In this issue of Blood, Li et al1 demonstrate that dietary iron restriction protects mice from major vascular complications and organ failure in mouse models of sickle cell disease (SCD).
SCD is a major inherited hemolytic disorder affecting >100 000 people in the United States and millions worldwide.2 SCD results from a single amino acid substitution of valine for glutamic acid in the gene encoding β-globin chain producing hemoglobin S (HbS). Deoxygenation of HbS results in polymerization of hemoglobin, leading to sickle deformity of red blood cells (RBCs) and premature breakdown or hemolysis, ultimately resulting in anemia. Chronic hemolysis in mouse SCD increases systemic iron demand for erythropoiesis, prompting intestines to increase iron absorption. Ultimately, over time, hyperabsorption of iron leads to secondary iron overload in mice. The sickling of RBCs leads to vaso-occlusive episodes (VOEs), which contribute to tissue injury and organ failure.3 Although the molecular underpinnings of anemia and iron overload in SCD are well characterized, the mechanisms that drive VOEs in SCD have not been completely elucidated. In mice, tissue iron accumulation, inflammation, and obstruction of capillaries by sickle RBCs are known to be critically involved in the multicellular cascade that initiates VOEs. Repeated VOEs eventually cause ischemic end organ damages due to obstruction in the microcirculation.
Previous studies have shown that restriction of intestinal iron absorption ameliorates anemia and iron overload in mouse SCD.4 These strategies have also been shown to be efficacious in other mouse models of hemoglobinopathies.5,6 Moreover, clinical evidence has suggested iron restriction can improve several clinical parameters of SCD.7 More recently, dietary iron restriction was shown to reduce RBC sickling and disease severity in SCD mouse models.8 To explore the connections between dietary iron and SCD pathogenesis, Li and colleagues placed SCD mice on iron-restricted diet for 3 to 4 months and found a significant reduction in serum iron, tissue iron, and white blood cell iron levels without worsening anemia in SCD mice. Reduced mean corpuscular hemoglobin concentration and mean corpuscular hemoglobin suggested that dietary iron restriction improves cellular HbS levels and RBC sickling in mice, whereas lower serum bilirubin levels indicated the improvement in hemolysis. Further analysis revealed that iron restriction improved SCD pathology by attenuating several key features of VOEs (specifically, vascular adhesion molecule levels, neutrophil aging, leukocyte adhesion, RBC velocity, and microvascular flow rate). The investigators further confirmed this in a mouse model of cytokine-induced vaso-occlusion where SCD mice on iron-restricted diet showed a robust improvement in survival following tumor necrosis factor-α injection compared with the SCD mice on normal iron diet.
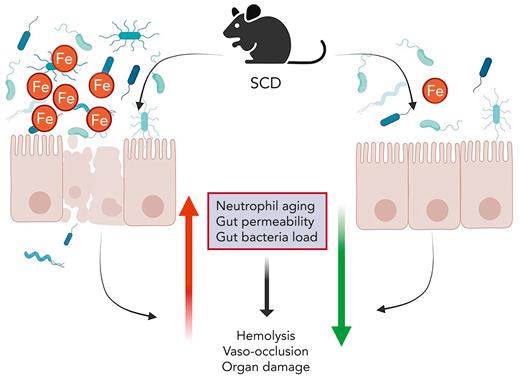
Li et al further demonstrated that SCD mice on iron-restricted diet exhibited significantly reduced hepatosplenomegaly and tissue leukocyte infiltration and improved liver function. The gut is home to a large number of microbial species that impact mammalian physiology. The epithelial barrier is essential to regulate interactions of luminal antigens and microbes with the underlying immune cells. Compromised barrier integrity is an essential mechanism that drives many local intestinal diseases and impacts other diseases. SCD mice have increased intestinal permeability and inflammation. An iron-restricted diet reduced intestinal inflammation and restored gut permeability in SCD mice. This suggests a dysregulation of the intestine/iron/microbiota cross talk in SCD. Indeed, an iron-restricted diet resulted in substantial reduction in fecal bacterial load in SCD mice. SCD mice were dysbiotic, and iron restriction increased the firmicutes/bacteroidetes ratio and reduced toll-like receptor 2 activity. This demonstrates that dietary iron restriction helps to restore gut epithelial integrity, improve microbiome composition, and prevent the systemic translocation of deleterious microbial metabolites and antigens, and it may contribute to the reduction of disease severity. However, more work is needed to definitively demonstrate the impact of the intestine and microbiota in SCD. Gut microbial communities have been shown to be significantly affected by dietary iron.
Probiotics, such as Lactobacillus species, are highly enriched following low-iron treatment.9 Indeed, Lactobacillus species have been shown to enhance barrier integrity.10 Further work in characterizing gut microbial communities, microbial metabolites, and germ-free or antibiotic experiments is needed to better define the impact of dietary iron on alterations on intestinal function and microbial changes in SCD.
Beneficial effects of iron restriction in anemia seem paradoxical, but it is well established that iron restriction strategies alleviate major SCD morbidities. This study provides new evidence that VOEs, tissue injury, and organ damage improve following dietary iron restriction (see figure). Moreover, a novel connection was identified, implicating the beneficial outcomes to changes in the host/microbiota interactions. This study shows that SCD affects gut microbial dysregulation, and it is linked with systemic iron levels, which can be successfully restored by dietary iron restriction (see figure). This study opens the possibilities of identifying novel microbial compounds with therapeutic potential for SCD management.
Iron restriction attenuates SCD pathologies in a mouse model. Iron plays a critical role in the pathogenesis of mouse SCD. Dietary iron restriction ameliorates neutrophil aging, gut epithelial disruption, and microbiota alteration, all of which impact SCD morbidities.
Iron restriction attenuates SCD pathologies in a mouse model. Iron plays a critical role in the pathogenesis of mouse SCD. Dietary iron restriction ameliorates neutrophil aging, gut epithelial disruption, and microbiota alteration, all of which impact SCD morbidities.
There are still many open questions that need to be addressed: How does iron restriction improve anemia in SCD? Is this dependent on decreasing the levels of HbS or does iron-induced oxidative stress in RBCs play a role? Are intestinal changes in SCD the mediator of local and systemic inflammatory and immune changes? What are the factors in the intestine, both host and microbial, that determine the extent of local and systemic tissue damage? Can we design studies in humans using information in mouse models to understand the efficacy of iron-restricted diets in patients with SCD?
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal