TO THE EDITOR:
Patients with B-cell malignancies who become infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are at an increased risk of morbidity and mortality due to advanced age, use of B-cell-depleting therapies, and immunodeficiency.1-3 Despite development of COVID-19 messenger RNA vaccines, the majority of patients with B-cell malignancies fail to develop anti-SARS-CoV-2 spike antibodies in response to vaccination, and mortality rates secondary to COVID-19 infection over 10% have been reported.4,5
Preexposure prophylaxis with AZD442/Evusheld (tixagevimab-cilgavimab) may be an alternative strategy to decrease the incidence or severity of COVID-19 for patients with B-cell malignancies. Tixagevimab-cilgavimab is a monoclonal antibody (MoAb) that inhibits attachment of the SARS-CoV-2 spike protein to the surface of cells, thereby preventing viral entry and infection by the COVID-19 virus.6 In December 2021, the Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for its use in individuals 12 years and older with moderate to severe immunodeficiency.6 Tixagevimab-cilgavimab was authorized based on results of the phase III PROVENT study, which demonstrated a breakthrough infection rate of 0.5% in unvaccinated adults at an increased risk of inadequate response or exposure to COVID-19.7 However, only 7% of patients had cancer or a history of cancer, and 3% were actively receiving immunosuppressive therapy.7
Omicron subvariants BA.1 and BA.1.1 were found to have decreased COVID-19 neutralization in response to tixagevimab-cilgavimab.6 Thus, in February 2022, the FDA revised the EUA to include an increase of the recommended dose from 150/150 mg to 300/300 mg based on data suggesting the higher dose would be more likely to prevent infection by these variants.6 The currently recommended dosing interval is every 6 months while SARS-CoV-2 remains in circulation. We sought to determine the real-world efficacy and incidence of COVID-19 breakthrough infections in patients with B-cell malignancies receiving preexposure prophylaxis with tixagevimab-cilgavimab.
We retrospectively analyzed medical records at our institution to define the incidence of COVID-19 infection in patients with B-cell malignancies who received preexposure prophylaxis with tixagevimab-cilgavimab from January 2022 to August 2022. Patients were included if they had been diagnosed with a B-cell malignancy, including chronic lymphocytic leukemia (CLL), B-cell lymphoma, multiple myeloma, or B-cell acute lymphoblastic leukemia and had received tixagevimab-cilgavimab prior to data cutoff. These patients were eligible for tixagevimab-cilgavimab per institutional protocol if they were receiving active treatment for a hematological malignancy (Bruton tyrosine kinase inhibitor [BTKi], anti-CD20 MoAb, anti-CD38 MoAb), were within 1 year of chimeric antigen receptor T-cell therapy or hematopoietic stem cell transplant (HSCT), or had a diagnosis of CLL with a negative antibody titer post vaccination. COVID-19 infection was defined by a confirmed positive SARS-CoV-2 polymerase chain reaction or rapid antigen testing. Immunoglobulin g (IgG) spike antibodies were measured with DiaSorin’s LIAISON SARS-CoV-2 TrimericS assay. Data including patient demographics, diagnosis, cancer treatment, COVID-19 vaccination history, and infection characteristics were collected from the electronic health record.
Among the 251 patients included, the median age was 66 years (range 18-91) and 149 (59%) patients were male. The median prior lines of therapy was 1 (range 0-14), and median follow-up time from the initial tixagevimab-cilgavimab dose was 141 days (range 8-221). Fourteen (5%) patients received a single 150/150-mg dose, and 237 (95%) received a cumulative first dose of 300/300 mg (58 patients received 2 separate administrations of 150/150 mg dose as a result the FDA revision to the EUA to increase of the recommended dose from 150/150 mg to 300/300 mg). Eleven (4%) patients received a repeat 300/300-mg dose 6 months after their first full dose. At a median of 79 days (range, 3-154), 53 (79%) patients tested for IgG spike antibodies before tixagevimab-cilgavimab had titers below 264 arbitrary units (AU)/mL, previously reported to adequately protect against SARS-CoV-2 infection in immunocompetent patients.8 All patients tested for IgG spike antibodies after tixagevimab-cilgavimab administration had antibody titers ≥264 AU/mL. Overall, tixagevimab-cilgavimab was well tolerated with 2 patients experiencing low-grade adverse reactions including diarrhea and rash. One patient with a history of epilepsy experienced a self-resolving seizure within minutes of administration. Detailed clinical characteristics are presented in Tables 1 and 2.
Characteristics of patients with B-cell malignancies who received tixagevimab-cilgavimab
| Patient variable . | SARS-CoV-2 negative (n =224) . | SARS-CoV-2 positive (n = 27) . |
|---|---|---|
| Median age, y (range) | 66 (18-91) | 67 (22-80) |
| ≥65 y, n (%) | 119 (53) | 10 (47) |
| Sex, n (%) | ||
| Male | 132 (59) | 18 (67) |
| Female | 92 (41) | 9 (33) |
| Diagnosis, n (%) | ||
| B-ALL | 8 (4) | 1 (4) |
| CLL | 52 (23) | 6 (22) |
| DLBCL | 48 (22) | 6 (22) |
| FL | 16 (7) | 3 (11) |
| MCL | 18 (8) | 3 (11) |
| MZL | 5 (3) | 0 (0) |
| MM | 32 (14) | 2 (7) |
| PCNSL | 4 (2) | 1 (4) |
| WM | 10 (4) | 1 (4) |
| Other | 31 (14) | 4 (15) |
| Performance status (ECOG), n (%) | ||
| 0-1 | 194 (87) | 23 (85) |
| 2 or higher | 30 (13) | 4 (15) |
| Median prior lines of therapy (range) | 1 (0-14) | 1 (1-6) |
| Prior hematopoietic stem cell transplant, n (%) | ||
| Allogeneic | 12 (5) | 2 (7) |
| Autologous | 43 (19) | 3 (11) |
| Prior CAR T-cell therapy, n (%) | ||
| NHL | 13 (6) | 0 (0) |
| MM | 6 (3) | 0 (0) |
| SARS-CoV-2 infection prior to tixagevimab-cilgavimab, n (%) | 70 (31) | 5 (19) |
| Median prior SARS-CoV-2 vaccines, n (range) | 3 (0-5) | 3 (0-5) |
| Patient variable . | SARS-CoV-2 negative (n =224) . | SARS-CoV-2 positive (n = 27) . |
|---|---|---|
| Median age, y (range) | 66 (18-91) | 67 (22-80) |
| ≥65 y, n (%) | 119 (53) | 10 (47) |
| Sex, n (%) | ||
| Male | 132 (59) | 18 (67) |
| Female | 92 (41) | 9 (33) |
| Diagnosis, n (%) | ||
| B-ALL | 8 (4) | 1 (4) |
| CLL | 52 (23) | 6 (22) |
| DLBCL | 48 (22) | 6 (22) |
| FL | 16 (7) | 3 (11) |
| MCL | 18 (8) | 3 (11) |
| MZL | 5 (3) | 0 (0) |
| MM | 32 (14) | 2 (7) |
| PCNSL | 4 (2) | 1 (4) |
| WM | 10 (4) | 1 (4) |
| Other | 31 (14) | 4 (15) |
| Performance status (ECOG), n (%) | ||
| 0-1 | 194 (87) | 23 (85) |
| 2 or higher | 30 (13) | 4 (15) |
| Median prior lines of therapy (range) | 1 (0-14) | 1 (1-6) |
| Prior hematopoietic stem cell transplant, n (%) | ||
| Allogeneic | 12 (5) | 2 (7) |
| Autologous | 43 (19) | 3 (11) |
| Prior CAR T-cell therapy, n (%) | ||
| NHL | 13 (6) | 0 (0) |
| MM | 6 (3) | 0 (0) |
| SARS-CoV-2 infection prior to tixagevimab-cilgavimab, n (%) | 70 (31) | 5 (19) |
| Median prior SARS-CoV-2 vaccines, n (range) | 3 (0-5) | 3 (0-5) |
B-ALL, B-cell acute lymphoblastic leukemia; DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Performance Group; FL, follicular lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; MZL, marginal zone lymphoma; NHL, non-Hodgkin lymphoma; PCNSL, primary central nervous system lymphoma; WM, Waldenström macroglobulinemia.
Clinical impact of IgG spike antibody titers and dose of tixagevimab-cilgavimab in patients with B-cell malignancies who received tixagevimab-cilgavimab
| . | SARS-CoV-2 negative . | SARS-CoV-2 positive . | P . |
|---|---|---|---|
| IgG spike antibody titer beforetixagevimab-cilgavimab(AU/mL) | 63 | 4 | .1901 |
| Median (range) | 250 (14-800) | 309 (41-643) | |
| ≥264, n (%) | 12 (19) | 2 (50) | |
| <264, n (%) | 51 (81) | 2 (50) | |
| IgG spike antibody titer after totixagevimab-cilgavimab(AU/mL) | 25 | 4 | >.999 |
| Median (range) | 800 (526-800) | 681 (588-800) | |
| ≥264, n (%) | 25 (100) | 4 (100) | |
| <264, n (%) | 0 (0) | 0 (0) | |
| Tixagevimab-cilgavimab dose (mg), n (%) | 224 | 27 | .1804 |
| 150/150 | 11 (5) | 3 (11) | |
| 300/300 | 213 (95) | 24 (88) |
| . | SARS-CoV-2 negative . | SARS-CoV-2 positive . | P . |
|---|---|---|---|
| IgG spike antibody titer beforetixagevimab-cilgavimab(AU/mL) | 63 | 4 | .1901 |
| Median (range) | 250 (14-800) | 309 (41-643) | |
| ≥264, n (%) | 12 (19) | 2 (50) | |
| <264, n (%) | 51 (81) | 2 (50) | |
| IgG spike antibody titer after totixagevimab-cilgavimab(AU/mL) | 25 | 4 | >.999 |
| Median (range) | 800 (526-800) | 681 (588-800) | |
| ≥264, n (%) | 25 (100) | 4 (100) | |
| <264, n (%) | 0 (0) | 0 (0) | |
| Tixagevimab-cilgavimab dose (mg), n (%) | 224 | 27 | .1804 |
| 150/150 | 11 (5) | 3 (11) | |
| 300/300 | 213 (95) | 24 (88) |
At a median of 91 days (range 3-162) from tixagevimab-cilgavimab administration, 27 (11%) patients experienced a confirmed COVID-19 breakthrough infection. Twenty-two (81%) infections occurred at least 30 days after tixagevimab-cilgavimab administration. Among infected patients, 63% (17/27) had received B-cell-depleting therapy (rituximab, obinutuzumab, or blinatumomab) within 3 months of infection. Three (11%) infected patients were on active therapy with a BTKi (acalabrutinib, zanubrutinib), and 4 (15%) were within 6 months of HSCT but not on immunosuppressive therapy at the time of infection. The majority (63%) of infected patients had received at least 3 prior COVID-19 vaccinations. Three (11%) infected individuals had only received a single 150/150-mg tixagevimab-cilgavimab dose. Although there was not a statistically significant difference between the incidence of COVID infections between patients who received a single 150/150-mg dose compared with patients who received a cumulative 300/300-mg first dose (P = .1804, Fisher exact test), this was likely a result of the small sample size; the clinically significant benefit that was seen reinforced the incorporation of FDA revision to increase the dose to 300/300 mg (Table 1). Twenty-three (85%) infections occurred between June and August 2022 when Omicron variant BA.5 was dominant among the local population.9 Eleven (41%), 2 (7%), and 4 (15%) infected patients received postexposure treatment with nirmatrelvir/ritonavir, molnupiravir, or remdesivir, respectively. Four patients (15%) were hospitalized for severe infections, but no mortalities were observed.
Our findings suggest patients with B-cell malignancies who received preexposure prophylaxis with tixagevimab-cilgavimab and B-cell-depleting therapy or HSCT within the past 3 to 6 months may still be at risk of breakthrough COVID-19 infection. Indeed, a previous report describing 52 patients with hematologic malignancies investigated activity of tixagevimab-cilgavimab, but at a median follow-up of 79 days, the investigators only found 2 (4%) cases of breakthrough infection, both in patients who had received a single 150/150-mg dose.10 A larger study of immunocompromised patients with a median follow-up of 63 days found a breakthrough infection rate of 4.4%, although the majority of included patients did not have a hematologic malignancy.11
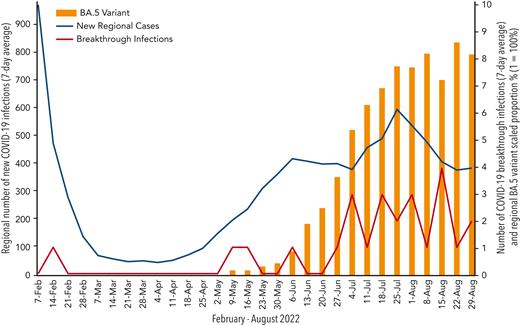
To our knowledge, this study is the first to report efficacy outcomes of the currently recommended 300/300-mg dose of tixagevimab-cilgavimab and demonstrate a clinically significant risk of breakthrough infections in patients with B-cell malignancies during the Omicron era. Our report suggests, despite vaccination and tixagevimab-cilgavimab, these patients are still at risk of infection and should continue to use precautions; this may be explained by the hypothesis of a dysregulated immune repertoire including both B and T lymphocytes seen in patients with B-cell malignancies. There does not seem to be a clinical correlation between timing of tixagevimab-cilgavimab administration and infection. More likely, the rate of breakthrough infections seems to correlate with the emergence of the Omicron BA.5 variant, which was the dominant strain in our region at the time of most infections (Figure 1). Notably, few patients with infections were hospitalized and no deaths occurred, suggesting preexposure prophylaxis with tixagevimab-cilgavimab may reduce the risk of severe infection and death in patients with breakthrough infections.
Chronological correlation of new regional cases, breakthrough cases, and COVID variant BA.5 incidence. The left vertical axis represents the 7-day average of new regional COVID-19 infections. The right vertical axis represents the 7-day average of breakthrough COVID-19 infections and the regional proportion of the BA.5 variant.
Chronological correlation of new regional cases, breakthrough cases, and COVID variant BA.5 incidence. The left vertical axis represents the 7-day average of new regional COVID-19 infections. The right vertical axis represents the 7-day average of breakthrough COVID-19 infections and the regional proportion of the BA.5 variant.
Our observations were limited by the retrospective nature of our study, a single-center population, and limitations of the data available in the electronic health record. COVID variant information was unavailable and has not been included. One patient in our infected cohort tested positive 3 days after tixagevimab-cilgavimab, which may not truly account for a breakthrough infection. In addition, we may have not have captured undiagnosed or unreported infections as patients were advised but not mandated to perform a COVID-19 test when symptomatic. A prospective, multicenter study with a larger patient population and long-term follow-up is needed to confirm our findings.
In conclusion, patients with B-cell malignancies, especially those receiving B-cell-depleting therapy or following HSCT, are at risk for breakthrough infections despite use of preexposure prophylaxis with tixagevimab-cilgavimab, although it should be emphasized hospitalization rates were low and no COVID-related deaths were observed. Our findings suggest these patients should maintain a multimodal prevention strategy including social distancing, vaccination, and tixagevimab-cilgavimab. Future studies are needed to demonstrate effectiveness of a repeat dosing strategy and whether response remains similar across viral variants.
Authorship
Contribution: J.A.D. wrote the manuscript, created the table/figure, and participated in data collection; K.G., K.R., D.S., K.J.G., M.M., A.C., A.T., A.H., L.H., H.H., and B.T.H. participated in data collection and manuscript revision.
Conflict-of-interest disclosure: B.T.H. has received consulting fees from ADC therapeutics and BMS. H.H. is on advisory boards of Sanofi, BMS, and Janssen. The remaining authors declare no competing financial interests.
Correspondence: James A. Davis, Medical University of South Carolina, 86 Jonathan Lucas St, Suite 219, Charleston, SC 29425; e-mail: davisjaa@musc.edu.
References
Author notes
There is a Blood Commentary on this article in this issue.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal