies. Vaccinations are able to elicit seroconversion in up to two-thirds of patients with B-cell malignancies.6 Tixagevimab and cilgavimab reduced the probability of developing a symptomatic infection before the BA.4 BA.5 Omicron variants became predominant,4 and early administration of antiviral agents within 3 to 7 days from the onset of symptoms offered an effective protection against severe disease in the majority of patients.
Sequence of interventions to prevent and successfully treat COVID-19 infection in patients with B-cell malignancies. Vaccinations are able to elicit seroconversion in up to two-thirds of patients with B-cell malignancies.6 Tixagevimab and cilgavimab reduced the probability of developing a symptomatic infection before the BA.4 BA.5 Omicron variants became predominant,4 and early administration of antiviral agents within 3 to 7 days from the onset of symptoms offered an effective protection against severe disease in the majority of patients.
Conflict-of-interest disclosure: L.S. received honoraria from AbbVie, AstraZeneca, BeiGene, and Janssen; speaker bureau for Octapharma. A.C. received honoraria from AbbVie, AstraZeneca, Gilead, Janssen.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal